Physiological and molecular responses of invasive cyanobacterium Raphidiopsis raciborskii to ambient phosphorus def iciency*
Junqiong SHI, Shuhan HE, Lu ZHAO, Lulu JI, Songqi YANG, Zhongxing WU
Key Laboratory of Eco-environments in Three Gorges Reservoir Region (Ministry of Education), Chongqing Key Laboratory of Plant Ecology and Resources Research in Three Gorges Reservoir Region, School of Life Sciences, Southwest University,Chongqing 400715, China
Abstract Raphidiopsis raciborskii can cause harmful cyanobacterial blooms when concentrations of environmental phosphorus (P) are very low, thus the physiological and molecular mechanisms involved in the acclimation to P need to be characterized better. The growth, chlorophyll f luorescence, alkaline phosphatase,and expression of genes directly involved in P assimilation were compared in the R. raciborskii FACHB 1496 strain grown with and without inorganic P. The specif ic growth rate ( μ), Chl a, and six f luorescence parameters (minimal f luorescence ( F 0), maximal f luorescence ( F m), maximal variable f luorescence ( F v),electron transport f lux (further than Q A) per RC (ET 0/RC), quantum yield of the electron transport in PSⅡ( Ø E0), and the probability that an electron from a trapped exciton is moved into the electron transport chain beyond QAˉ ( ψ 0)) markedly decreased in R. raciborskii in response to experimental P-def iciency. In contrast, the relative variable f luorescence at the J-step ( V J), trapped energy f lux (leading to Q A reduction)per RC (TR 0/RC), and alkaline phosphatase activity signif icantly increased. In addition, gene expressions involved in the alkaline phosphatase ( phoA1 and phoA2), high-affi nity inorganic P transporter ( pstS1),phosphonate transporter and metabolism ( phnD and phnM), and nucleotidase ( nucH) were signif icantly upregulated under P def iciency. However, physiological and molecular responses were resumed rapidly after P re-supplementation following P-def icient conditions. Our results highlight that R. raciborskii can perform coordinated and complex cellular and physiological responses to cope with P def iciency, ref lecting R. raciborskii’s multi-faceted machinery to respond to environmental P f luctuations.
Keyword: phosphorus def iciency; Raphidiopsis raciborskii; gene expression; chlorophyll f luorescence;alkaline phosphatase
1 INTRODUCTION
As an essential nutrient, phosphorus (P) is not only involved in cellular structure and metabolic processes(Karl, 2014; Lin et al., 2016), but inf luences the growth and proliferation of phytoplankton (Dyhrman, 2016).A shift in the phytoplankton community towards cyanobacteria dominance occurs when freshwater bodies are enriched with phosphorus (Burford et al.,2014; Chislock et al., 2014; Wan et al., 2019). Therefore,P availability is hypothesized to be responsible for the survival and vitality of phytoplankton, as well as the proliferation of cyanobacteria (Wu et al., 2000; Young et al., 2010; Li et al., 2016).
Phosphorus supply, however, is aff ected by redoxdependent P retention in sediments (Welch and Cooke, 1995), land use types in watersheds (Downing and McCauley, 1992; Carpenter et al., 1998), and the food web structure of lakes and streams (Elser et al.,1988). As a result, these lakes and streams often have very low P availability (Nausch et al., 2004). For example, during the summer months in temperate lakes, dissolved inorganic phosphorus (DIP), a preferred form of P to phytoplankton, often fails to meet the demand of phytoplankton growth (Smith,1983; Wu et al., 2000; Karl, 2014). To survive under low-P conditions, phytoplankton has developed a series of encoding regulatory mechanisms to control the uptake, storage, and metabolism of phosphate(Su et al., 2007; Tetu et al., 2009; Liu et al., 2015;Luo et al., 2017; Shi et al., 2017; Zhang et al., 2019).Harke et al. (2012) found that genes involved in high-affi nity phosphate-binding proteins and putative alkaline phosphatase were signif icantly upregulated(50- to 400-fold) whenMicrocystisaeruginosa, a bloom-forming cyanobacterium, was cultured under low inorganic phosphate conditions. Additionally,a set of genes involved in high-affi nity phosphate uptake and dissolved organic phosphate (DOP)hydrolysis, e.g., the P-specif ic transport (Pst) system(Vershinina and Znamenskaya, 2002), P-inorganic transport (Pit) system (Vershinina and Znamenskaya,2002; Ilikchyan et al., 2009), and DOP hydrolysis genes,phoXandphoA(Moore et al., 2005; Wu et al.,2007; Sebastian and Ammerman, 2009) have been reported to cope with P def iciency. These f indings suggest phytoplankton can effi ciently transport phosphate and exploit organic phosphate sources to adapt to P-def icient environments. Wan et al. (2019)has suggested that diff erent cyanobacterial species,i.e.,MicrocystisandDolichospermum, have distinct responses to P availability. Therefore, molecular patterns presented by diff erent cyanobacteria in response to P availability will help understand the variation of P-acclimation mechanisms. Owing to genome data scarcity, however, studies regarding molecular responses to P have mostly focused on a few marine and even fewer freshwater cyanobacteria(Vershinina and Znamenskaya, 2002; Su et al., 2003;Tetu et al., 2009; Harke et al., 2012).
Raphidiopsisraciborskiiis an invasive and notorious cyanobacterial species because it has recently become prevalent in temperate regions(Padisák, 1997; Saker and Griffi ths, 2001; Burford et al., 2016). Wu et al. (2009) and Posselt et al. (2009)showed that P availability had an essential role in the occurrence and invasive behavior ofR.raciborskii.For example, its high DIP uptake affi nity and P storage capacity (Isvánovics et al., 2000; Willis et al., 2017), as well as superior ability to utilize DOP(Bai et al., 2014, 2020), may helpR.raciborskiioutcompete other species. Several studies found thatR.raciborskiicould grow under conditions of low and/or variable P availability (Piccini et al.,2011; Wu et al., 2012; Prentice et al., 2015; Willis et al., 2015, 2019; Guedes et al., 2019; Xiao et al.,2020), ref lecting that the ability to adapt to low and variable P supply might also be a crucial factor driving the dominance ofR.raciborskii(Amaral et al., 2014). Although previous studies indicated that this cyanobacterium could effi ciently regulate its physiological metabolism to adapt to ambient inorganic phosphate availability (Wu et al., 2012;Willis et al., 2015, 2019; Guedes et al., 2019; Xiao et al., 2020), the adaptive mechanisms, especially in molecular responses, to ambient P def iciency, are not well characterized inR.raciborskii.
