The impact of the accumulation of algal blooms on reed wetlands in the littoral zones of Chaohu Lake*
Shuzhan MA , Yue WU , Siwen CHEN , Bingfa CHEN , Cheng LIU ,Xiaozhi GU , Xiaoli SHI , Kaining CHEN ,**
1 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Institute of Agricultural Resources and Environmental Sciences, Jiangsu Academy of Agricultural Sciences, Nanjing 210014,China
4 Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, Suzhou University of Science and Technology, Suzhou 215009, China
Abstract In a large eutrophic lake, the littoral zone is normally an area with high-density elevated aquatic plant life, including algal blooms, where the presence of reed wetlands allows the accumulation of algae. In this study, the impact of accumulated algal blooms in reed wetlands in the littoral zones of Chaohu Lake was investigated seasonally from 2018 to 2019. The concentrations of chlorophyll a (Chl a), total nitrogen (TN), and total phosphorus (TP) were much higher in the reed-covered littoral zones (RCLZ) than in the unvegetated littoral zones (ULZ), indicating that more algal biomass was trapped and accumulated in the RCLZ. Algal biomass could be horizontally transported to downwind littoral zones under low wind speeds, favoring the establishment of blooms. Algal accumulation levels were highest in summer due to high water temperatures and algal biomasses. Likewise, the northern littoral zones were conducive to the development of large algal blooms because of the wind pattern. The values of TN, TP, Chl a, and loss on ignition in surface sediments were higher in the RCLZ than in the ULZ. Moreover, the diff usive f luxes of ammonium and soluble reactive phosphorus were also higher in the RCLZ than in the ULZ. Considering the capability of reed wetlands to trap algae, mechanical salvage and other physical methods should be adopted to eliminate algal biomass when massive blooms accumulate in the RCLZ.
Keyword: Chaohu Lake; littoral zone; reed wetlands; algal blooms
1 INTRODUCTION
Eutrophication and associated algal blooms have been increasing in frequency and intensity in lakes and reservoirs worldwide in recent decades due mainly to human-induced nutrient (nitrogen and phosphorus) over-enrichment (Xu et al., 2010; Ho et al., 2019; Wurtsbaugh et al., 2019) and partly because of climate change (e.g., increased temperature) (Paerl and Huisman, 2008). The overproliferation of algae causes serious ecological and socioeconomic problems, including oxygen def iciency, bad odors,degradation of aquatic ecosystems, and losses of biodiversity (Kudari and Kanamadi, 2008; Paerl and Huisman, 2008; Von Sperling et al., 2008; Paerl and Otten, 2013). Thus, the removal and control of algal blooms is key to the restoration of the health and the functioning of lake ecosystems (Wu et al., 2019).

Fig.1 The distribution of sampling sites
The littoral zone of a lake is the nearshore region,which can support large stands of aquatic macrophyte and phytoplankton growth (Xiang et al., 2014; Zhu et al., 2020; Wong et al., 2021). The intensity of algal accumulation varies with the characteristics of landforms and vegetation in littoral zones (Cai et al.,2018). Common reed (Phragmitesaustralis) is a cane-like perennial grass with a worldwide distribution in littoral zones (Hao et al., 2013). Reed is considered an eff ective macrophyte for removing nutrients from lakes (Fukuhara et al., 2007; Sollie et al., 2008; Wang et al., 2011). However, in eutrophic lakes, the presence of reeds causes the accumulation of algae in littoral zones (Wang et al., 2006a), negatively aff ecting water quality (Wang et al., 2006b; Xiang et al., 2014; Zhu et al., 2020).
Chaohu Lake is the f ifth-largest freshwater lake in China and has suff ered from increasingly severe algal blooms in recent decades due to rapid growth of the local human population as well as economic development (Xu et al., 2003; Kong et al., 2013;Zhang and Kong, 2015). Massive algal bloom outbreaks have occurred in this lake almost every year since the 1980s (Jiang et al., 2010, 2014; Cai and Kong, 2013; Kong et al., 2013; Yu et al., 2014). In Chaohu Lake, f loating-leaved and submerged macrophytes are rarely present, and reeds are the dominant macrophytes in the littoral zones. The littoral zones are the region most frequently aff ected by human activities (Cai et al., 2018), and algae in these littoral zones may serve as a seed stock for subsequent blooms (Gu, 2012; Chen et al., 2016).Thus, the accumulation of algal blooms in the littoral zones of shallow lakes have received much attention(Xiang et al., 2014; Qi et al., 2017). However, most studies have only focused on the eff ects of algal blooms on water quality (Sollie et al., 2008; Li et al.,2010) but have underestimated the impact of algal accumulation on the emergent macrophyte wetlands.Thus, knowledge related to the pattern of algal accumulation in reed wetlands is important for understanding the biogeochemical cycle of algae in Chaohu Lake.
