Overview of the distribution and adaptation of a bloomforming cyanobacterium Raphidiopsis raciborskii:integrating genomics, toxicity, and ecophysiology*
Zhongxing WU , Songqi YANG, Junqiong SHI
Abstract Raphidiopsis raciborskii is a notorious bloom-forming and f ilamentous cyanobacterium that has been extensively investigated into its toxicity, phylogeny, and spreading potential. Studies have demonstrated that this species has spanned diff erent climates from tropical zones to temperate regions,suggesting that R. raciborskii is becoming a cosmopolitan species in freshwater systems around the world. In fact, it has been proposed that several characteristics of R. raciborskii may explain its spread and dominance. In particular, R. raciborskii is known to display a high extent of physiological plasticity regarding nutrients, light regimes, and temperatures. Moreover, this species illustrates diff erent ecotypes with distinct environmental requirements. Here, we present an overview of R. raciborskii’s global distribution and adaptation strategy based on the recent f indings from genome variance, toxicity, and ecophysiology.The expansion of its geographical distribution can be linked to its genome, toxicity, and ecophysiology.The variable genes are mainly associated with the stress response, phage defense, DNA repair, cell cycle control, and membrane transport, illustrating the species’ adaptability in response to changing environments.In fact, the species shows rapid adaptability to low and/or variable nutrient availability, especially changing phosphorus availability. Moreover, the variabilities of strains within the population extend their f lexibility to adapt and acclimate to ambient environment. In addition, cylindrospermopsins (CYN) appear to have a potential biological role in facilitating theirs dominance or bloom. These strategies of R. raciborskii make it a challenge to manage in a freshwater system, ref lecting the management of its bloom from further evidence of the complex ecophysiology, toxicity, and genome of this species.
Keyword: distribution; ecophysiology; genome variance; Raphidiopsis raciborskii; toxicity
1 INTRODUCTION
Raphidiopsisraciborskii(basonymCylindrospermopsisraciborskii; Aguilera et al.,2018) is a solitary, planktonic, and f ilamentous diazotrophic cyanobacterium belonging to the order Nostocales. Because it has two terminal heterocysts,R.raciborskiiwas originally described asAnabaenaraciborskiiWołoszyńska from the samples collected in Java, Indonesia, in 1912 (Wołoszyńska, 1912).Original observations were limited to the Indo-Malayan realm, soR.raciborskiiwas considered to be a tropical species. However, an increasing number of reports have been made from every continent except Antarctica (Padisák, 1997; Sinha et al., 2012) thatR.raciborskiiforms bloom in approximately 18% of freshwater lakes, reservoirs, and rivers (Xiao et al.,2020a). These results make it reasonable to assume thatR.raciborskiiis an invasive species (Padisák,1997; Briand et al., 2004; Antunes et al., 2015).
Fig.1 The global geographic distribution of Raphidiopsis raciborski
Apart from its dispersal potential,R.raciborskiihas attracted scientif ic interest due to its association with toxic eff ects. The cyanotoxin alkaloid cylindrospermopsin (CYN) was f irst identif ied by Ohtani et al. (1992) isolated fromR.raciborskii,after which more CYN variants were found (Li et al.,2001; Rzymski and Poniedziałek, 2014; Wimmer et al., 2014). Recently, some strains ofR.raciborskiihave also been found that can produce other toxins,including alkaloid saxitoxin (STX; Lagos et al.,1999; Zingone and Enevoldsen, 2000; Neilan et al.,2003; Miotto et al., 2017) and lipophilic congeners of phorbol 12-myristate 13-acetate (PMA; Rzymski et al., 2017).
Several studies have highlighted the distinctive features of this species that aid its succession and dominance. For example, laboratory studies have observed thatR.raciborskiican utilize all kinds of diff erent nitrogen sources (Harris and Baxter,1996; Moisander et al., 2012; Ammar et al., 2014)and a high uptake affi nity and storage capacity for phosphorus (Isvánovics et al., 2000; Posselt et al.,2009; Wu et al., 2009; Xiao et al., 2020a), as well as can thrive in a wide range of light intensities (Padisák and Istvánovics, 1997; Padisák and Reynolds, 1998;Briand et al., 2002; Pierangelini et al., 2014). Therefore,information on the ecology, phylogeography, and toxicology of this species has been reviewed (Padisák and Istvánovics, 1997; Griffi ths and Saker, 2003;Antunes et al., 2015; Burford et al., 2016, 2018).However, as interest inR.raciborskiihas increased,some issues regarding its phylogeography, molecular selection, and ecophysiological adaptation have been questioned. Here, the latest proposals on its distribution and adaptation through the integration of genomics, toxicity, and ecophysiology are discussed.As well, managing implications forR.raciborskiiare also presented according to new insights into the success of this species in diff erent environments.