Genomic comparisons of nine co-occurringRaphidiopsisstrains from Lake Wivenhoe,Queensland, Australia, revealed that the core genome content is 85% similar and is much conserved in the main P metabolic pathways among the nine strains (Sinha et al., 2014; Willis et al., 2018).However, Willis et al. (2019) showed that two strains ofR.raciborskii, CS-505 and CS-506,showed a marked diff erence in the gene expression response to P-free conditions, which suggests that the diff erent responses to P might result in diverse ecotypes (Burford et al., 2018; Guedes et al., 2019).Therefore, the molecular mechanisms responding to P acclimation in the diverse ecotypes ofR.raciborskiineed to be studied from diff erent regions. Posselt et al. (2009) indicated that the cell concentrations and biovolume ofR.raciborskiisignif icantly increased with periodic pulsed addition of DIP. Amaral et al.(2014) also found that the growth rate increased by 2-3-fold due to multiple pulses of DIP relative to a single pulse. These f indings have highlighted the ability ofR.raciborskiito use pulsed P supplies supports its succession and dominance in lakes and reservoirs. However, little information about the molecular responses to P-Pulse conditions has been obtained forR.raciborskiiexposed to P def iciency.Here, we hypothesized that (i) P def iciency aff ects the growth, photosynthesis, and gene expression inR.raciborskii; (ii)R.raciborskiican recover rapidly to grow when P was resupplied; and (iii) genes encoding phosphate uptake and transporters are upregulated signif icantly under P-def iciency condition, whereas rapidly downregulated transcriptions occur when P is resupplied. In the present study, therefore, the growth,chlorophyll f luorescence, alkaline phosphatase activity, and gene expression inR.raciborskiiwere characterized to reveal the adaptation strategyofR.raciborskiito ambient P def iciency and the response mechanisms to P-re-supplementation.
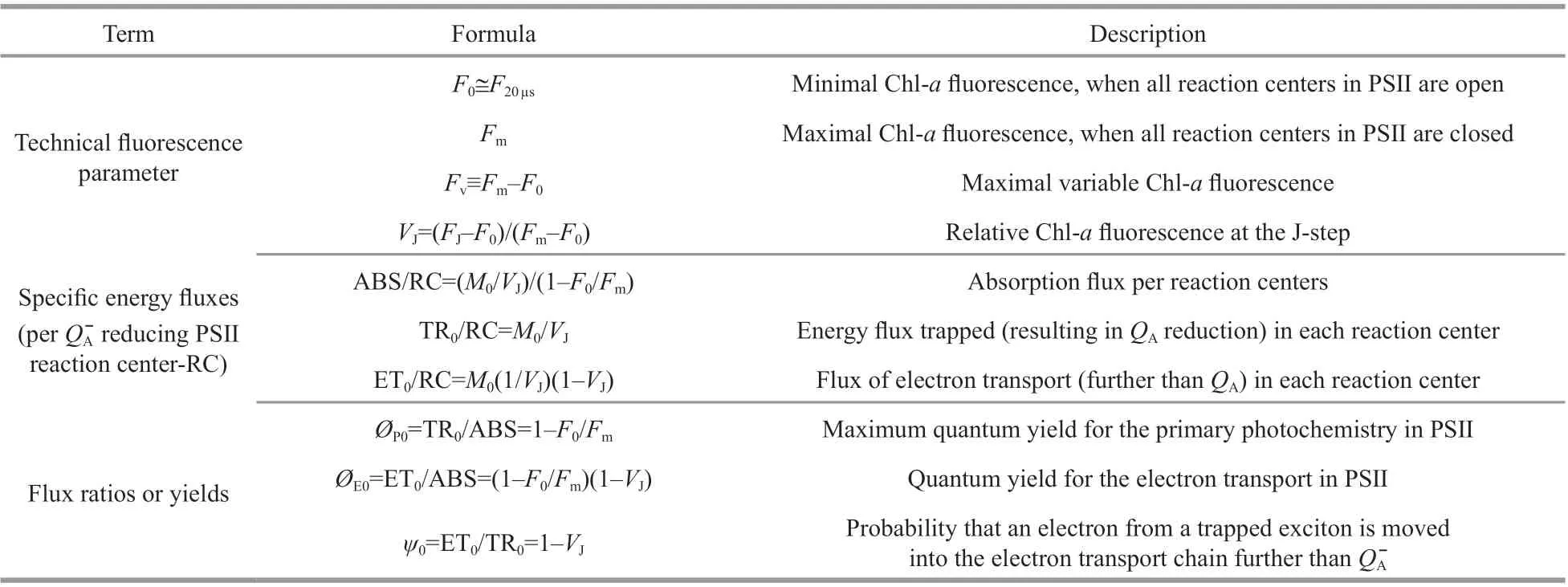
Table 1 Formulae and description of Chl- a f luorescence parameters in this study (Strasser and Strasser, 1995)
2 MATERIAL AND METHOD
2.1 Culture conditions and experimental design
RaphidiopsisraciborskiiFACHB 1496 was selected as the experimental alga and was cultured in a liquid CT medium (Ichimura, 1979) under 30 μE/(m2·s) white light intensity at 25±1 °C, with a 12-h∶12-h light∶dark cycle. Cells were harvested in the logarithmic growth phase, washed twice with sterile P-free CT medium, and inoculated in a fresh P-free CT medium for f ive days to exhaust intracellular P(Bai et al., 2014).