In this study, the physicochemical features of water and sediment were investigated seasonally in reed wetlands and surrounding unvegetated lake regions.The purpose of this study is to clarify the factors inf luencing the accumulation of algal blooms in reed wetlands and to evaluate how the accumulation of algal biomass aff ects the physicochemical environmental parameters of reed wetlands. Finally,management recommendations for the prevention and control of algal blooms in the littoral zones of Chaohu Lake are proposed.
2 MATERIAL AND METHOD
2.1 Study area
Chaohu Lake, which is located in the lower Changjiang (Yangtze) River Basin (31°25′28″N-31°43′28″N, 117°16′54″E-117°51′46″E), features a 774-km2surface area and a 184.66-km-long shoreline(Yu et al., 2011; Wang et al., 2018). Lake depth is usually less than 4 m but depends on hydrological conditions, with a maximum depth of 6.78 m and an average value of 3 m (Zhang et al., 2016). The volume of Chaohu Lake is 3.23×109m3in the rainy season,with a residence time of approximately 150 days. In contrast, the volume is only 1.72×109m3in the dry season, with a residence time of approximately 210 days (Duan et al., 2017). Chaohu Lake has a basin area of 13 350 km2and is characterized by a subtropical monsoon climate with an average annual rainfall of 1 100 mm. The annual water temperature averages approximately 15 °C, and the lowest and highest temperatures are 2-3 °C in January and 28-30 °C in July, respectively. The dominant wind directions are southeast in summer and northwest in winter (Yu et al., 2011). This lake performs multiple functions, including aquaculture, agricultural irrigation, f lood control, tourism, and drinking water supply. This lake has undergone rapid eutrophication and frequent cyanobacterial blooms with nutrient inputs from urban sewerage and agricultural nonpoint sources over the past three decades (Shang et al.,2015), and cyanobacterial blooms are primarily dominated byMicrocystisandAnabaena(Jiang et al.,2010, 2014).
2.2 Field sampling
In this study, samples were collected monthly from f ifteen sites ( 1#- 15#) in open water areas in Chaohu Lake from April 2018 to November 2019 (Fig.1).Three littoral zones (C1-C3), representing diff erent downwind directions, were sampled seasonally in April 2018, July 2018, January 2019, and November 2019. Every littoral zone included the reed-covered littoral zone (RCLZ) and its surrounding unvegetated littoral zone (ULZ). The reed densities in the three RCLZ averaged 120 plants per square meter, with a standard deviation of 15 plants per square meter.Surface water samples, including samples from open water areas and three littoral zones, were collected and stored under low-temperature conditions (4 °C)for analysis in the laboratory. Sediment cores were also collected from zones (C1-C3) using a gravity corer sampling apparatus (90 mm in diameter ×500 mm in length; Rigo Co., Ltd., Saitama, Japan).Each sediment core was immediately sealed with a rubber stopper and sealing f ilm.
2.3 Physicochemical properties of the water column
Dissolved oxygen (DO) and temperature of surface water (WT) were measured in situ with a Yellow Springs Instruments (YSI) 6600 V2 multisensor sonde. Environmental parameters, including total nitrogen (TN), total phosphorus (TP), and chlorophylla(Chla), were determined in the laboratory-based on standard methods. TN was determined using the alkaline potassium persulfate digestion method in unf iltered water (State Environmental Protection Administration and Editorial Board of Water and Wastewater Monitoring and Analysis Methods, 2002).TP was determined using the molybdenum blue colorimetric method in unf iltered water (State Environmental Protection Administration and Editorial Board of Water and Wastewater Monitoring and Analysis Methods, 2002). Chlawas determined photometrically at wavelengths of 630, 645, 665, and 750 nm after f iltration on Whatman GF/F glass f ilters and 24 h extraction in hot 90% acetone (Chen et al.,2006). In this study, the concentration of Chlawas used as the indicator to characterize the intensity of algal accumulation.
