Stoichiometric f lexibility regulates the co-metabolism eff ect during organic carbon mineralization in eutrophic lacustrine sediments*
Jie MA , Fei HE , Xingcheng YAN , Ruijie SHI , Ming JI , Bin XU , Xiaodong WU ,Zhichun LI , Xiaoguang XU ,**, Guoxiang WANG
1 Nanjing Institute of Environment Sciences, Ministry of Ecology and Environment, Nanjing 210042, China 2 Sorbonne University, Paris, UMR 7619, METIS, Paris 75005, France
3 School of Environment, Nanjing Normal University, Nanjing 210023, China
4 College of Urban and Environmental Sciences, Hubei Normal University, Huangshi 435002, China
5 School of Environment and Surveying Engineering, Soochow University, Suzhou 234000, China
Keyword: co-metabolism eff ect; stoichiometric; carbon cycling; eutrophic lake; decomposition; organic carbon
1 INTRODUCTION
Organic carbon (OC) burial in lacustrine sediments represent an important sink in global carbon cycling(Cole et al., 2007; Gudasz et al., 2010). Although lakes cover only about 2% of Earth’s surface, annual OC burial in lake sediments can amount to approximately 50% of the annual OC burial in marine sediments (Sobek et al., 2009). Aquatic macrophytes are one of the primary sources of OC, which deposit residues composed of cellulose, hemicellulose,lignins, tannins, and cuticular matrix onto the surface of sediments (Li et al., 2013). The relatively recalcitrant OC accumulates gradually and then increases with the lake ecosystem succession(Hanamachi et al., 2008). However, as eutrophication becomes a more widespread environmental problem,an increasing number of shallow lakes have shifted from a clear-water state dominated by macrophytes to a turbid-water state dominated by phytoplankton (i.e.,“retrogressive succession”) (Scheff er et al., 1993,2001). Compared with the slow accumulation and decomposition of macrophyte-dominated lakes, the turnover of phytoplankton-dominated lakes is more rapid (Hanamachi et al., 2008). That is, the rate of succession may decrease in phytoplankton-dominated eutrophication lakes. Clarifying the underlying response mechanism of inputing phytoplanktonderived OC into sediments is crucial for understanding the direction and magnitude of succession in eutrophication lacustrine ecosystems.
One vital mechanism driving changes in OC reserves in terrestrial ecosystems is the priming eff ect,wherein labile OC input aff ects the extent of microbial activity mediating native OC decomposition(Kuzyakov et al., 2000; Blagodatskaya and Kuzyakov,2008; Fang et al., 2018). This process is sometimes referred to as the ‘‘co-metabolism eff ect’’ because similar observations have not been made under aseptic conditions (Horvath, 1972; Wakeham and Canuel, 2006; Van Nugteren et al., 2009). Labile sources of any form, including manure, plant residues,and root exudates (Dai et al., 2009; Phillips et al.,2012; Qiao et al., 2014; Zhu et al., 2018), can aid microbial community composition and enhance the decomposition of indigenous OC (Fontaine et al.,2004; De Graaff et al., 2010). Although the cometabolism eff ect has been widely reported in terrestrial ecosystems, this eff ect has been virtually ignored in the study of aquatic ecosystems, especially lacustrine ecosystems (Guenet et al., 2010a). A few studies have mentioned that algae may stimulate the co-metabolism eff ect in the water column (Bianchi,2011; Bianchi et al., 2015). In contrast, lake sediments collect a large amount of OC from a wide range of sources and of complex composition (Xu et al., 2015),consequently, the potential co-metabolism eff ect of phytoplankton detritus and OC in sediments may be more spatially heterogeneous. With global climate warming and eutrophication, recurrent algae blooms have been frequently observed in freshwater lakes with continuous and considerable deposition onto the surface of sediments after their decay, which provides ideal conditions for the co-metabolism eff ect between detritus and OC in sediments (Yan et al., 2017; Qin et al., 2018). In addition, the input of residual phytoplankton also maintains sediment nutrient levels in the surrounding environment (Zhao et al., 2019).Despite experimental evidence showing that nitrogen addition increases carbon dioxide (CO2) emissions and that phosphorus (P) addition inhibits methane(CH4) emissions (Huang et al., 2019; Wang et al.,2019; Ma et al., 2020), few studies have linked the co-metabolism eff ect with nutrient supply and nutrient stoichiometric control in lacustrine ecosystems.