The experiment included two treatments, each in triplicate, with an initial cell density of 2×106cells/L.At the beginning of the experiment, medium with 0-and 0.6-mg/L K2HPO4were used for the P-def icient(-P) and P-replete (+P) treatment, respectively. The cells were harvested at 0, 24, 72, 120, and 144 h to monitor Chla, alkaline phosphatase activity,orthophosphate concentrations, and RNA expression.After 144 h of culture, 0.6-mg/L K2HPO4was added to both treatments to determine how phosphorus replenishment aff ected the parameters mentioned above after another 24 h.
2.2 Alkaline phosphatase and Chl- a detection
ELF®97 phosphate dye (ELFP, USA) was used for the microscopic detection of external phosphatases in the strain. The dye method for external phosphatase detection followed the protocol of Wu et al. (2009). In brief, the cells were incubated into the ELFP solution(f inal concentration 27 mmol/L) at darkness and 25 °C for 4 h, followed by f iltration with 0.22-μm pore size f ilters, and 2-3 times washing. Samples were observed with a f luorescence microscope with UV-excitation(Nikon, Japan). The activities of alkaline phosphatase were determined according to the method of Shen and Song (2007) usingp-nitrophenyl phosphate(pNPP, USA) as a substrate. Chlawas extracted with 90% acetone and measured spectrophotometrically according to Nusch (1980). Based on the cell density,the specif ic growth rate (μ) was analyzed according to the following equation:μ=(lnCt2-lnCt1)/(t2-t1), in whichCt2andCt1are cell densities att2(24 h) andt1(0 h) for the -P treatment,t2(72 h) andt1(24 h) for the +P treatment, andt2(168 h) andt1(144 h) for the P-added treatment.
2.3 PSII f luorescence measurement
A Plant Effi ciency Analyzer (PEA, UK) was used to conduct Chl-af luorescence assays with a 3 000 μmol photons (photosynthetically active radiation, PAR)/(m2·s) actinic light after all samples were darkadapted for 20 min. The f luorescence signals were recorded according to Yang et al. (2020). Each transient was analyzed according to the JIP-test using the following original data:F0,F300μs(FK),F2ms(FJ),andF30ms(FI) def ined as the f luorescence intensity at 20 μs, 300 μs, 2 ms, and 30 ms, respectively, as well as the maximum f luorescence intensity (Fm) (Strasser and Strasser, 1995). The parameters selected for the quantif ication of PSII activity are shown in Table 1.
2.4 DNA extraction, amplif ication, and sequencing
Total genomic DNAs ofRaphidiopsiswereextracted according to Wu et al. (2010). The PCR primers designed for seven genes are listed in Table 2. PCR was carried out in a PTC-100 thermal cycler (MJ Research Inc., USA). Fifty-microliter volume (containing 5-10-ng DNA, 1-UTaqDNA polymerase (TaKaRa, Japan), 10 pmol of each primer, and 200 μmol/L of each deoxyribonucleoside triphosphate) was amplif ied with the program of 94 °C for 5 min, 35 cycles (94 °C for 40 s, 60 °C for 50 s, and 72°C for 2 min), and 72 °C for 5 min. The amplif ication products were purif ied and then cloned using a pGEM-T vector (Promega, USA) based on the protocol of the manufacturer. An ABI 3730 Automated Sequencer (Perkin-Elmer Biosystems,USA) was used to sequence all products.
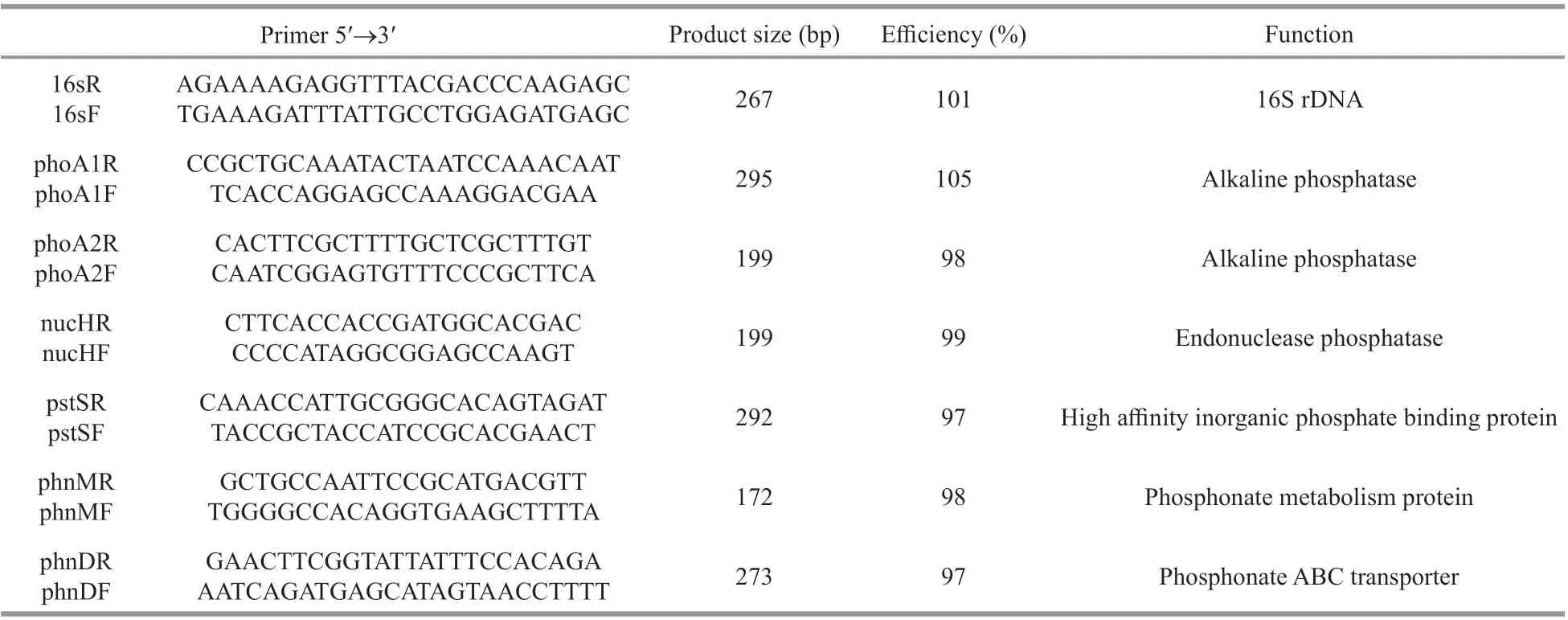
Table 2 Primer sequences and product sizes for quantitative PCR reactions in this study
2.5 RNA isolation and reverse transcription
Pelleted cells harvested by centrifuging at 7 000 r/min were re-suspended in Trizol reagent (Invitrogen, USA).Total RNAs were extracted following the Trizol reagent manual (Invitrogen, USA) after pellets were homogenized with a mini-bead beater. RQ1 RNasefree DNase (Promega, USA) was used to digest total RNAs. The random primersp(dN)9and a reverse transcriptase kit (Generay, China) were employed to reverse-transcribe DNase-treated RNA to cDNA.