2.4 Physicochemical properties of the surface sediment
All sediment cores were segmented at 2-cm intervals. The segmented sediment samples were then freeze-dried in a vacuum freeze drier (Biosafer,Nanjing, China). The dried sediment samples were ground in an agate mortar and sieved through a 100-mesh screen (0.15 mm) before analysis. TN in the sediment was extracted using alkaline potassium persulfate and then analyzed with an UVeVis spectrophotometer (State Environmental Protection Administration and Editorial Board of Water and Wastewater Monitoring and Analysis Methods, 2002).TP in the sediment was analyzed by inductively coupled plasma atomic emission spectrometry (ICPAES, Perkin-Elmer DV4300, USA) (Yin et al., 2018).The detailed pretreatment procedure and method for determining Chl-aconcentration in the sediment were described by Yan et al. (2004). The water content of the sediment was determined by weight loss after drying at 105 °C in an oven (Senxin DGG-9240A,Shanghai, China) for 24 h, and sediment porosity was calculated from the water content (Cermelj et al.,1997). Organic matter in the sediment was measured by loss on ignition (LOI), which was determined after ignition at 550 °C for 5 h (Dean, 1974). All the analytical methods described above were used consistently during the long-term investigation.
2.5 Pore water diff usive f lux calculations
The pore water in the sediment cores was sampled using a series of mini-peepers (Ding et al., 2010).Each peeper contained 30 vertically disposed dialysis cells at a vertical resolution of 4 mm. The capacity of each cell was approximately 300 μL. Before each peeper was used, a nitric f iber f ilter membrane with an aperture of 0.45 μm was f ixed on both sides of the peeper, and the peeper was placed in deionized water and f illed with nitrogen for 24 h to discharge the oxygen from the peeper. All of the peepers were inserted into the sediment with approximately 3-5 cm exposed above the sediment-water interface (SWI) to obtain an intact interstitial water-overlying water prof ile and allowed to stabilize for three days, after which the peepers were pulled gently from the sediment and f lushed with oxygen-free deionized water before analysis for ammonia nitrogen (NH4+-N)and soluble reactive phosphorus (SRP). The balanced pore water in each peeper was removed with an enzyme marker plate. The concentrations of NH4+-N and SRP were analyzed using a microtiter plate reader(Biotek Epoch, Winooski, VT, USA) by a miniaturized photometrical method (Laskov et al., 2007). Based on the analyzed pore water prof iles, the diff usive f luxes of NH4+-N and SRP across the SWI were calculated using Fick’s f irst law of diff usion (Ullman and Aller,1982). The detailed method for calculating the diff usive f luxes was described by Liu et al. (2016).
2.6 Meteorological data
Meteorological data, including wind speed and wind direction in April 2018, July 2018, January 2019, and November 2019, were obtained from the National Meteorological Data Service Center website,(http://data.cma.cn/en), using daily records from meteorological station #58326 of the China Meteorological Administration.
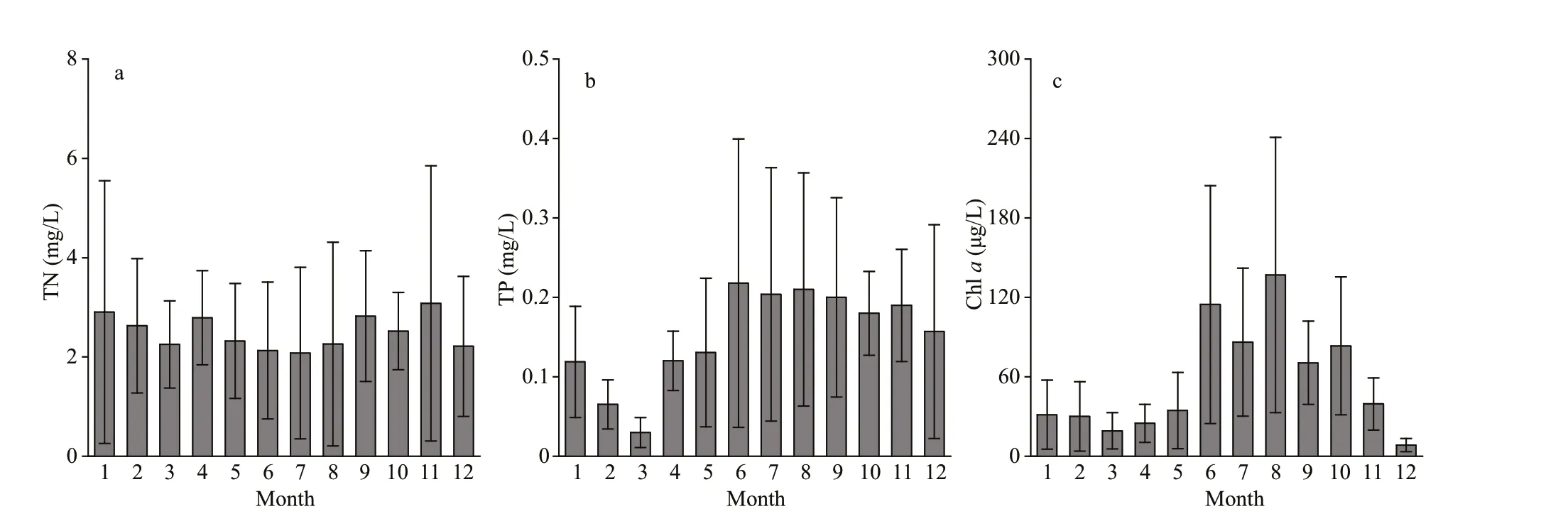
Fig.2 The temporal variation in total nitrogen (TN), total phosphorus (TP), and chlorophyll- a (Chl-a) concentrations in Chaohu Lake
2.7 Statistical analysis
Visualization and spatial interpolation were performed using Origin 9.0 software (OriginLab,Northampton, MA, USA) and ArcGIS (Kriging method, ESRI, Redlands), respectively. Analysis of covariance (ANCOVA) was carried out using SPSS 23.0 (IBM, New York, NY, USA) statistical software.The signif icance levels are reported as not signif icant(P>0.05) or signif icant (P<0.05) for all tests.