Two distinct mechanisms have been proposed in terrestrial ecosystems to explain the co-metabolism eff ect caused by nutrient availability and OC inputs:“microbial nutrient mining” and “stoichiometry decomposition” (Chen et al., 2014). In the former, the nutrient-acquiring microbes use input carbon as an energy source to enhance OC decomposition. That is,when the nutrient supply is low, microorganisms facilitate the acquisition of nutrients via native OC mineralization (Razanamalala et al., 2018). In contrast,the co-metabolism eff ect generated by “stoichiometric decomposition” theory predicts a correlation between stoichiometric C source and nutrient ratios. In other words, decomposition rates are maximized if C and nutrient inputs match microbial demands (Fang et al.,2018). Although many studies have focused on the mechanisms by which nutrient availability aff ects the co-metabolism eff ect in terrestrial ecosystems, these underlying mechanisms have not yet been validated in aquatic ecosystems.
This study aimed to reveal the exogenous organic C, nutrient input impact on co-metabolism eff ect of sediment OC in lacustrine ecosystems. Specif ically,our objectives of this study were to (i) link nutrient availability and the co-metabolism eff ect and (ii) test the applicability of two associated but competing hypotheses of how nutrients aff ect the co-metabolism eff ect. Addressing these knowledge gaps is critical for improving the prediction of OC models in eutrophic lakes and for identifying labile OC and stoichiometric changes that can increase sediment OC.
2 MATERIAL AND METHOD
2.1 Field sampling
Fig.1 The sampling site in Taihu Lake

Table 1 Description of the C and nutrient (N, P) input treatments
Taihu Lake (30°56′N-31°33′N, 119°54′E-120°36′E) is located in the Changjiang (Yangtze)River delta in East China. It is a typical large shallow lake with an area of 2 338 km2with a mean depth of 1.9 m (Qin et al., 2007). In recent years, large-scale cyanobacteria blooms in Taihu Lake have occurred frequently because of eutrophication, especially along the northwestern bay and shorelines, which induced a serious ecological and environmental problem (Liu et al., 2015). In September 2019, surface sediments (0-10 cm) were sampled around Maodu Port in the west of Taihu Lake (Fig.1) where the sediment has accumulated to a high degree by a steady stream of algae organic detritus. The sediments were air-dried and sieved to pass through 2-mm mesh. The values of TN, TP, and TOC in sediments were 3.39±0.24,2.51±0.41, and 50.19±2.19 mg/g DW (dry weight),respectively. The TOC and TN in sediments were determined by an Element Analyzer (EA, VARIO EL,OSIC, Germany), and TP were measured by Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) (Ji et al., 2021).
2.2 Incubation experiment
Thirty grams of dry sediment were weighed in 500-mL Schott bottles. Water was added to 2 cm above the sediment surface. All treatments were preincubated at 25 °C. Thereafter, water or13C-labeled glucose (10.5 atom%13C) solution was evenly added dropwise to the sediment surface using an injector.Before the incubation, the labeled glucose was gently and uniformly mixed, and added to the sediment at two levels. This is equivalent to adding 0.23 and 0.93 mg/g DW carbon to the sediment.There were seven treatments: low and high input levels of the labeled glucose with and without two levels of nutrients and a control without nutrient and glucose inputs (Table 1). Each treatment had three replicates. All treatments were incubated at 25 °C.The headspace gas was collected at Days 3, 7, 13,21, 32, and 45 for total CO2-C and δ13C-CO2analyses.
2.3 CO 2 analysis
Headspace samples were collected using a 25-mL gas-tight syringe and stored in pre-evacuated 35-mL glass bottles (Yan et al., 2017). The CO2concentrations were measured using an Agilent 7890B gas chromatograph (Agilent Technologies, Alto Palo,California, USA) equipped with f lame ionization,f lame photometric, electron capture detectors, and a nickel catalyst methanizer. The CO2emission was expressed as cumulative production. In addition, the stable C isotope composition in the headspace samples was analyzed using a Picarro G2101-i CO2cavity ring-down isotope spectroscope (Picarro Inc.,Sunnyvale, California, USA).
2.4 Extracellular enzyme activities
Extracellular enzyme activities ref lect the functions of decomposer communities. Three enzymes:β-glucosidase (AG), chitinase (CH), and phosphatase(PHOS), were determined using a modif ied 96-well plate assay method (Bell et al., 2013). β-glucosidase is a commonly measured enzyme responsible for degrading cellulose. Chitinase is a common enzyme involved in degrading organic N compounds. Artif icial substrates labeled with the methylumbelliferone f luorophore were used to estimate the activities of three enzymes involved in sediment organic C, N, and P degradation.