2.6 Quantitative real-time PCR
Final volumes of 20 μL including 0.2 μL(10 pmol/μL) of both forward primer and reverse primer (Table 2), 1-μL cDNA, 10-μL Master Mix(SYBR Green, TOYOBO, Japan), and 8.6-μL ddH2O,were used in the amplif ication reactions of quantitative real-time PCR with an iCycler Iq (Bio-Rad, Hercules,CA). The amplif ication conditions were 95 °C for 3 min, followed by 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s for 40 cycles. The expression levels of target genes from quantitative real-time PCR(QPCR) were evaluated using theCtvalue (Livak and Schmittgen, 2001) by the normalization of the housekeeping gene 16S rRNA. The fold change of transcription was calculated using the ΔΔCtmethod,where ΔΔCt=Ct,targetgene-Ct,16S. Samples were processed in triplicate reactions. Amplif ication effi ciencies of 90%-110% and a ΔCtof the two slopes less than 0.1 were considered acceptable.
2.7 Statistical analysis
The Chl-aconcentration, f luorescence parameters,alkaline phosphatase activity, and gene transcription ofR.raciborskiiunder -P and +P treatments were evaluated by pairedt-tests. All Data were analyzed using one-way analyses of variance (ANOVA). The ANOVAs were performed by pairwise multiple comparisons using least signif icant diff erences (LSD)analysis. Signif icance levels ofP<0.05 were adopted for all tests. Before running statistical analyses,gene transcriptions were normalized relative to the housekeeping gene 16S rRNA and all data were tested for homogeneity of variance and normal distribution of residuals. All analyses and graphs were performed in SPSS version 26.0 (IBM, USA) and Origin version 2021 (Origin Lab Corporation, USA).
3 RESULT
3.1 Growth experiment

Fig.1 Chl a, specif ic growth rate ( μ), and alkaline phosphatase activity (APA) responses of R. raciborskii to changing ambient phosphorus
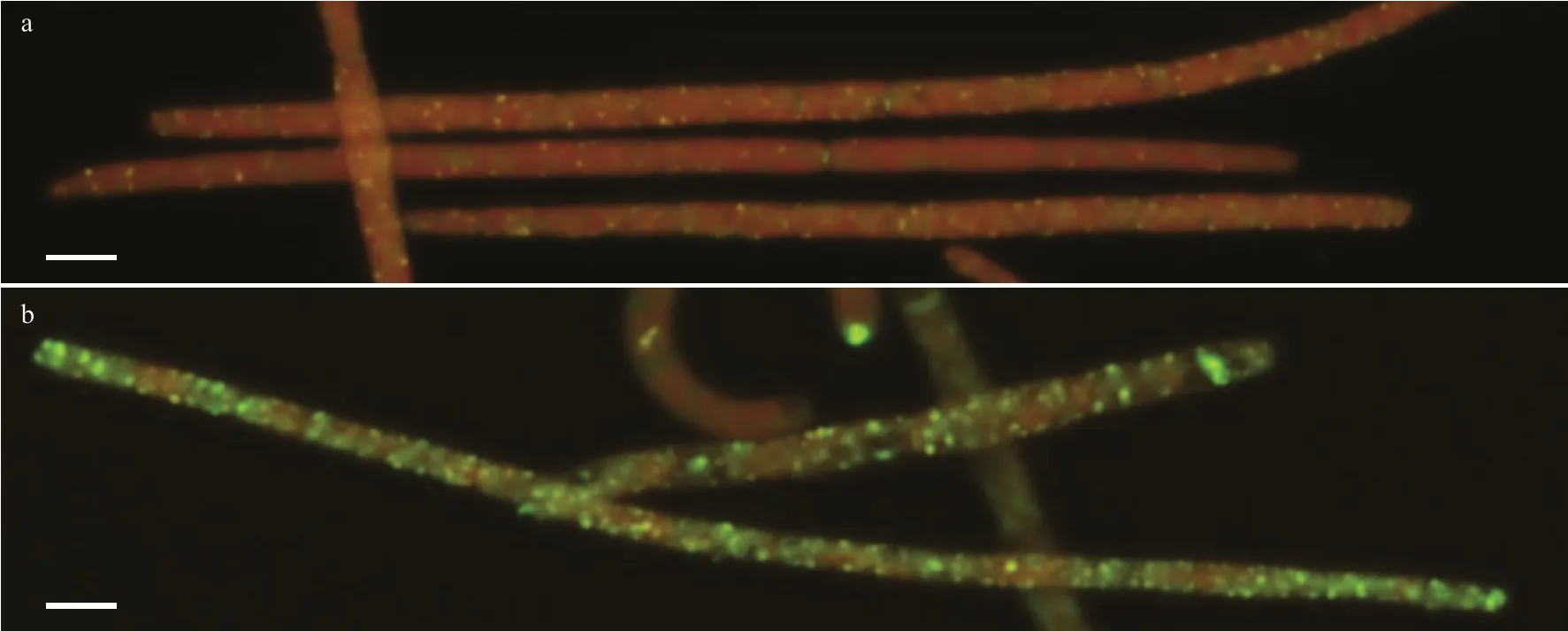
Fig.2 ELF-APA labeling of f ilaments of R. raciborskii cultured at +P and -P conditions
The specif ic growth rate (μ) ofR.raciborskiiwas signif icantly lower in -P than in +P treatments, and Chlawas signif icantly higher in the +P treatment(Fig.1a & b) (ANOVA,P<0.05). When P was added at the end of the experiments, both treatments showed comparable Chl-aresponses, and the signif icant diff erences in absolute Chl-alevels between both treatments remained.