2 DISTRIBUTION
Raphidiopsisraciborskiiwas f irst identif ied by Wołoszyńska (1912) in 1899-1900 from samples taken from the lakes in Java, Indonesia. In Europe,R.raciborskiiwas f irst observed in Lake Kastoria,Greece (Skuja, 1937), and later in Hungary (Padisák,1997). In Africa,R.raciborskiiwas recorded in detail in Lake Victoria by Komá rek and Kling (1991)and was probably f irst detected in 1938 by Huber-Pestalozzi (1938). Additionally, this species was f irst reported in America in 1955 (Prescott and Andrews,1955), in Australia in 1979 (Hawkins et al., 1985), and in the Middle East in 1998 (Zohary, 2004). To date,an increasing number of observations have localized this species in rivers, lakes, reservoirs, and shallow waters in the northern and southern hemispheres(Fig.1). This species has been found, for example, in Spain (Romo and Miracle, 1994), Thailand (Li et al.,2001), New Zealand (Stirling and Quilliam, 2001),Germany (Fastner et al., 2003), Japan (Chonudomkul et al., 2004; Zarenezhad et al., 2012), Brazil (Soto-Liebe et al., 2010; Stucken et al., 2010), Poland(Kokociński et al., 2010), Italy (Messineo et al.,2010), Russia (Vinogradska, 1974; Babanazarova et al., 2015; Sidelev et al., 2020), Vietnam (Dao et al.,2010; Nguyen et al., 2017), USA (Yilmaz and Phlips,2011), and Myanmar (Ballot et al., 2020; Swe et al.,2021). In China,R.raciborskiiwas f irst reported in a f ish pond in Kunming, Yunnan, in 2006 (Wu et al.,2011); thereafter, this species was found to be present in freshwater bodies in Guangdong (Lei et al., 2014;Yu et al., 2014), Hubei (Wu et al., 2011, Jiang et al.,2014), Shanghai (Wu et al., 2011), Guizhou (Chen et al., 2011), Jiangsu (Wu et al., 2012a), Taiwan(Yamamoto and Shiah, 2012), Fujian (Lv et al., 2013;Jiang et al., 2014; Tan et al., 2021), Beijing (Xie et al.,2018), Sichuang (Tao et al., 2016), Shangdong (Wang,2019), Chongqing (Zhang, 2019), and Zhejiang (Chao et al., 2021).
2.1 Phylogeography and dispersal route
Several hypotheses have been proposed to explain the origin and dispersal routes ofR.raciborskiifrom tropical/subtropical zones to northern latitudes.The “radiation center” hypothesis was proposed by Padisák (1997) only based on the high diversity and salinity tolerance characteristics ofR.raciborskii.Padisák and Istvánovics (1997) suggested that two radiation centers, Africa as the primary center and Australia as the secondary center, were responsible for expansion in Central America and Asia, respectively.Two possible routes, such as an oceanic route to the America by migratory birds or by unintentional human activities and a continental route to Central Asia and then to European by river course or by birds,are thought to explain the expansion ofR.raciborskiifrom Australia to temperate regions (Padisák and Istvánovics, 1997; Moreira et al., 2011).
Another “refuge” hypothesis for the current geographic distribution was proff ered by Gugger et al. (2005) based on a phylogeographic study. They found that three clusters ofR.raciborskiistrains were grouped: (i) America, (ⅱ) Europe, and (ⅲ) Africa and Australia using the 16S-23S internally transcribed spacer (ITS1) sequences. Therefore, they suggested that recent spread ofR.raciborskiiacross the Americas and Europe occurred from restricted warm refuge areas rather than through intercontinental exchanges. Wood et al. (2014) indicated that cryptic akinetes ofR.raciborskiiwere already present in lake sediment layers in New Zealand long before they were discovered as phytoplankton in 2003. A similar f inding was found in the Blanca subtropical lagoon (De La Escalera et al., 2014). However, the refuge hypothesis has been repeatedly challenged as signif icant genetic diff erences were found in strains ofR.raciborskiifrom southern Europe (Spain, Greece,and Italy) and northern Europe (Germany, Hungary,and Russia) (Cirés et al., 2014; Panou et al., 2018;Sidelev et al., 2020).
Later, a new hypothesis was raised by Haande et al. (2008) and Moreira et al. (2015) through the phylogeographic analysis of strains from all f ive continents based on three genetic markers, 16S rRNA gene, 16S-23S rRNA larger fragment (ITS-L), and RNA polymerase (rpoC1). They postulated that the primary evolutionary center ofR.raciborskiiwas the tropical area of America, from where this species spread to the African continent, followed by Australia,Asia, and Europe. The hypothesis was supported by recent studies which indicated that strains from Spain,Greece, Italy, Tunisia, Russia, and New Zealand are more genetically similar to strains from the Americas(Cirés et al., 2014; Wood et al., 2014; Panou et al.,2018).
Although various hypotheses have been raised to explain the spread ofR.raciborskii, there is a lack of high-quality paleontological evidence to support each hypothesis (Padisák et al., 2016; Kokociński et al., 2017). Sidelev et al. (2020) suggested that close genetic relatedness between the southern European,Tunisian, and American strains, as well as between the African and Australian strains, may be the result of the ancient origin of the species inhabiting the continents, rather than new transport in some cases through birds, insects, humans, or rivers in some cases.Recently, however, Vico et al. (2020) showed Central Africa as the primary center of distribution based on the analysis of 354 orthologous genes from all available genomes and ITS sequences. A nested clade analysis (NCA; Posada et al., 2006) was performed to test the phylogeography of 96 strains ofR.raciborskiifrom diff erent continents in our laboratory (Fig.2).Our results revealed that these strains isolated from Uganda, Senegal, and Australia formed a tight cluster,conf irming the result of Padisák and Istvánovics (1997)and Vico et al. (2020). It suggests thatR.raciborskiican spread from the tropical zone to temperate and northern regions (Padisák and Istvánovics, 1997).However, closer relationships between some Chinese strains and other strains (e.g. European and American strains) were also noted in our results. It suggests that the biogeography ofR.raciborskiihas become even more confused with the increasing studies of more strains being isolated from around the world.