3 RESULT
3.1 Spatiotemporal patterns of nutrients and chlorophyll- a concentrations and wind in Chaohu Lake
The concentrations of TN varied from 2.08 to 3.10 mg/L at the 15 sampling sites in Chaohu Lake from 2018-2019 (Fig.2a). The average TN concentrations in spring, summer, autumn, and winter were 2.46±0.29, 2.16±0.09, 2.81±0.28, and 2.58±0.35 mg/L, respectively. The value of TP ranged from 0.03 to 0.22 mg/L (Fig.2b). The mean values of TP in spring, summer, autumn, and winter were 0.09±0.06,0.21±0.01, 0.19±0.01, and 0.08±0.06 mg/L,respectively. Chl-aconcentration varied from 8.44 to 136.82 μg/L, with mean values of 26.21±7.74,112.53±25.38, 64.50±22.53, and 23.29±12.87 μg/L in spring, summer, autumn, and winter, respectively(Fig.2c). There were two distinct peaks: the f irst was in June, and the second was in August.
The TN and TP concentrations gradually decreased from the western to the eastern lake region (Fig.3a-b).In spring, relatively high values of Chlawere observed in the northeastern region, with the highest concentration of 101.03 μg/L at site 11# (Fig.3c). In contrast, the concentrations of Chlain summer decreased slowly from the western to the eastern lake region, with the highest value detected at site 2#(Fig.3d). The highest value of Chlawas observed at site 2# in autumn, with higher values detected in the southeastern region (Fig.3e). In winter, the values of Chlawere higher in the southern region than in the northern region (Fig.3f).
Wind roses displaying seasonal patterns of wind at Chaohu Lake were produced for April 2018, July 2018, January 2019, and November 2019 (Fig.3c-f).In spring, the prevailing winds were from the northwest or east-southeast. Wind speeds of 1-3 m/s occurred at a frequency greater than 60%, and wind speeds >3 m/s occurred at a frequency less than 30%.In summer, the prevailing winds were from the west or east-southeast. Wind speeds were typically <3 m/s,with this range representing approximately 80% of wind speeds recorded. The wind rose charts showed a typical pattern of dominant southeast or east-southeast winds in autumn. Wind speeds were typically <3 m/s,at approximately 20% frequency for each of the prevailing wind direction. The prevailing winds during winter were primarily from the east-southeast or east-northeast. Wind speeds of 1-3 m/s constituted approximately 90% of recorded wind speeds; wind speeds >3 m/s accounted for approximately 10%.