2.5 Sediment microbial community abundance analysis
Total genomic DNA of each sediment sample was used for DNA extraction using a soil DNA isolation kit (OMEGA Bio-tek, Inc.). DNA concentrations were measured with a spectrophotometer (Nanodrop ND-2000; NanoDrop Technologies). The extracted DNA was stored at-20 °C until further analysis.
PCR amplif ication was conducted for Illumina MiSeq sequencing using bacterial primers. The forward primer 5′-ACTCCTACGGGAGGCAGCA-3′ and the universal reverse primer 3′-GGACTACHVGG-GTWTCTAAT-5′ targeted the V3V4 hypervariable regions of bacterial 16S rRNA genes. PCR amplif ication was performed in triplicate using an ABI 2720 PCR System. The 25-μL PCR mixture contained 5 μL of 5×buff er, 5 μL of 5×GC buff er, 2 μL of dNTP (2.5 mmol/L), 1 μL of forward primer (10 μmol/L), 1 μL of reverse primer(10 μmol/L), 2 μL of DNA template, 8.75 μL of ddH2O, and 0.25 μL of Q5 DNA polymerase.Cycling parameters were as follows: initial denaturation 98 ℃ for 2 min, denaturation 98 ℃ for 15 s, annealing 55 ℃ for 30 s, extension 72 ℃ for 30 s, with a f inal extension of 10 min at 72 °C. For each sample, the products of three parallel PCRs were mixed. The PCR amplicons were pooled together and purif ied using a PCR purif ication kit.The purif ied amplicons were merged at equal concentrations for all samples with a KAPA Library Quantif ication Kit. The purif ied amplicons were sequenced on the Illumina MiSeq platform.
2.6 Calculations and statistics
Because of the diff erent δ13C signatures between sediment and labeled glucose, the total CO2emissions can be separated into sediment-derived CO2emissions(CSOC) and glucose-derived CO2emissions (Cglu) using the following equations (Grasset et al., 2018):
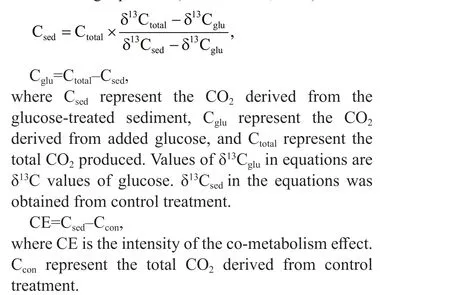
The repeated measures Anova test method was used to identify the diff erences for cumulative CO2emissions and CE in diff erent treatment groups by R language, version 3.5.1. Moreover, One-way ANOVAs test method was used to compare the accumulated sediment-derived CO2emissions and glucose-derived CO2emissions in diff erent treatment groups at f inal stage. The multifactorial analysis was used to explain time, C, N, and P inputs to cumulative CE. The criteria ofP<0.05 andP<0.01 were used to determine statistical signif icance at the 0.05 and 0.01 levels, respectively.
3 RESULT
3.1 Cumulative CO 2 emissions
CO2emissions from the background sediments gradually increased to 2 112.82 μg/g DW during the 45-day incubation period (Fig.2a). The repeated measures Anova test showed that the glucose addition led to a signif icant increase in CO2emissions relative to background levels (P<0.05). High-C treatments caused 40.52% higher CO2emissions than low-C treatments during incubation. Glucose with high nitrogen treatments led to higher CO2emissions compared with the no nutrient treatments. By the end of incubation, both high nitrogen input treatments led to higher cumulative CO2emissions than low nitrogen treatments. The CO2released from each treatment increased rapidly at the beginning of the incubation and then increased slowly after 13 days. The cumulative CO2emissions ranged from 2 112.82-3 794.23 μg/g DW. However, nutrient input signif icantly increased glucose-C mineralization at the high-C input level relative to the no nutrient treatment but not at low-glucose input. The cumulative CO2driven by glucose ranged from 154.47-793.27 μg/g DW, which was equivalent to adding 4.33%-17.70% of CO2emissions across all treatments(Fig.2b).