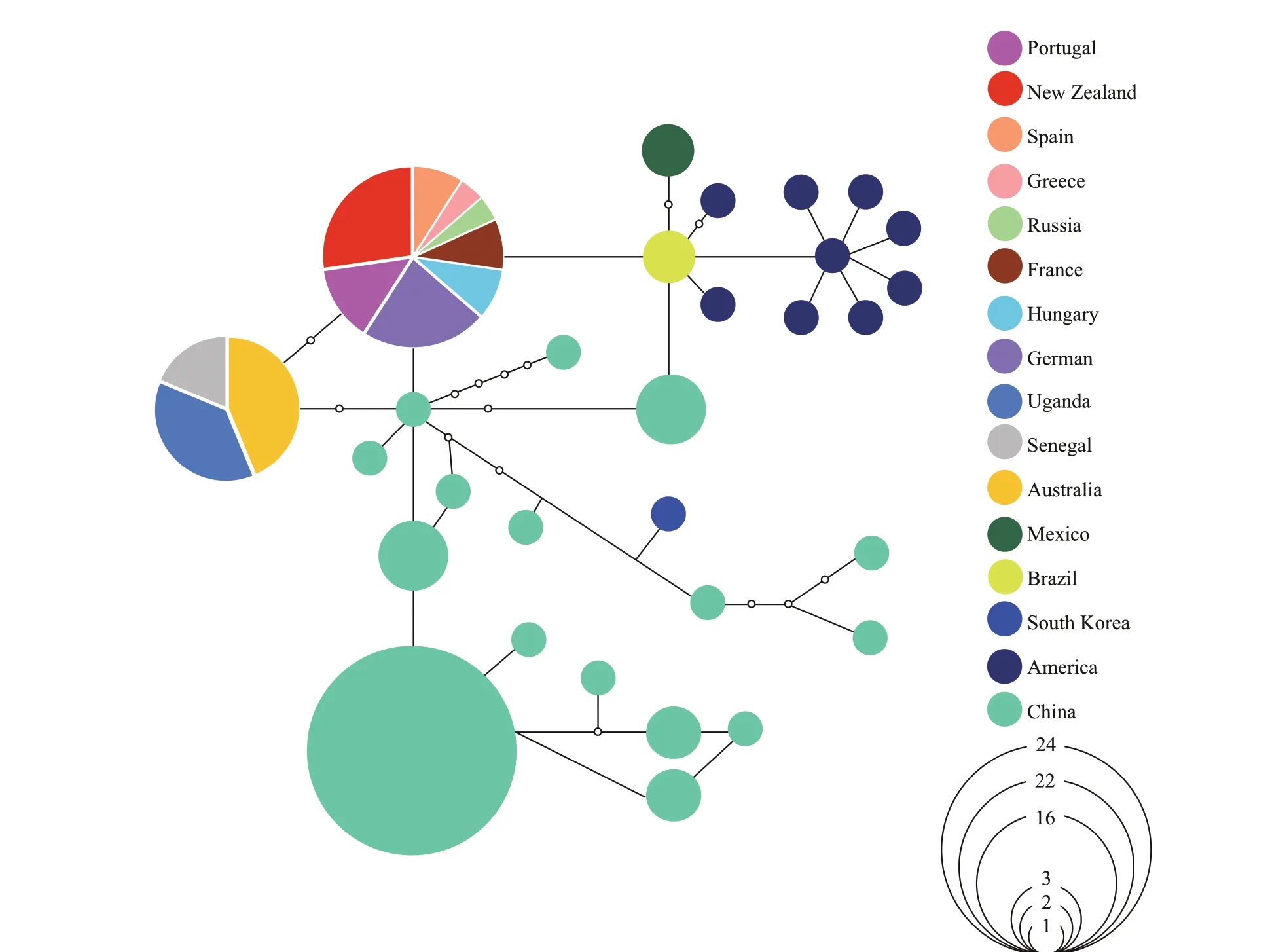
Fig.2 A nested clade analysis (NCA) of nif gene for 96 strains of R. raciborskii isolated from diff erent continents
2.2 Toxicity and dispersal route
The cyanotoxin cylindrospermopsin (CYN) f irst became known in scientif ic documents as the “Palm Island Mystery Disease,” which occurred in 1979 on Palm Island, Australia. One hundred forty-eight persons were hospitalized with severe symptoms of anorexia, vomiting, and tender livers after consumption of cyanobacterial bloom water treated with copper sulfate (Byth, 1980; Ohtani et al.,1992). Another implication ofR.raciborskiiin the poisoning was in northern Queensland, Australia,13 cattle died in 1992 after drinking from a water source with a heavy cyanobacterial bloom (Thomas et al., 1998).
A novel structure for CYN was proposed by Ohtani et al. (1992). To date, four diff erent CYN variants, 7-epicylindrospermopsin (7-epi-CYN),7-deoxy-cylindrospermopsin (7-deoxy-CYN),7-deoxy-desulfo-cylindrospermopsin, and 7-deoxydesulfo-12-acetyl-cylindrospermopsin, have been described (Norris et al., 1999; Banker et al., 2000;Rzymski and Poniedziałek, 2014; Wimmer et al.,2014). Their novel structures, chemical properties,and toxicological eff ects and occurrences have been extensively reviewed (see reviews by De La Cruz et al., 2013; Burford et al., 2016; Adamski et al., 2020;Yang et al., 2021). Moreover,R.raciborskiican also produce neurotoxic STX and its analogs (i.e.,neo-STX, gonyautoxins 2 and 3 [GTX-2 and GTX-3], decarbamoyl STX [dc-STX], and decarbamoylneo-saxitoxin [dc-neo-STX]), collectively known as paralytic shellf ish toxins (PST) (Lagos et al., 1999;Li et al., 2001; Griffi ths and Saker, 2003; Molica et al., 2005; Soto-Liebe et al., 2010). A 43-kbcyrgene cluster (Stucken et al., 2014) and a 35-kbstxgene cluster (Kellmann et al., 2008) were responsible for the production of CYN and STX, respectively.Recently, another toxic compound, polymethoxy-1-alkene (PMA) was reported from strains isolated from North America (Rzymski et al., 2017).