3.2 Diff erences in physicochemical parameters and algal biomass in the water column in the littoral zones
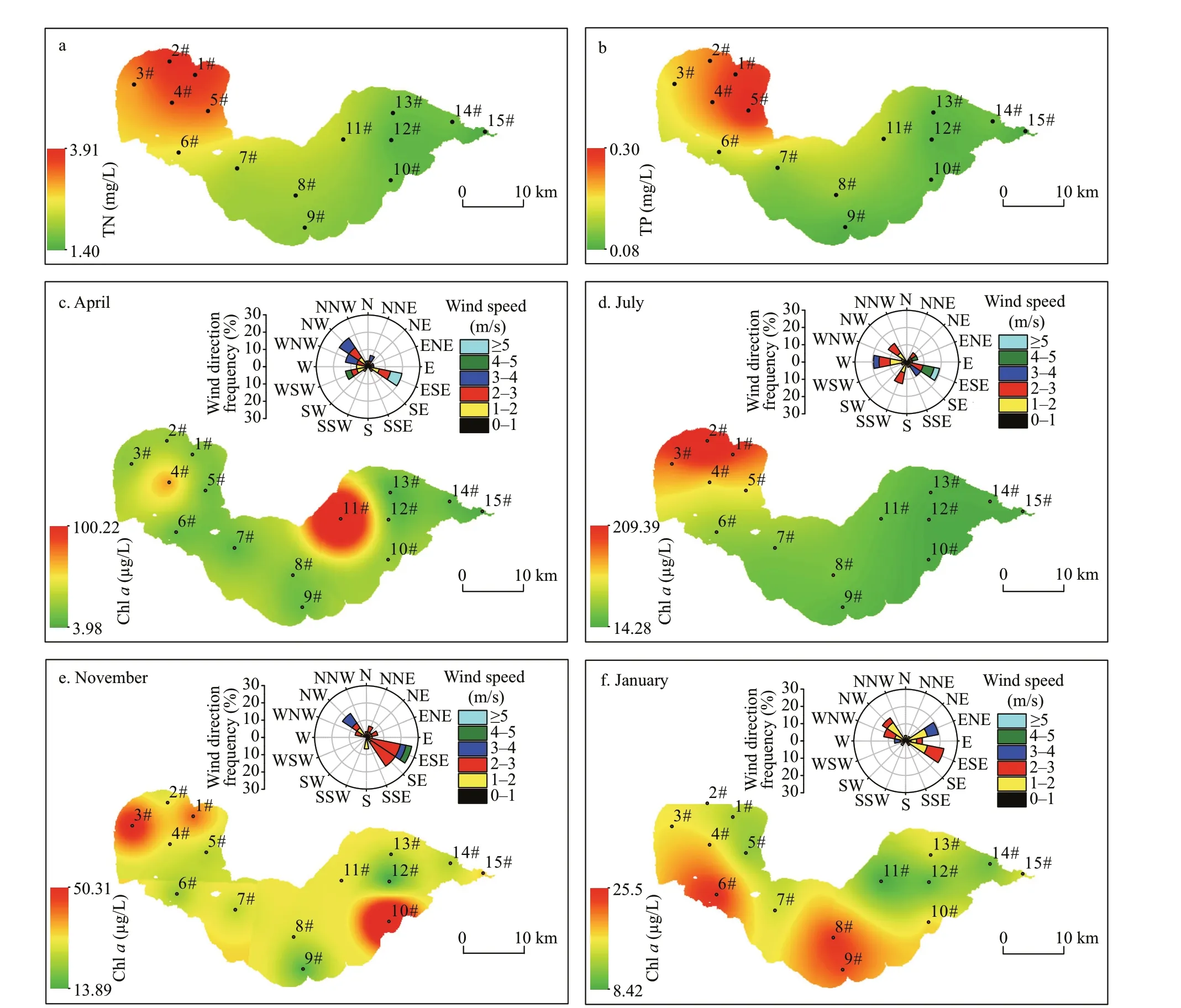
Fig.3 The spatial distribution of total nitrogen (TN), total phosphorus (TP) and Chl- a concentrations, and wind roses in Chaohu Lake
The littoral zone included the RCLZ (V+) and its surrounding ULZ (V-). Water temperatures ranged between 3.5 °C and 34.6 °C over the entire year in the littoral zones (Fig.4). The DO concentrations varied from 0.73 to 12.15 mg/L, with relatively high values(>10.86 mg/L) in winter. In addition, the concentrations of TN, TP, and Chlain the littoral zones were generally higher during summer than during spring, autumn, and winter. The mean concentration of TN was 340.71 mg/L in summer,which was 62.57, 163.59, and 134.74 times higher than the values in spring, autumn, and winter,respectively. The mean value of TP was 33.33 mg/L in summer, which was 118.04, 415.63, and 157.71 times higher than that in spring, autumn, and winter,respectively. The average concentration of Chlawas 15 086.72 μg/L in summer, followed by lower contents in spring, autumn, and winter.
The lowest concentration of DO (0.73 mg/L) was observed in summer in the RCLZ. Moreover, the concentrations of Chlawere much higher in the RCLZ than in the ULZ, particularly in spring and summer, indicating that algal blooms could be trapped and accumulate dramatically in the RCLZ compared with more restrained growth in the ULZ. However,the Chl-aconcentrations in the RCLZ were close to those in the ULZ in autumn and winter. Likewise, the concentrations of TN and TP were also higher in the RCLZ than in the ULZ during spring and summer.