3.2 Alkaline phosphatase activities (APAs) and Chl- a f luorescence
After 144 h, a signif icant diff erence was found in alkaline phosphatase activity between the -P and +P treatment (Fig.1c). After 144 h, APAs in -P treatment were signif icantly higher (7.32- and 6.01-fold)than those at 0 h and in the +P treatment at 144 h,respectively (P<0.05). When phosphorus was added to the -P treatment at the end of the experiment, APAs showed a remarkable decrease. However, APAs did not signif icantly respond to the P addition in the +P treatment (P>0.05). In the +P treatment, only red chlorophyll auto-f luorescence was determined in the f ilaments (Fig.2a). However, predominantly green f luorescent products were observed in f ilaments whenR.raciborskiiwas labeled by ELF in the -P treatment(Fig.2b).
After 144-h culture, Chl-af luorescence parameters ofR.raciborskiishowed signif icant diff erences between treatments (Table 3). Technical f luorescence parameters, such as minimal f luorescence (F0),maximal f luorescence (Fm), and maximal variable f luorescence (Fv), had signif icantly lower values in-P treatments than in +P treatments (P<0.05). Similar trends were observed for the f lux of electron transport(further thanQA) in each reaction center (ET0/RC),quantum yield for the electron transport (ØE0) of PSⅡ,and the probability that an electron from a trapped exciton is moved into the electron transport chain further thanQAˉ (ψ0) (ANOVA,P<0.05). However,signif icantly higher values of relative variable f luorescence at the J-step (VJ) and trapped energy f lux (leading toQAreduction) per RC (TR0/RC) were found in -P than in +P treatments. In a simple energy f lux model (Fig.3), a markedly lower energy f lux was observed in -P than in +P treatments (P<0.05).
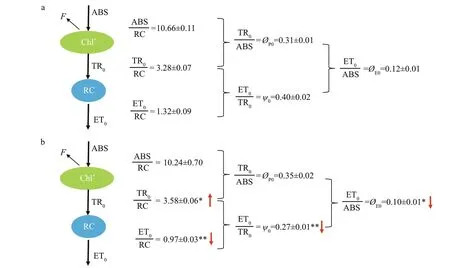
Fig.3 A simple energy f lux model of PSⅡ in R. raciborskii cultured at +P and -P conditions
3.3 Gene responses to P def iciency
The expression of six genes involved in the phosphorus assimilation process was examined after 0, 24, 72, and 144 h in the -P and +P treatment. The expression ofphoA1encoding alkaline phosphatase was signif icantly upregulated in -P compared to +P treatments at all sampling times (P<0.01; Fig.4a). The expressions at 24, 72, and 144 h in the -P treatment were 15-, 19-, and 54-fold higher than 0 h, respectively.
Moreover, the expressions ofphoA1were 7.5-,6.46-, and 2.70-fold higher in -P treatments at 24, 72,and 144 h, respectively, than those in +P treatments.However, the expression at 168 h decreased signif icantly in the -P treatment after phosphorus had been added to the medium at 144 h (P<0.01; Fig.4a),and the gene expression showed no signif icant diff erence between -P and +P treatments (P>0.05;Fig.4a).PhoA2encoding alkaline phosphatase-like in the -P treatment was signif icantly upregulated at 72 and 144 h (P<0.01; Fig.4b). The expressions ofphoA2at 72 and 144 h were 1.72 and 1.76 times higher than those at 0 h in the -P treatments, while the expressions ofphoA2in the -P treatment at 72 and 144 h were 1.55 and 1.74 times higher than those in the +P treatment, respectively.
Signif icantly higher expressions in the genespstS1encoding a high-affi nity inorganic phosphatebinding protein,phnDencoding a phosphonate ATP-binding cassette (ABC) transporter,phnMencoding a phosphonate metabolism protein, andnucHencoding the endonuclease phosphatase were found inR.raciborskiiin the -P treatment at 24 to 144 h than at 0 h (P<0.01; Fig.4c-f). Moreover, the-P treatment exhibited general increases in gene expressions ofpstS1andphnDover time compared to the +P treatment (P<0.01; Fig.4c-d). However,the genesphnMandnucHshowed a constant, high expression level at 72 and 144 h relative to 0 h(P<0.01; Fig.4e-f). Compared with the +P treatment,the gene expressions ofpstS1,phnD,phnM, andnucHwere markedly upregulated in the -P treatment(P<0.05, Fig.4c-f). However, the expressions at 168 h decreased signif icantly in the -P treatment when phosphorus had been added to the cultures at 144 h(P<0.01, Fig.4c-f).