Most studies on the dispersal route ofR.raciborskiiare based on molecular genetic markers (i.e., 16S rRNA, ITS, PC-IGS,nifH, andrpoC1), and did not consider the phenotypes and genotypes of their toxicity (Vico et al., 2020). Studies have shown thatR.raciborskii’s ability to produce toxins appears to show a geographic pattern. For example, CYNs isolated from Australia, New Zealand, and Asia can be produced (Hawkins et al., 1997; Saker et al., 2003;Wood and Stirling, 2003; Chonudomkul et al., 2004;Jiang et al., 2014; Lu et al., 2021), while the South American strains are associated with STX producers(Lagos et al., 1999; Antunes et al., 2015). In contrast,strains from Africa, Europe, and North America are neither PST nor CYN- producers (Fastner et al., 2003;Neilan et al., 2003; Kellmann et al., 2006; Yılmaz et al., 2008; Mowe et al., 2015). Vico et al. (2020)found that the strains analyzed were divided into two clades, one with the South American strains (mostly PSP-producers) and another with the non-toxic strains isolated from Europe and Sun-Saharan Africa and CYN-producers isolated from Oceania. A similar result was also reported by Jiang et al. (2020), who indicated that Clade IV included all PST-producing strains, while CYN-producing strains were divided into two clusters, Clade II and Clade V.
Nevertheless, partial sequences ofcyrgenes are determined in American non-CYN producing strains(Piccini et al., 2011) and in PST-producing strains from Brazil (Hoff -Risseti et al., 2013). Recently,Vico et al. (2020) found that the partial genes ofcyrA,cyrB, andcyrCare present again in the strains isolated from South America. Meanwhile, Yilmaz and Phlips (2011) found thatcyrgenes exhibit more exchange changes within North American strains of CYN-producingAphanizomenon(Chrysosporum)ovalisporumthan between species. A hypothesis raised by Vico et al. (2020), is therefore that (i)non-toxicR.raciborskiispread early from tropical Africa as the primary evolutionary center to North Africa, North America, and Mediterranean Europe;(ii) a secondary evolutionary event was involved to acquire the cluster for CYN synthesis. These CYNproducing species spread warm climates across sub-Saharan Africa, Oceania, and South America.Later, the populations in South America somehow lost thecyrcluster and acquired thestxcluster through horizontal gene transfer, then the STXproducing species migrated to North America. In fact, the secondary evolutionary event mentioned by Vico et al. (2020) was the result of a phylogenetic analysis based on the ribosomal ITS of the species.Moreover, the estimated divergence time calculated forRaphidioposismay coincide with the time when Gondwana was split into Oceania and the South American continent. However, strains isolated from North America, Europe, Africa, and the Middle East have not been reported to produce CYN (Neilan et al., 2003; Yılmaz et al., 2008; Alster et al., 2010),which does not support this secondary evolutionary evidence that African strains are a source of the CYN gene cluster.
Recently, Jiang et al. (2020) found that strains ofR.raciborskiiisolated from China (i.e., CHAB3409,CHAB3422, and CHAB3426) produce STX, neo-STX, and dc-STX, and these strains and American strains have been clustered into diff erent clades,further supporting the recent intercontinental spread events of toxicR.raciborskii(Antunes et al., 2015),but not the geographic origin of the strains. In fact,other cyanobacterial genera, includingChrysosporum,Aphanizomenon,Anabaena,Umezakia,Microseira,andOscillatoria, have been reported to produce CYN(Rzymski and Poniedziałek, 2014). Hence, a complex history of acquisition and loss in thecyrgene cluster may be associated with its intra- and intergenomic transfers (Jiang et al., 2014, 2020; Burford et al., 2016). Similarly, Moustafa et al. (2009) also suggested that STX is a common ancestral trait ofR.raciborskiistrains. Therefore, to answer these questions, additional genomic data from these diverse lineages, including closely related toxic and non-toxic strains, are needed.
Based on the literature and our NCA analysis(Fig.2), we partially agree with Padisák’s early hypothesis of the tropical region as the evolutionary center ofR.raciborskii, but do not support the f inding of Africa and Australia as the primary and secondary centers, and the high genetic and toxic diversity of Chinese strains indicates a high heterogeneity of theR.raciborskiipopulation (Cirés et al., 2014; Moreira et al., 2015; Panou et al., 2018). Willis et al. (2018)also suggested thatR.raciborskiiexhibits high plasticity due to frequent gain or loss of genes. These ref lect that the ability ofR.raciborskiito produce toxins may not be a geographical pattern but the result of environmental responses and adaptations(Willis et al., 2019; Jiang et al., 2020). Therefore,a larger number of strains with diff erent toxicity are required to test the comprehensive biogeography of this species, as previously suggested (Cirés et al.,2014).