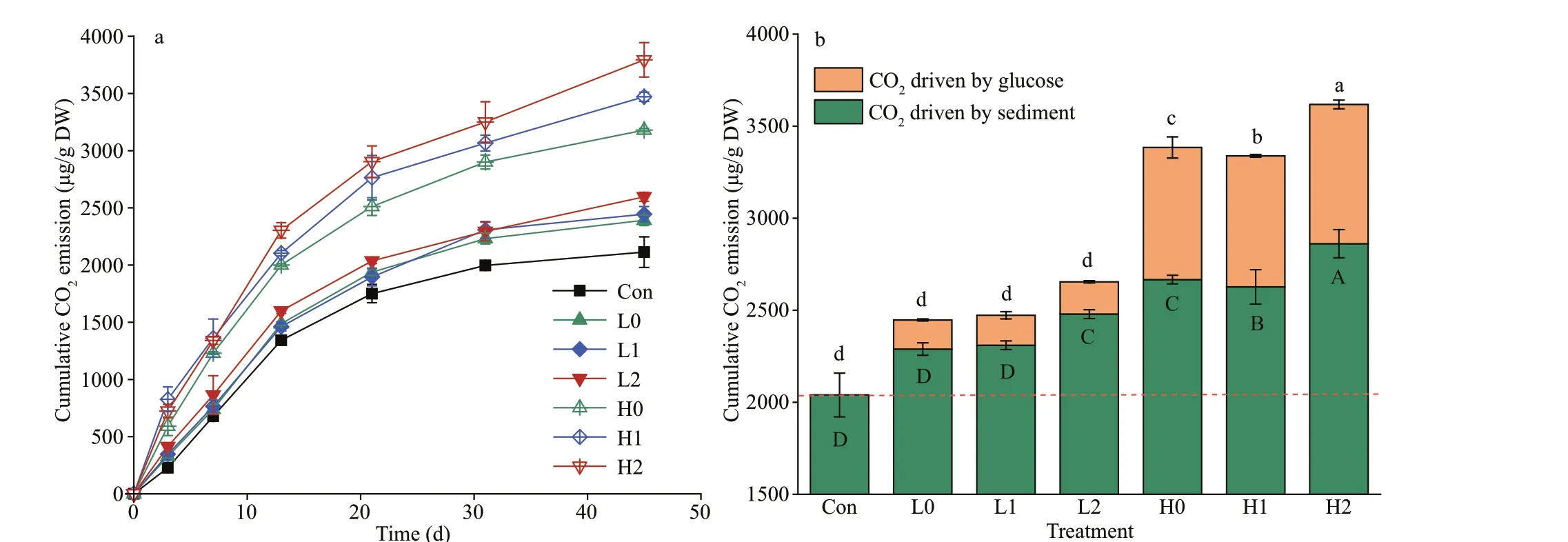
Fig.2 T exh peecrui mmeunlta t(ibv)e CO 2 emissions in each treatment over time (a) and the cumulative CO 2 emissions at the end of the
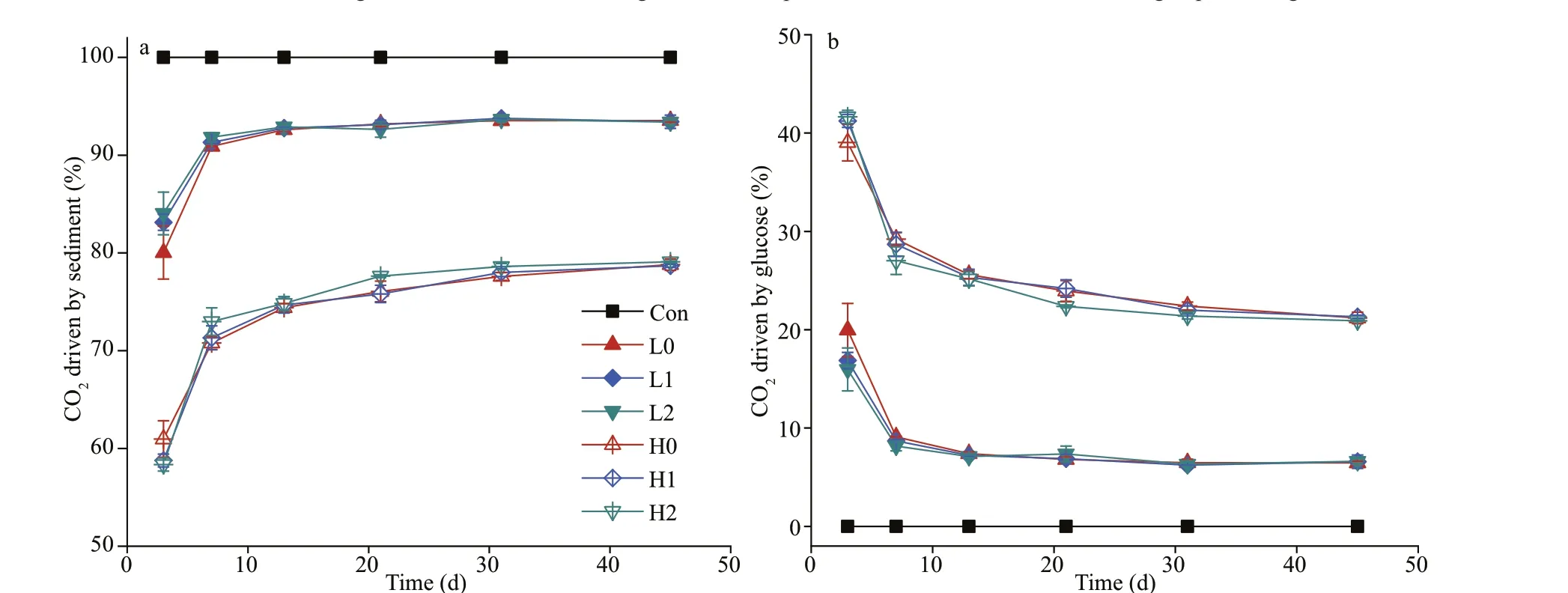
Fig.3 The percentage of CO2 driven by sediment (a) and CO2 driven by glucose (b) in each treatment
The percentage of CO2emission driven by sediment and glucose during the decomposition was shown in Fig.3a & b, respectively. The percentage of CO2driven by glucose in each treatment continually decline over time, while the percentage of CO2driven by sediment showed an opposite direction (Fig.3).Noteworthily, the proportion of CO2emission in low C treatment driven by sediment and glucose reached dynamic equilibrium around Day 13. As a contrast,similar dynamic equilibrium in high C treatment was reached in about 30 days.
3.2 Cumulative CE of labile C addition on CO 2 production
All treatments with added glucose with or without nutrients had a signif icantly positive co-metabolism eff ect of sediment OC mineralization over the 45-day period (P<0.05). The co-metabolism eff ect generally remained positive until the end of the experiment(except for the occurrence of negative rates on Days 7 and 13) (Fig.4). During the f irst three days, the positive co-metabolism eff ect increased rapidly in all treatment groups and reached 34.12-258.17 μg/g DW. All treatments except for the high C and high nitrogen treatment (H2) declined to varying degrees (i.e., the rate of OC decomposition in sediments reduced).However, the positive