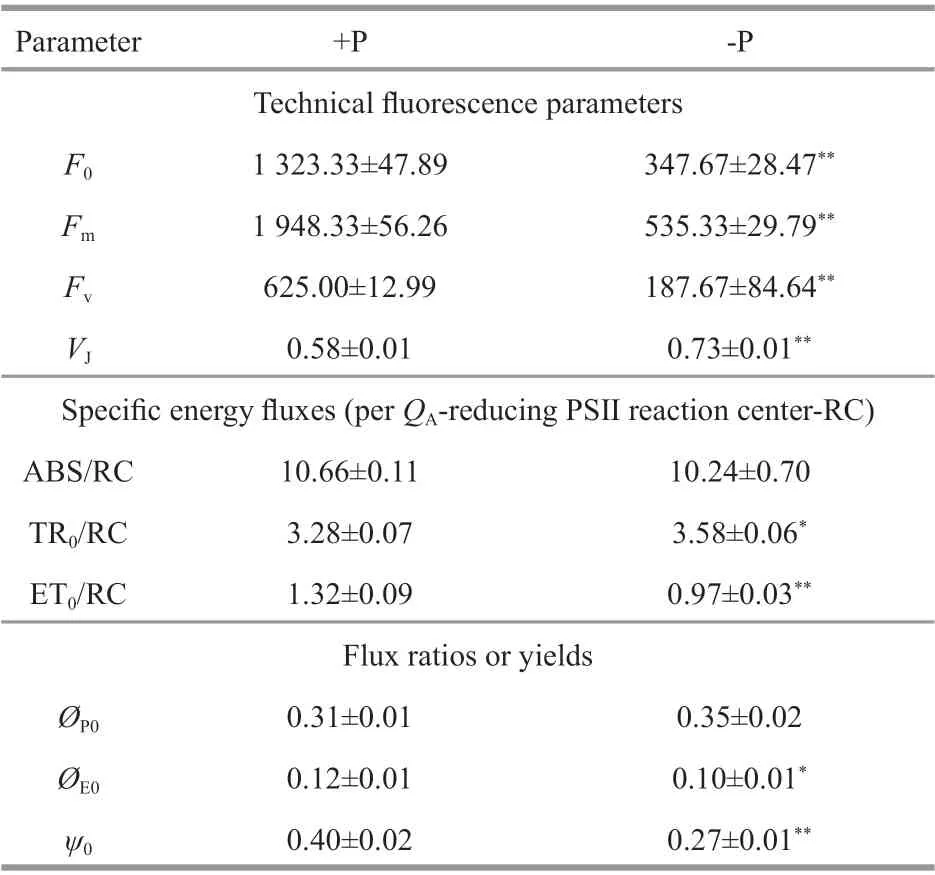
Table 3 Diff erent responses of Chl- a f luorescence parameters in R. raciborskii to presence and absence phosphorus in 144-h culture
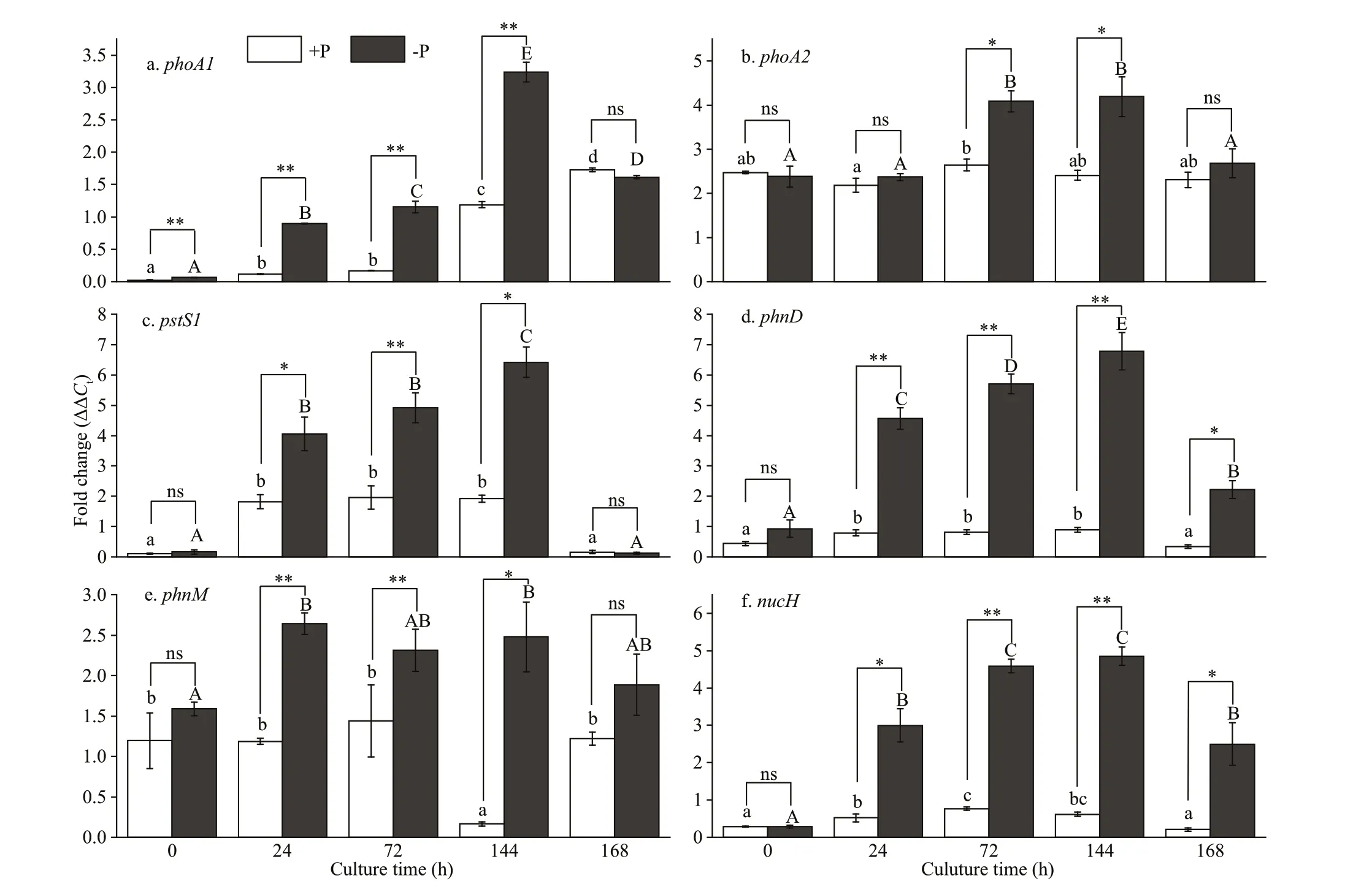
Fig.4 Relative quantitative gene expression for phosphorus metabolism genes encoding alkaline phosphatase ( phoA1 and phoA2), high-affi nity phosphate binding proteins ( pstS1), phosphonate transporter and metabolism ( phnD and phnM),and nucleotidase ( nucH)
4 DISCUSSION
4.1 Physiological responses of R. raciborskii to P variations
Phosphorus is a critical factor controlling the physiological ecology of phytoplankton (Lin et al., 2016; Zhang et al., 2018). However, dissolved inorganic phosphorus (DIP) in many waters often falls below the level limiting the growth of phytoplankton(Dyhrman, 2016). In the present study, P-depleted cultures grew slower and entered the stationary phase earlier than the P-enriched cultures (Fig.1a-b),supporting previous studies (Wu et al., 2012; Bai et al., 2014). Similar results were presented by Guedes et al. (2019), who found that f iveR.raciborskiistrains could grow under P def iciency for 10 d by reductions in growth rate and Chl-ayield, suggesting that internally stored phosphorus, i.e., polyphosphate,might have been used during the 10-d endurance of P def iciency (Guedes et al., 2019). Droop (1973)proposed that the storage could be expended theoretically for 3-4 generations when grown under P-depleted conditions. In our experiment, the culture P-depleted for 5 d probably was not depleted enough to exhaust intracellular P, which resulted in a slight increase in Chlain 24 h, while a signif icant diff erence was observed between -P and +P treatments.
The production of extracellular phosphatases is one possible mechanism allowing cyanobacteria to overcome P limitation (Dyhrman et al., 2012; Lin et al.,2012; Zhang et al., 2016; Shi et al., 2017). Under the P-def icient culture conditions, alkaline phosphatase activities were markedly elevated (Fig.1c). After P supplementation to previously limited cultures,alkaline phosphatase activity was signif icantly downregulated after 24 h (Fig.1c). Predominantly green f luorescent products were observed whenR.raciborskiiwas cultured in a P-def icient medium(Fig.2), further supporting the f indings of González-Gil et al. (1998), who showed that an intensive green f luorescence ELF alcohol (ELFA) was formed and tagged at or near the sites of enzymatic activity in cells when phosphatases and enzyme-labeled f luorescence phosphate (ELFP) were present, suggesting that phytoplankton can produce alkaline phosphatase