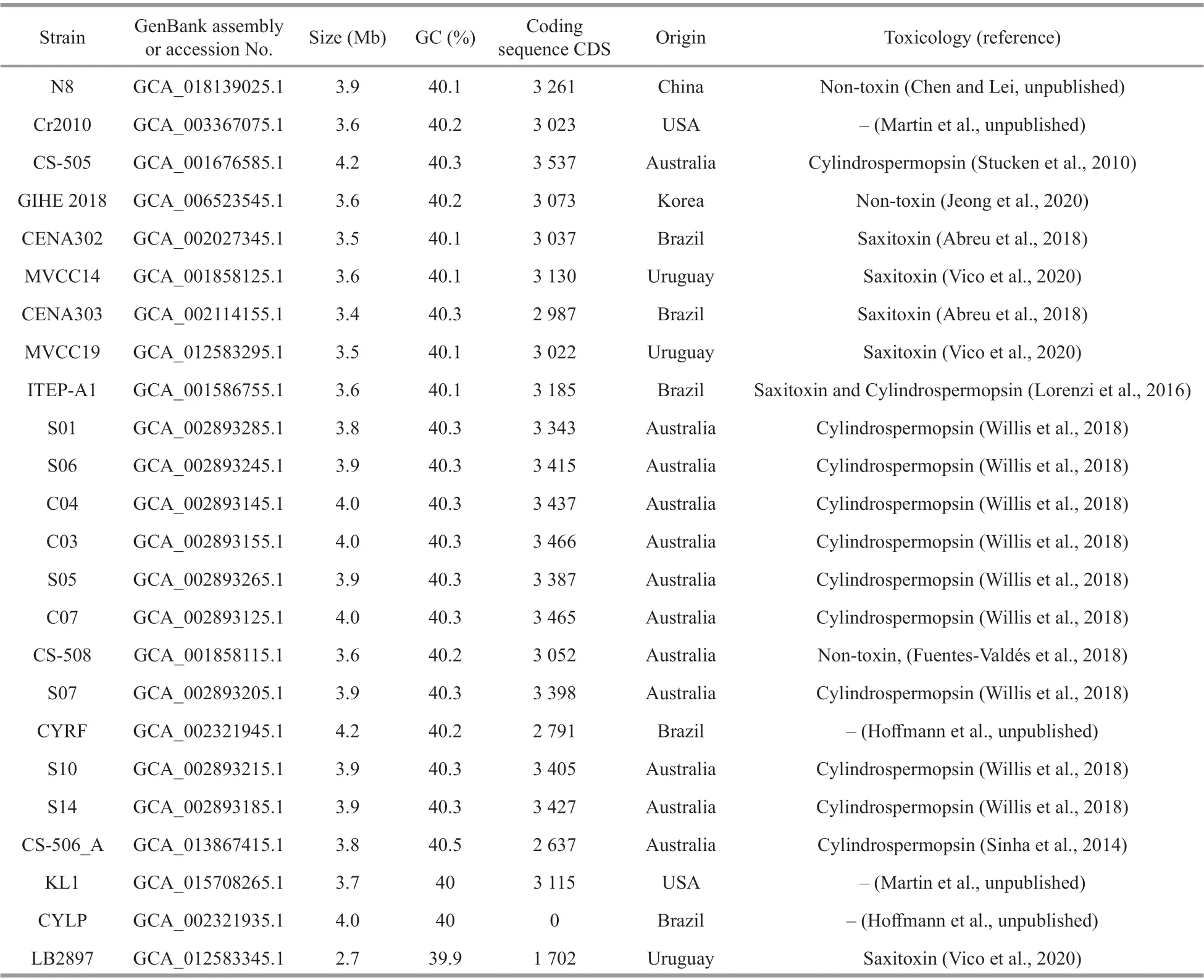
Table 1 Genome assembly and toxins statistics in R. raciborskii strains
3 ADAPTATION AND ACCLIMATION
3.1 Genome variations and adaptation
Based on ecological and genomic studies, new insights into the genomic adaptation of marine picocyanobacteria to the local environment have been provided (Kashtan et al., 2014; Larsson et al.,2014; Biller et al., 2015). However, due to the lack of balanced genomic samples, the genomic adaptation of cyanobacteria to a wide variety of environments is still poorly understood (Chen et al., 2021). The f irstR.raciborskiigenome was sequenced from the toxigenic strain CS-505 (Stucken et al., 2010),followed shortly thereafter by those of CS-506 and CS-509 (Sinha et al., 2014). To our knowledge, only oneR.raciborskiigenome has been closed as late as 2021. However, several draft genomes isolated from diff erent locations were subsequently sequenced. To date, 23 drafts and 1 closed genomes sequenced from isolated strains from Australia, Brazil, United States,Uruguay, China, and Korea were available from NCBI (see Table 1 for detailed information). The average nucleotide identity (ANI) between strains was 0.997 6 (range 0.995 0-0.998 7, between genome pairs; Willis and Woodhouse, 2020).
Compared to other cyanobacteria (e.g.,Microcystis,Nostoc,Dolichospermum, andAphanizomenon),a smaller genome was found inR.raciborskiiwith a genome size of 3.74±0.24 Mb, 40.24%±0.15%G+C content, and 3 144±292.35 coding sequences(Table 1). Stucken et al. (2010) has suggested that a small genome found inR.raciborskiimay be in the process of reducing superf luous functions. Typically,a downsizing of a genome is seen as an indication of an evolutionary adaptation strategy to diff erent environments (Rocap et al., 2003; Shi and Falkowski,2008; Larsson et al., 2011; Willis et al., 2018). It suggests that genome variants inR.raciborskiiareresponsible for the global expansion into new habitats.
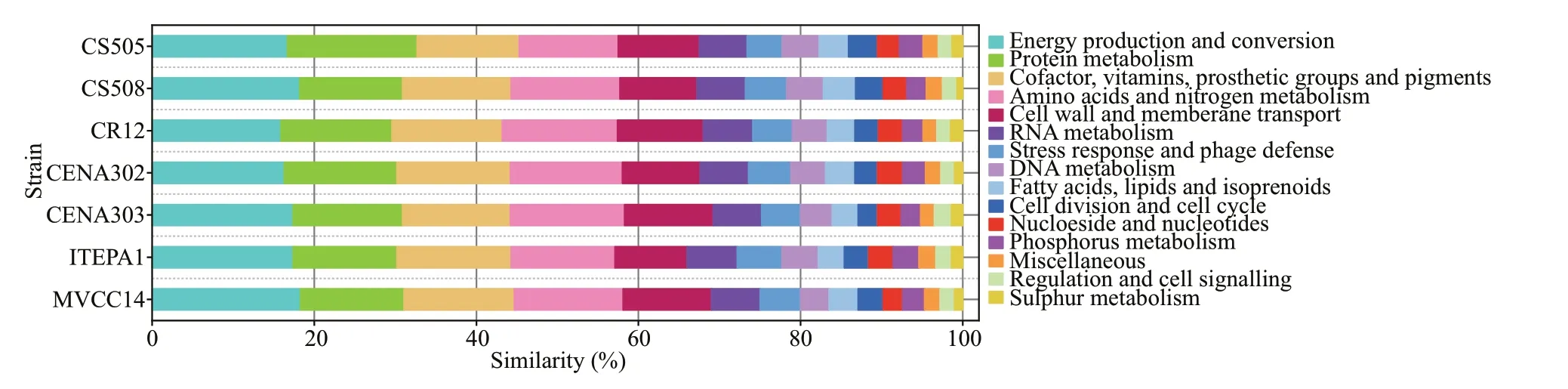
Fig.3 Comparison of strain-specif ic (or non-homologous) genes in Raphidiopsis raciborskii strains
Willis et al. (2018) stated that theR.raciborskiipan-genome contains about 16% ofR.raciborskiigenome with 847 variables and 433 strain-specif ic orthologous groups, suggesting that there is greater genetic diversity inR.raciborskiistrains. In addition,variation, arrangement, or shifting of genes are always found inR.raciborskiiwhen seven strains ofR.raciborskiiare compared with the strainRaphidiopsisbrookiiD9 strain. These genes are involved in natural product biosynthesis, heterocyst glycolipid formation, nitrogen f ixation, and toxin production. Similar results are reported by Abreu et al. (2018), who found variable genes involved in amino sugar metabolism, DNA modif ication, and carbohydrate biosynthesis. Shi and Falkowski (2008)suggested that selective pressures and evolution can aff ect the core and variable genes, resulting in strain variability in diff erent environments (Kashtan et al.,2014).