co-metabolism eff ect of each group increased gradually after Day 13. By the end of incubation, the cumulative co-metabolism eff ect reached 178.25-888.14 μg/g DW. The positive cometabolism eff ect was consistently higher at high C inputs, which was 152% more than low C inputs across the nutrient treatments. The multifactorial analysis showed that C input and time are signif icant positive correlation with the co-metabolism eff ect (P<0.05).
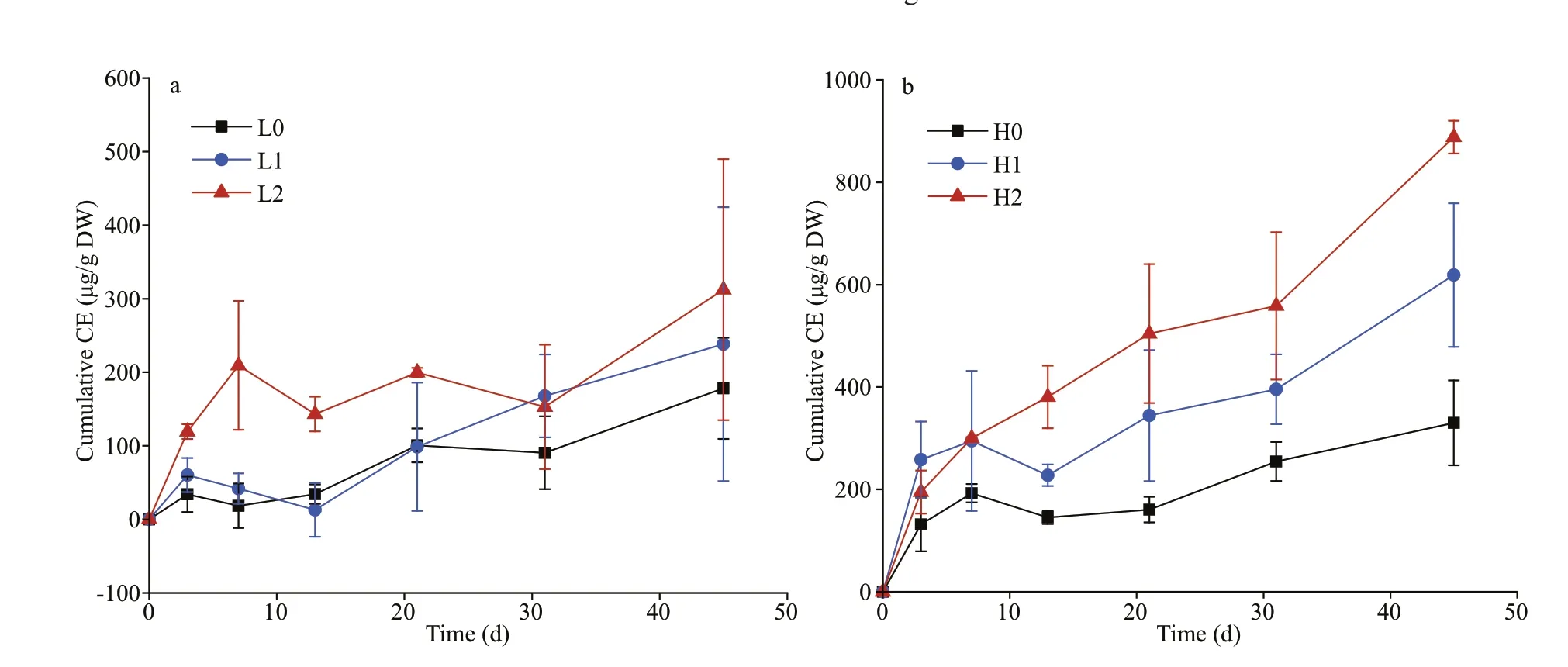
Fig.4 The cumulative co-metabolism eff ect in the low glucose treatment (a) and high glucose treatment (b)
3.3 Extracellular enzyme activities
All treatments with added labile C at both low and high levels led to increases in the activities of all measured enzymes on Days 3, 10, and 45 (Fig.5). The activity of β-glucosidase has no signif icant diff erences between treatments on Day 3. However, treatments with the addition of N and P were higher with the only glucose treatments on Days 10 and 45 (P<0.05). In both high C and low C addition treatments, increasing nutrient input levels generally increased the activity of phosphatase on Day 3, but no signif icant diff erences were observed among treatments. The activity of chitinase almost continuously decreased over time,which was signif icantly higher in C input treatment than control treatment on Day 3 (P<0.05).
3.4 Gene abundance