to hydrolyze organic P to compensate for P def iciency when ambient P is scarce (Zhang et al., 2019).Prentice et al. (2019) suggested that 89% of the total P demand resulted from alkaline phosphatase activity in f ield populations dominated byR.raciborskii.This suggests that high alkaline phosphatase activity could be induced as a response to the P starvation inR.raciborskii, supporting f indings of Wu et al. (2012),Posselt et al. (2009), and Lu et al. (2021). However,Burford et al. (2018) observed thatR.raciborskiistrains isolated in Australia did not increase alkaline phosphatase activity in response to P def iciency,ref lecting thatR.raciborskiistrains showed intraspecif ic variations in alkaline phosphatase (Guedes et al., 2019). This suggested thatR.raciborskiihas developed various strategies to adapt to P-def icient environments (Guedes et al., 2019; Xiao et al., 2020).
The reduction of the parametersF0,Fm,Fv, and ET0/RC showed that the electron transport was blocked in the photosynthetic system II (PSII). Additionally,φE0andψ0also decreased in P def icient treatments relative to P supplemented treatments. However, an increased trapped energy f lux per reaction center(TR0/RC) was found in P def icient treatments(Table 2; Fig.3), suggesting thatR.raciborskiiin P def icient treatments could dissipate excess excitation energy by thermal and f luorescence forms to avoid the potential for photo-oxidative damage (Perron et al., 2012). Jacob and Lawlor (1993) also showed that non-photochemical dissipation of energy was raised, and PSII activity was downregulated when phytoplankton suff ered long-term P def iciency.Therefore, P starvation might suppress the photophosphorylation ofR.raciborskiicells, resulting in an increased excitation of the thylakoid membrane and a decreased probability of PSII excitation energy transfer from the antenna to the RCs, as well as relatively ineffi cient photosynthesis (Jacob, 1995;Wu et al., 2012). However, Pierangelini et al. (2014)proposed that the regulation of excitation energy transfer is likely driven by state transitions rather than by heat dissipation in twoR.raciborskiistrains exposed to 100 μmol photons/(m2·s), indicating thatR.raciborskiican develop diff erent acclimation strategies toward diff erent environmental factors.Additionally, the responses to P-depleted conditions were compared among f iveR.raciborskiiand f iveMicrocystisaeruginosastrains by Guedes et al.(2019), who observed that only oneR.raciborskiiand allM.aeruginosastrains showed a decrease in PSII, further supporting that intra-specif ic variation occurs inR.raciborskiipopulations.