A comparative genome analysis showed that strains ofR.raciborskiicontained a variety of strain-specif ic (or non-homologous) genes (Stucken et al., 2010; Sinha et al., 2014; Abreu et al., 2018).These genes are involved in energy production and conversion, stress response and phage defense, DNA repair and recombination, cell cycle control, and the nutrients transport and uptake (Fig.3), all of which are largely related to environmental response and adaptation. Moreover, the gene clusters associated with toxin production and heterocyst diff erentiation(i.e., hassallidin [hass], cylindrospermopsin [cyr],saxitoxin [sxt], heterocyte glycolipid [hgl], and nitrogen f ixation [nif,fdxN,hesAandB, andfeoaA])also indicate phenotypic plasticity (Sinha et al.,2014; Abreu et al., 2018). Stucken et al. (2010)suggested that the absence or loss of thecyrcluster,rather than indicating mutations or partial deletions,was associated with the absence of toxicity in some strains ofR.raciborskii. However, several reports have found that someR.raciborskiistrains retained the partialcyrcluster, i.e.,cyrA,cyrB, or/andcyrCare still unable to produce CYN (Kellmann et al.,2006; Rasmussen et al., 2008; Hoff -Risseti et al.,2013). This supports that horizontal gene transfer or subsequent loss of thecyrgene is responsible for the acquisition of thecyrgenes (Christiansen et al.,2008; Moustafa et al., 2009). Willis et al. (2018) has observed that 21 proteins, particularly those involved in sugar transport, phosphonate substrate binding, and CRISPR/Cas phage-defense systems, yield a greater copy number in the coiled compared to the straight morphotypes ofR.raciborskii. Larsson et al. (2011)proposed that gene duplication can expand phenotype and adaptive behavior in cyanobacteria.
In short, comparative genomics provides new insights into the genotypic and phenotypic plasticity of the speciesR.raciborskii. A high proportion of variable strain-specif ic genes associated with environmental responses and adaptation, particularly in some key cellular processes (e.g., cell regulation,biosynthesis, and transport), are found in this species,ref lecting that successful adaptation to specif ic habitat inR.raciborskiimay allow the exploration of a wide range of environmental conditions. Furthermore, the co-existence of multiple strains within aR.raciborskiipopulation in a single water sample can confer f itness advantages to this species in variable environments by eliciting their niche adaptation (Piccini et al.,2011; Willis et al., 2018). In addition, Abreu et al.(2018) found that the comparative genome analysis showed that the f ive South American genomes CENA302, CENA303, ITEP-A1, MVCC14, and D9(Brazil and Uruguay) are slightly smaller and more conserved than the non-South American CS-505, CS-508, and CR12 (Australia and Singapore) genomes,suggesting that genomes from South America underwent gene loss events. However, due to the lack of genome sequences in European and African strains, no more precise conclusions can be drawn about the inf luence of the geographic environment on their genomic plasticity. Therefore, in order to explain the very diff erent strategies for genomic organization and adaptation mechanisms inR.raciborskii, more strains from a range of habitats or regions needed to be sequenced and compared in the future.
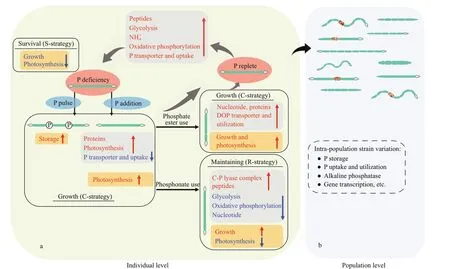
Fig.4 Diagram for the response to changing ambient phosphorus in Raphidiopsis raciborskii
3.2 Ecophysiology and adaptation
3.2.1 Phosphorus
Phosphorus is considered a key factor in the ecophysiology and dominance ofR.raciborskii. A positive or negative correlation betweenR.raciborskiicell densities and phosphorus concentrations has been reported in f ield studies (Bonilla et al., 2012; Muhid et al., 2013; Soares et al., 2013a; Zhao et al., 2017).Several studies have illustrated thatR.raciborskiihas a high uptake affi nity for dissolved inorganic phosphorus (Isvánovics et al., 2000; Wu et al., 2009)and a high phosphorus storage capacity (Posselt et al., 2009; Willis et al., 2017), as well as a superior scavenger for dissolved organic phosphorus (Bai et al., 2014). Furthermore, both uptake and conversion of phosphorus were more eff ective inR.raciborskiithan inMicrocystisaeruginosaandAphanizomenonf los-aquae(Wu et al., 2009). Therefore, these traits are favorable for the dominance ofR.raciborskiipopulations (Isvánovics et al., 2000), which is supported by the results of Chislock et al. (2014), who indicated thatR.raciborskiican dominate at diff erent phosphorus concentrations.