The dominant phyla in this study were Proteobacteria, Firmicutes, Actinobacteria,Bacteroidetes, and Armatimonadete (Fig.6). In treatments with low C input, Proteobacteria generally increased, especially on Day 3, and then decreased to the same level as in the high C input treatments.Furthermore, almost no Acidobacteri a were observed in each treatment group on Day 3. However, the relative abundance of Acidobacteria increased over time. Although the relative abundance changed, there was no signif icant diff erence at the phylum level in each treatment.
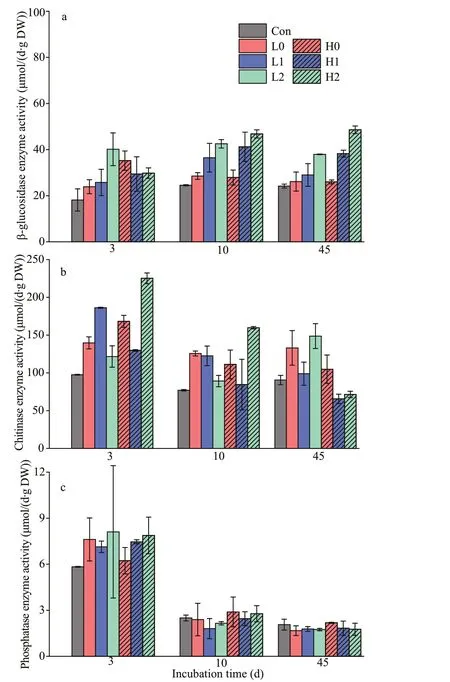
Fig.5 Activity of the extracellular enzymes β-glucosidase(a), chitinase (b), and phosphatase (c) in sediments on days 3, 10, and 45
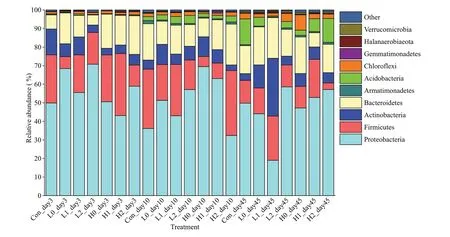
Fig.6 Bacterial community composition of the sediment layers at the phylum level
4 DISCUSSION
4.1 Co-metabolism eff ect in response to labile C addition
Increases and decreases in OC decomposition upon labile C input (a readily available energy source) have been commonly observed (Lö hnis, 1926; Kuzyakov et al., 2000). The recent renewed interest in this phenomenon—the priming eff ect—has still not penetrated many disciplines. However, it has been virtually ignored in aquatic ecosystems. As a subcomponent of priming (Bianchi, 2011), the cometabolism eff ect primarily emphasized the role of microorganisms in OC decomposition, and excluded other bioprocess such as biodigestion, biodisturbance,etc. (Dai et al., 2009; Ma et al., 2020). Here, we proposed a method to evaluate the co-metabolism eff ect based on the end-member mixing model to calculate the fraction of CO2from labile C added and refractory OC derived from sediment in shallow lake ecosystems.