4.2 Molecular mechanisms of responses to P variation
In a P-def icient environment, many phytoplankton organisms tend to increase phosphate uptake by regulating the expression of phosphate transporters(Frischkorn et al., 2014; Alexander et al., 2015;Liu et al., 2015; Shi et al., 2017; Zhang et al.,2019). All the six genes involved in the phosphorus assimilation process showed signif icant upregulation after 24, 72, and 144 h of P-def icient culture.However, the expression patterns diff ered among the six genes (Fig.4). Genes encoding alkaline phosphatase (or -like),phoA1andphoA2, were signif icantly upregulated, which was consistent with the result of APAs. A similar result was obtained forR.raciborskiiCS-505 and CS-506 (Willis et al.,2019). Previous observations have indicated thatR.raciborskiihas the highest recorded active uptake rates (Willis et al., 2017). Active P uptake is below a threshold of 4.75-μg/L phosphate (Prentice et al.,2015). This suggested that the transcript expression of alkaline phosphatase allowed the acquisition of P from organic P-molecules with ester bonds inR.raciborskii(Liu and Wu, 2012; Bai et al., 2014).However, the diff erent expressions ofphoA1andphoA2were found at 24-, 72-, and 144-h P-free conditions (Fig.4a-b), ref lecting that diff erential regulation of the two genes inR.raciborskiimay be a result of their diff erent affi nities for P assimilation(Su et al., 2007; Beszteri et al., 2012).
The genepstS1encoding a high-affi nity phosphatebinding protein was upregulated in this study under P-def icient conditions (Fig.4c). This is consistent with the f indings of Willis et al. (2019). However,the timing of upregulation was diff erent from the Australian strains CS-505 and CS-506, which indicates that the timing of upregulation varies greatly among diff erent strains ofR.raciborskii(Vershinina and Znamenskaya, 2002). Usually, the genepstS1has multiple copies in the genome of cyanobacteria and pico-cyanobacteria (Su et al., 2007), but only one copy may respond to changes in P availability (Harke and Gobler, 2013). InR.raciborskii, however, there is only one copy ofpstS1in a complete operon in the form ofpstS-C-A-B(Sinha et al., 2014; Fuentes-Valdés et al., 2016), which suggests that the presence and regulation ofpstS1are responsible for howR.raciborskiiadapts and responds to P-limited or P-depleted conditions (Moore et al., 2005).
Like in the genomes ofNostocsp. PCC7120 andTrichodesmiumIMS101 (Su et al., 2007), in the genome ofR.raciborskii, orthologous genes of the phosphonate transporter complex and the C-P lyase were found (Stucken et al., 2010). After 24-h culture in a P-def icient medium, the genesphnDencoding phosphonate ABC transporter andphnMencoding phosphonate metabolism protein were markedly upregulated (Fig.4d-e), suggesting that phosphonate compounds might be an important phosphorus source toR.raciborskiiin this low DIP system. These results support a previous study showing thatR.raciborskiican grow with phosphonate as the only available P source (Bai et al., 2014). However, Willis et al.(2019) found the genephnDdid not upregulate in CS-505, but a signif icant upregulation was found in CS-506, ref lecting that intra-specif ic variation of the phosphonate pathway might occur inR.raciborskiipopulations, and that this pathway is active under P-def icient conditions. Dyhrman et al. (2006) showed that the ability to utilize phosphonates played an important role in the prevalence and competitive advantage ofTrichodesmium, indicating that phosphonate metabolism might represent a crucial niche adaptation for the invasion ofR.raciborskii.
In this study, the gene expressions ofnucH,encoding extracellular nuclease, were markedly upregulated in P-def icient treatments in contrast to P supplemented treatments (Fig.4f). Suzuki et al. (2004)proposed that thephoA-nucHoperon inSynechocystisPCC6803 is activated under P limitation. Luo et al.(2017) also found 5′-nucleotidase activities and transcript expression were upregulated when the dinof lagellateKareniamikimotoiwas cultured with ATP as the only available P source, further supporting that nucleotidase was able to cleave PO43ˉ from ATP(Dyhrman and Palenik, 2003; Dyhrman et al., 2012).This implies thatnucHmight play a crucial role in the utilization of the P moiety in nucleic acids in the environment (Su et al., 2007).
4.3 Implications for the management of R. raciborskii blooms
Input reduction of nutrients, e.g., nitrogen (N) and P, has been widely recognized as an eff ective way of controlling cyanobacterial blooms in lakes and reservoirs (Qin et al., 2007; Hamilton et al., 2016).Reducing N or P inputs or both combined to prevent blooms eff ectively is a controversial issue (Xu et al.,2010; Paerl et al., 2011). Wan et al. (2019) proposed that P reduction was more important for controlling blooms dominated by diazotrophic cyanobacteria than by non-diazotrophic cyanobacteria. However,our results indicated thatR.raciborskiicould endure P def iciency conditions for 144 h (6 d) by reducing its growth and photosynthesis and increasing its alkaline phosphatase (AP) activity and gene transcriptions of phosphate uptake and transporters. These results support thatR.raciborskiican regulate physiological and molecular responses to low P availability by an increase in P uptake, the utilization of organic compounds and phosphorus storage compounds, and the induction of alkaline phosphatase (Bai et al., 2014;Guedes et al., 2019; Willis et al., 2019). Additionally,Xiao et al. (2020) indicated that P luxury storage was a strategy for the persistence ofR.raciborskiipopulations under P stress conditions. Therefore,P reduction may not always controlR.raciborskiiblooms, especially in lakes and reservoirs with intermittent P pulses.
5 CONCLUSION
Here, we investigated the responses ofR.raciborskiito ambient P def iciency and resupply at the physiological and molecular levels. The growth and photosynthesis ofR.raciborskiiwere markedly inhibited, while alkaline phosphatase signif icantly increased under P-def iciency. The expression of genes encoding alkaline phosphatase, high-affi nity phosphate-binding proteins, the phosphonate transporter, metabolism, and nucleotidase exhibited a general increase under P-def icient conditions.However, physiological and molecular responses occurred rapidly after DIP re-supplementation of P-def icient treatments, indicating thatR.raciborskiihas a strong ability to regulate its utilization of P to adapt to low P environments. However, diff erent strains ofR.raciborskiimay have diff erent capacities for metabolizing diff erent P sources. Therefore,variations between strains regarding P metabolization need to be further clarif ied to understand the global distribution patterns ofR.raciborskii.
6 DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the f indings of this study are available within the article.The raw data that support the f indings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