Physiological and molecular studies have suggested thatR.raciborskiican evolve a variety strategies in response to environmental phosphorus(Fig.4a). Under phosphorus def icient conditions,strains show little metabolic activity to keep sustain themselves (i.e., “S-adapted strains”). In this environment, the growth and photosynthesis of these strains are signif icantly inhibited and the genes encoding photosynthesis and protein synthesis are markedly downregulated, while alkaline phosphatase and the genes encoding phosphate uptake and transport, ATP-consumption, and energy metabolism are markedly upregulated (Wu et al., 2012a; Bai et al., 2014; Willis et al., 2018; Shi et al., 2022). Under organic phosphate conditions, the strains showed a rapid growth (i.e., “C-adapted strains or K-adapted strains”). In this state, rapid growth is noted, which is caused by a signif icant increase in genes encoding phosphate-specif ic transporters, alkaline phosphatase,and ribosomes (Bai et al., 2014; Willis et al., 2018; Shi et al., 2022). However, under phosphonate conditions(i.e., “R-adapted strains or r-adapted strains”), a slight inhibition of growth and photosynthesis is observed because alkaline phosphatase and the genes encoding carbon-phosphorus lyase, genetic information, and environmental information are dramatically upregulated (Willis et al., 2018; Shi et al., 2022). Additionally, sinceR.raciborskiihas a high phosphorus storage capacity, pulsed additions of dissolved inorganic phosphorus are more favorable for the growth of this species compared to constant feeds of dissolved inorganic phosphorus (Posselt et al., 2009; Marinho et al., 2013; Amaral et al., 2014),referred to as “C-adapted strains or K-adapted strains”(Xiao et al., 2020a).
Recent studies have provided evidence that diff erent strains in aR.raciborskiipopulation exhibit signif icant diff erences in growth, storage, and molecular response to phosphorus concentrations and pulses (Fig.4b, Amaral et al., 2014; Willis et al.,2015, 2017, 2019; Guedes et al., 2019; Xiao et al.,2020a). For example, Xiao et al. (2020a) showed that phosphorus storage capacity can vary four-fold in six toxic strains ofR.raciborskii. Willis et al. (2019) have also pointed out that gene copy number and expression patterns for phosphorus metabolism show diff erences between the coiled and straightR.raciborskiistrains under phosphorus replete and def iciency conditions.This f inding suggests that the intraspecif ic variability ofR.raciborskiican lead to changes in the proportion of strains within a population (Burford et al., 2018).In addition, it has been suggested thatR.raciborskiidominance can be promoted under both high and low nitrogen-to-phosphorus ratios (Posselt et al., 2009;Chislock et al., 2014).
Moreover, previous studies have conf irmed that P availability can aff ect intracellular CYN concentration(QCYNS) or a shift in the proportion of toxic and nontoxicR.raciborskii. For example, Mohamed and Al-Shehri (2013) found that QCYNSofR.raciborskiicells was increased when P concentrations were higher in a Saudi lake. Burford et al. (2014) also indicated that the proportion of toxicR.raciborskiistrains increases with increasing phosphorus availability, regardless of whether N was supplied using a mesocosm study.Lu et al. (2021) showed that phosphorus def iciency stimulatesR.raciborskiidominance by facilitating CYN-induced alkaline phosphatase secretion. A similar f inding was reported by Bar-Yosef et al. (2010)in CYN-producing cyanobacteria,Chrysosporumovalisporum. The results may indicate that CYN may be facilitated by the dominance ofR.raciborskiiunder P def iciency.
3.2.2 Nitrogen
Raphidiopsis(Cylindrospermopsis)raciborskiiwas originally described as a Nostocales species with heterocytes, distinguished from otherRaphidiopsisby its lack of heterocytes and nitrogen-f ixing ability(Padisák, 1997). However, Abreu et al. (2018) found that theC.raciborskiistrain CENA303 isolated from Brazil does not diff erentiate heterocytes due to the absence ofnifandhglgene clusters involved in nitrogen f ixation and thick heterocyte glycolipid envelope formation, respectively. Therefore,CylindrospermopsisandRaphidiopsisare considered to be a unifying genus, withRaphidiopsisusing a morphological, ultrastructural, physiological,and molecular approach (Aguilera et al., 2018). In general, nitrogen-f ixing ability is often associated with an ecological advantage ofR.raciborskiiover non-nitrogen-f ixing species (Harris and Baxter, 1996;Hadas et al., 2012). Studies have indicated that the terminal heterocyst cells ofR.raciborskiican f ix nitrogen, allowing this species to survive in low dissolved nitrogen environments (Harris and Baxter,1996; Présing et al., 1996; Padisák and Istvánovice,1997; McGregor and Fabbro, 2000; Spröber et al.,2003; Plominsky et al., 2013; Willis et al., 2016).However, a preference for diff erent forms of dissolved nitrogen (i.e., ammonia, nitrate, and urea) has now been demonstrated inR.raciborskii(Hawkins et al.,2001; Saker and Neilan, 2001; Burford et al., 2006;Ammar et al., 2014; Figueredo et al., 2014; Yu et al.,2014). Ammar et al. (2014) showed thatR.raciborskiican grow faster than the speciesPlanktothixagardhii,a perennial biomass and phytoplankton community dominant in a Tunisian reservoir, at high ammonia concentrations. Dai et al. (2015) also found that the growth ofR.raciborskiiwas signif icantly inhibited under conditions of low nitrogen (<0.5 mg/L).