The labile C addition caused a rapid increase in the positive co-metabolism eff ect at the f irst 7 days of incubation (Fig.4). This increase is generally attributed to the activation of microorganisms from starvation states to active states by labile OC (Blagodatskaya and Kuzyakov, 2008). We observed enzyme production rapidly increasing during the initial stage, which caused the microbial switch from dormancy to activity(Fig.5). Especially on Day 10, the higher β-glucosidase activity after nutrient addition, which, in turn,promoted the OC mineralization in sediments (Figs.2& 5a). On a time scale, the sediment OC mineralization was inhibited by glucose addition during early incubation (the f irst 3-13 days) (Fig.4). This inhibition likely stems from the fact that the preference of microorganisms for substrate switched from the relatively recalcitrant OC in sediments to the available energy source, thus causing a negative co-metabolism eff ect (Kuzyakov and Bol, 2006; Zhu et al., 2018).Moreover, the proportion of CO2emissions from glucose in the total emissions decreased gradually,which was attributable to microorganisms preferentially utilizing glucose (Fig.3). However, as more glucose was consumed, the microorganisms had to utilize sediment OC, which then enhanced the cometabolism eff ect (Fang et al., 2018).
Generally, the co-metabolism eff ect is positively correlated with OC input level (Guenet et al., 2010b;Di Lonardo et al., 2019; Lyu et al., 2019). This f inding may result from the alleviation of energy limitation,thus leading to increased microbial activities and nutrient demand (Fang et al., 2018). By the end of incubation, the co-metabolism eff ect with high C input was almost double that at low C input, which was not proportional to the applied dose of glucose(4 times higher in the high glucose compared with the low glucose treatment) (Fig.4). Nevertheless, several studies indicate that the occurrence of the positive cometabolism eff ect requires the amount of labile OC to reach a threshold, but this threshold diff ers between substrates (Bastida et al., 2013; Qiao et al., 2014). In addition, if the threshold is exceeded, further addition may not lead to increases in mineralization (Bremer and Kuikman, 1994). Microbial growth has been shown to strongly depend on the quality and size of available substrates (Wang et al., 2015); however, the underlying mechanisms need to be clarif ied for our understanding of sediment OC decomposition and turnover in eutrophic lakes to be enhanced.
4.2 Co-metabolism eff ect in response to N and P addition
There is an interactive eff ect between labile C and nutrient input on the co-metabolism eff ect. We observed diff erent impacts of nutrients on the cometabolism eff ect under diff erent C inputs, which were supported by previous study in paddy soil ecosystem (Wang et al., 2019). Under high C treatments, a signif icant impact of nutrients on the magnitude of the co-metabolism eff ect was observed.However, there was no obvious impact of nutrients on co-metabolism under low C input treatments (Fig.4).In general, nutrient addition may induce shifts in the abundance and diversity of bacteria, extracellular enzyme activities with increasing C turnover and sediment OC mineralization (Luo et al., 2019). A study showed that nutrient input did not increase the co-metabolism eff ect of maize straw after a 9-day incubation period (Chen et al., 2014). This result implies that microbial growth was constrained by C availability more than by nutrient availability (Heuck et al., 2018), which is supported by the changes in enzyme activities observed in this study, especially during the initial phase (Fig.5). Similarly, another study reported that nutrient inputs did not aff ect the co-metabolism eff ect under low-residue input levels(Fang et al., 2018).