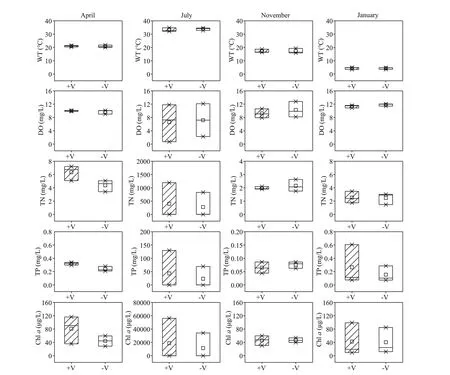
Fig.4 Seasonal variation in dissolved oxygen (DO), water temperature (WT), total nitrogen (TN), total phosphorus (TP),and Chl- a concentrations in the littoral zones
Relatively low concentrations of DO were observed in the northern (C2) region, with the lowest value(0.73 mg/L) observed in the littoral zones during the investigation period occurring in this region (Fig.5).Additionally, the concentrations of TN, TP, and Chlain the littoral zones of the C2 region were much higher than values in the northeastern (C1) and southern regions (C3). For instance, the mean concentrations of TN, TP, and Chlawere 255.79 mg/L, 25.01 mg/L, and 11 324.19 μg/L in the C2 region, which were 76.05,95.19, and 229.07 times higher than those in the C3 region, respectively. In addition, the water temperatures and DO concentrations of the RCLZ were close to those of the ULZ. Furthermore, the concentrations of Chlain the RCLZ were 58.1% and 62.3% higher than those in the ULZ in the C1 and C2 regions, respectively.The concentrations of TN and TP were also higher in the RCLZ than in the ULZ. However, the concentrations of TN, TP, and Chlawere similar between the RCLZ and ULZ in the C3 region.
3.3 Diff erences in physicochemical properties of the surface sediment in the littoral zones
TN, TP, Chla, and LOI for the surface sediment were much higher during autumn than during spring,summer, and winter in the littoral zones (Fig.6), and the diff erences were signif icant (P<0.05), except for that for the content of LOI. For example, the mean values of TN, TP, Chla, and LOI in autumn were 1 085.64 mg/kg, 259.68 mg/kg, 2453.95 ng/g, and 1.44%, which were 1.81, 1.87, 4.28, and 0.97 times higher than those in winter, respectively. Moreover,seasonal scale analysis showed that the values of TN,TP, Chla, and LOI in the surface sediments were higher in the RCLZ than in the ULZ. For instance, the values of TN, TP, Chla, and LOI in the surface sediments of the RCLZ were 30.5%, 31.4%, 15.4%,and 2.8%, respectively, higher than those in the ULZ in autumn. In addition, the water content and porosity of the surface sediment were very similar between the RCLZ and ULZ during the research period.
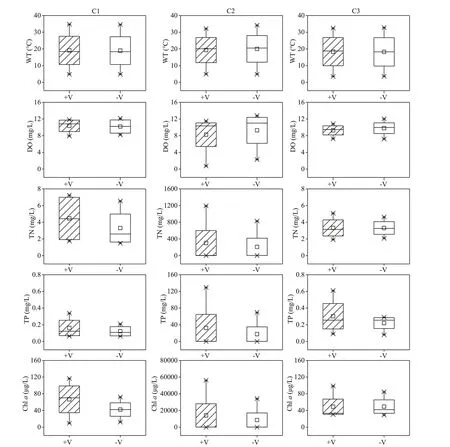
Fig.5 Spatial variation in dissolved oxygen (DO), water temperature (WT), total nitrogen (TN), total phosphorus (TP), and Chl- a concentrations in the littoral zones
3.4 Diff erences in diff usive f luxes of N and P across the SWI in the littoral zones
The diff usive f lux of NH4+-N in the littoral zones was higher during summer and autumn than during spring and winter (Fig.7a). Furthermore, the diff usive f lux of NH4+-N was higher in the RCLZ than in the ULZ at the seasonal scale. Specif ically, the values for the diff usive f lux of NH4+-N in the RCLZ in spring,summer, autumn, and winter were 42.6%, 45.8%,71.0%, and 90%, respectively, higher than those in the ULZ. However, the diff usive f lux of SRP in the littoral zones was higher during spring and summer than during autumn and winter (Fig.7b). Moreover,the seasonal-scale results showed that the diff usive f lux of SRP was also higher in the RCLZ than in the ULZ.