The relationship between CYNs concentrations and nitrogen has led to conf licting conclusions.For example, Saker and Neilan (2001) found that the highest and lowest CYNs concentrations were determined inR.raciborskiigrown in the absence of a f ixed N source and ammonium, respectively.Rigamonti et al. (2018) also indicated that a positive association between CYN production and nitrogen f ixation was observed inR.raciborskii. In contrast,Vico et al. (2016) showed that nitrate availability is not related to the biosynthesis of saxitoxin and analogs inR.raciborskii. However, compared to nitrate uptake,nitrogen f ixation is an ineffi cient and energetically expensive process (Shaf ik et al., 2001; Burford et al.,2006). Abreu et al. (2018) found that the non-nitrogenf ixing strainR.raciborskiiCENA303 lacks the nitrogen f ixation (nif) and heterocyte glycolipid (hgl)gene clusters. Therefore, nitrogen availability can shift the proportion of toxic and non-toxicR.raciborskiiand regulate the formation ofR.raciborskiibloom.Switching between dissolved nitrogen assimilation and nitrogen f ixation inR.raciborskiiis an adaptive strategy to respond to f luctuations in environmental nitrogen (Moisander et al., 2012).
3.2.3 Other factor
Raphidiopsisraciborskiihas shown a wide tolerance to diff erent temperatures and light intensities.This species can grow at light intensities as low as tens to hundreds of μmol photons/(m2·s) (Saker et al.,1999; Shaf ik et al., 2001; Griffi ths and Saker, 2003;Briand et al., 2004; Dyble et al., 2006; Mehnert et al., 2010; Yu et al., 2014). Field studies have shown thatR.raciborskiican form blooms under low light intensity, which has advantages for its shade tolerance and light acclimatizaion (Padisák, 1997; Padisák and Reynolds, 1998; Briand et al., 2002; Mehnert et al.,2012). Moreover, stratif ied water column conditions are generally considered favorable forR.raciborskii,although it is typically dispersed throughout the water column (Bouvy et al., 1999, 2003; McGregor and Fabbro, 2000; Berger et al., 2006). This could be a factor contributing to the success ofR.raciborskii(Antunes et al., 2015; Burford et al., 2016).
Raphidiopsisraciborskiialso exhibits a wide tolerance to diff erent temperatures (Briand et al.,2004; Chonudomkul et al., 2004; Everson et al., 2011;Bonilla et al., 2012). A model analysis revealed thatR.raciborskiiblooms can occur in the temperature range of 25 °C to 32 °C (Recknagel et al., 2014),suggesting that increasing temperature favor the bloom formation of this species (Soares et al.,2012). Studies have shown that rising temperatures are benef icial for the spread ofR.raciborskii, as the akinete germination of this species is aff ected by early spring warming in temperate habitats (Padisák, 1997;Briand et al., 2002; Wiedner et al., 2007; Mehnert et al., 2012; Yu et al., 2014). Saker and Neilan (2001)observed that temperate strains can produce more akinetes than those in tropical strains. These results suggest that the interplay between ecophysiology and genetic evolution may have an impact on the spread ofR.raciborskii. A recent study also demonstrates that temperature and light have a synergistic eff ect on the growth rates ofR.raciborskii(Kehoe et al., 2015;Xiao et al., 2020b).
In addition, the ecological performance and selection ofR.raciborskiican also be inf luenced by anthropogenic CO2(Wu et al., 2012b; Pierangelini et al., 2014), pH (Bonilla et al., 2012; Holland et al.,2012), salinity (Moisander et al., 2012), allelopathy(Figueredo et al., 2007; Leão et al., 2009; Antunes et al., 2012; Mello et al., 2012), multiple disturbance(Yang et al., 2017), zooplankton (Soares et al., 2010;Bednarska et al., 2014), and other biotas (Sukenik et al., 2012; Bagatini et al., 2014; Guedes et al., 2019;Bai et al., 2020).
4 CONCLUSION AND IMPLICATION
The abundance of studies from around the world has provided our understanding of the biogeography,toxicity, genome, and ecophysiology ofR.raciborskii.Considering all the evidence, the biogeography of this species is credited with an early spread from a tropical zone to temperate regions, while the scenario of refuge and secondary radiation centers has yet to be conf irmed by exploring further strains from all continents or paleontological evidence. CYNproducing strains have been identif ied in a limited number of country, while a geographic spread of toxic strains or a complex history of acquisition and loss in thecyrorstxgene cluster is not excluded from the intra- and inter-genomic transfers based on current studies. Studies have shown that the production and export of CYN inR.raciborskiican be a functional strategy for competition with other phytoplankton.More direct evidence is still needed to support CYN’s potential biological role to facilitate its dominance or bloom.
It is obvious that this species shows f lexible adaptation strategies (“C-adapted, R-adapted, and S-adapted”) in nutrient dynamics based on laboratory experiments, which are very crucial for their expansion behavior. However, f ield studies always indicate negative or positive eff ects of nitrogen or phosphorus on this species or its dominance, ref lecting that an interaction of nitrogen or phosphorus is likely to be underestimated. Moreover, it is clear that genome variation and ecotypes exist between co-occurring strains in a water sample. Therefore, supplementary reports on interaction eff ects, phenotypic diff erences,and population plasticity can be expected in this species. Furthermore, the impact of global climate change on the physiological resilience or existence of distinct ecotypes in this species remains unclear.
Overall, there is no doubt that rising temperatures can be associated with the spread and proliferation of this species. Moreover, f lexible strategy and signif icant intra-population strain variation in nitrogen and phosphorus dynamics provide better resilience of a population under changing environmental nutrients.Therefore, controllingR.raciborskiibloom may not be achievable with a simple reduction in nitrogen or phosphorus loading, particularly in intermittent nutrient pulses and mixed water columns. Future eff orts are essential for a comprehensive understanding of the ecophysiology ofR.raciborskiiin diff erent scenarios in order to f ind an effi cient means of the control.
5 DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the f indings of this study are available within the article.The raw data that support the f indings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