In this study, no increase in the co-metabolism eff ect during the f irst 3-13 days was observed in all treatments except for the high C with high nitrogen treatment (Fig.4). This f inding may stem from the fact that the initial nutrients were not limited, which increased the decomposition of glucose during the early period (Kuzyakov and Bol, 2006). As available nutrients were depleted, nutrient limitation might lead to a shift in microorganisms when they degrade recalcitrant compounds in sediment OC in later stages(Kuzyakov et al., 2000). The microorganisms could then decompose more stable OC for nutrient acquisition and induce a higher mineralization of OC(positive co-metabolism eff ect), which is explained by the domination of “microbial nutrient mining”theory (Blagodatskaya et al., 2009). We also observed that the co-metabolism eff ect increased continuously in the high C with high nitrogen treatment and induced the largest co-metabolism eff ect by the end of the incubation period (Fig.4), which contradicts microbial“nutrient mining” predicting a lower co-metabolism eff ect at higher nutrient availability (Chen et al.,2014). Another mechanism called “stoichiometric decomposition” (Craine et al., 2007) might explain the co-metabolism eff ect under high C levels and nutrient availability in the substrate. Diff ering from the “microbial nutrient mining” theory, the“stoichiometry decomposition” theory suggests that native OC decomposition can be enhanced as nutrient limitation is alleviated. Due to suffi cient amount of nutrients and labile C may promote the growth of microbial communities. That is, decomposition rates are increased if C and nutrient inputs match microbial demands. In our study, chitinase activity in high nitrogen with high C treatment was signif icantly higher than other groups during early incubation(Fig.5), which may stimulate the growth and activity of microbial communities.
4.3 Implications for eutrophic lacustrine ecosystems
In this study, we tested the occurrence of the cometabolism eff ect in lake sediments. The availability of nutrients may lead to diff erent co-metabolism eff ect mechanisms, even though decomposers can degrade sediment OC by using labile C substrate as a source of energy. The microorganisms maintained their stoichiometric responses to labile C input,indicating that the acquisition of nutrients may be constrained by the environment. Thus, the nutrient requirements of microorganisms are likely benef icial for sediment OC decomposition. In this study, a positive co-metabolism eff ect was also observed without nutrient addition (Fig.4). Alternatively, the sediment microbes may be f lexible and shift their elemental balance in response to such labile C input(Kuzyakov et al., 2000). Hence, the highest cometabolism eff ect was observed in the high C with high nitrogen treatment.
In lacustrine ecosystems, the dramatic increase in nutrient inputs has accelerated eutrophication (Qin et al., 2019). An increase in nutrient availability further stimulates the primary production of algae in shallow lakes, making the large algae blooms during summer months increasingly severe (Shi et al., 2015; Ma et al., 2016) and resulting in the deposition of massive amounts of algae detritus (Yan et al., 2017). In this case, algae detritus may be a preferred energy source for sediment microorganisms given that it is better suited to their requirements. Such an energy source also contributes to the decomposition of recalcitrant OC (mainly macrophyte residue and humus) in sediments. This f inding could also be explained by an increase in microorganism production because of labile C and the nutrient supply (Guenet et al., 2014).This positive co-metabolism eff ect might be common in most shallow lakes, where phytoplankton is primarily dominated by algae blooms. In addition,massive algae decomposition has also led to increases in nutrients, which may be one of the important factors underlying the self-maintenance of eutrophic lakes, such as Taihu Lake in China, especially at the northwestern shorelines. The algal blooms accumulate, decay, and release nitrogen and phosphorus (Otten et al., 2012), which might further aff ect the metabolism of OC in sediments. For example, with suffi cient C sources and nutrients, the stoichiometric ratio can be adjusted according to microbial demands, maintain activity and further facilitate OC mineralization in sediments (Fang et al.,2018). The co-metabolism eff ect of algae detritus and sediment OC further weakened the function of the lake sediment carbon sink. The amount of glucose added in this study was based on the accumulation of algae in the context of eutrophication lakes (Liu et al.,2017; Yan et al., 2017).
5 CONCLUSION
New evidence indicating that diff erent dominant mechanisms can co-exist in the same system and coinf luence the strength of the co-metabolism eff ect,probably because the availability of labile C and nutrients in sediments can be explained by the correlation of the co-metabolism eff ect with key microbial variables. Compared with the low nutrient treatments, larges co-metabolism eff ect under high C with high nutrient treatment was observed by the end of the incubation. And in the low C treatment, the amount of nitrogen had limited impact on cometabolism eff ect. Higher nutrition further promotes the decomposition of sediment OC, which should be considered in the future management of aquatic ecosystems. Considering that the occurrence intensity,frequency, and duration of algae blooms were still enhanced, this study may underestimate the cometabolism eff ect in eutrophic lacustrine ecosystems.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