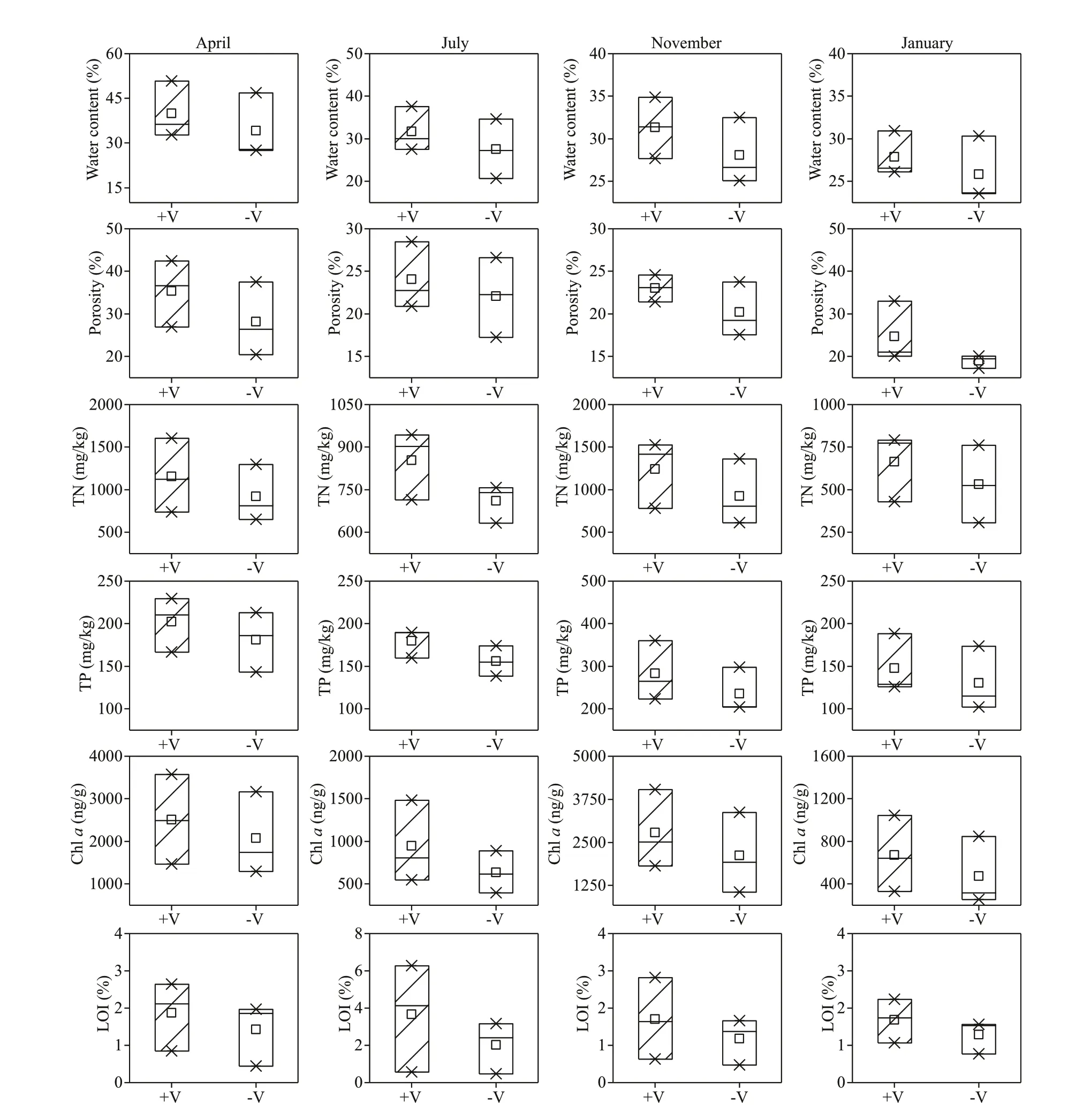
Fig.6 Seasonal variation in physicochemical properties of the surface sediments in the littoral zones
4 DISCUSSION
4.1 Inf luence of algal accumulation in the water column in the littoral zones
There was a seasonal pattern in the Chl-aconcentration in the littoral zone water columns(Fig.4). Our results indicate that Chl-aconcentration was higher in summer than in other seasons (Figs.2-4). Thus, the level of algal accumulation in the littoral zones is a more serious concern during summer than during spring, autumn, or winter. Previous studies have conf irmed that the formation of a surface water bloom is determined by suffi cient biomass of buoyant algae (Paerl and Otten, 2013). Furthermore, the initial algal biomass in the water column can inf luence the thickness of the algal patch that forms and its viscosity at the water surface (Deng et al., 2016). Water temperature has been found to be an important factor aff ecting algal blooms (Trombetta et al., 2019), with warm temperatures normally favoring algae (Jöhnk et al., 2008). Moreover, our data also showed a clear trend of higher algal biomasses in the warmer seasons(Fig.4). In addition, nitrogen and phosphorus are important nutrients for lake algal blooms. In Chaohu Lake, nutrient concentrations were suffi ciently high to meet algal growth requirements (Fig.2), which provided favorable conditions for massive algal proliferation (Xu et al., 2003).
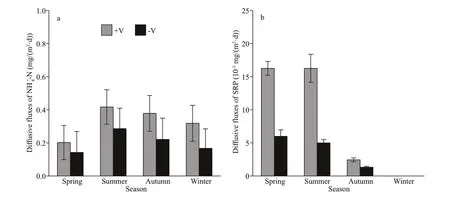
Fig.7 Diff usive f luxes of NH 4 +-N (a) and SRP (b) across the SWI in the littoral zones
We observed spatial variation in Chl-aconcentration in the water among the littoral zone regions, with much higher concentrations of Chlain the C2 region than in the C1 and C3 regions (Fig.5). Our results showed that more algal biomass was trapped and accumulated in the C2 region than in the C1 and C3 regions. Most cyanobacterial species have gas vacuoles, organelles of low density that provide buoyancy (Oliver and Walsby, 1984; O’Brien et al.,2004). When the wind blows in one direction, these positively buoyant cyanobacteria easily concentrate along the downwind shore (Cyr, 2017). Moreover,wind speed and wind direction can aff ect the horizontal distribution of algae (Chen et al., 2003;Deng et al., 2016; Xu and Chen, 2020). Cao et al.(2006) found that algal blooms in Taihu Lake were formed at wind speeds under a critical value of 3.1 m/s. Wu et al. (2015) reported that a decline in wind speed promoted the extension of algal bloom areas. In addition, previous f ield studies have demonstrated that algae are horizontally transported to downwind zones by wind-driven currents under critical wind speeds (Bai et al., 2005; Moreno-Ostos et al., 2009; Deng et al., 2016). In this study, the wind speed was predominantly less than 3.0 m/s, and wind direction was mainly from the east to southeast at Chaohu Lake (Fig.3). The wind pattern was capable of transporting algae to downwind zones. Thus,compared with the C1 and C3 regions, the C2 region was conducive to high algae accumulation.
We observed an obvious capture eff ect on algal blooms by reed wetlands (Figs.4-5). The reasons for the capture by reed wetlands of algal blooms are as follows. 1) Aff ected by the barrier and adsorption properties of reed wetlands in the littoral zones, algae can easily adsorb onto reeds once they enter reed wetlands. 2) Reed wetlands can weaken stormy waves in the littoral zones; thus, algae that already appear within these regions are less likely to be transported out. Reed wetlands continue to exert a capture eff ect in autumn and winter. The reason may be that withered reeds can still weaken wind and waves when algal biomass is low. Therefore, reed wetlands in the littoral zones can undoubtedly play a signif icant role in the mechanics of algal accumulation in eutrophic lakes.
The accumulation of algae in the littoral zones,directly and indirectly, aff ects a variety of water quality parameters. The growth and decay of algae could in turn lead to changes in DO and nutrient concentrations (Zhu et al., 2013). However, our data show that the DO level was inf luenced only by algal accumulation in summer, since the RCLZ of the C2 region formed an anaerobic environment (DO<2 mg/L, Figs.4-5). A possible explanation is that photosynthetic rates and DO strongly increased during the accumulation phase, but oxygen was rapidly consumed during decomposition, especially at night. The accumulation of algae may be responsible for elevated TN and TP levels. Zhu et al. (2013)reported that the trends for both TN and TP tracked those of Chl-aconcentration in the littoral zones of Taihu Lake, consistent with our results. A previous study indicated that algal blooms might also contribute to increased levels of particulate phosphorus (Zhu et al., 2008). In addition, we observed higher concentrations of TN and TP in the RCLZ than in the ULZ (Figs.4-5). Thus, we suggest that reed wetlands could aggravate the enriched levels of nitrogen and phosphorus in the water column.
4.2 Inf luence of algal accumulation on surface sediment in the littoral zones
Diff erent seasons show diff erent responses to algal accumulation in the littoral zones in terms of overall nutrient levels and organic matter content in surface sediment. We found that the values of TN, TP, Chla,and LOI for surface sediment were higher in autumn than in other seasons (Fig.6). Algae that accumulated dramatically in summer did not settle on the sediment surface at that time; in contrast, they settled rapidly on the sediment surface and decomposed in autumn. In addition, compared with the ULZ, we observed higher values of TN, TP, Chla, and LOI for the RCLZ. This phenomenon may be due to a high rate of primary production and a reduced rate of decomposition under anaerobic conditions (Aerts et al., 1999). Thus, reed wetlands could aggravate the deposition of pollutants in surface sediments.
Compared with rates in spring and winter, we observed higher rates of NH4+-N diff usion in summer and autumn during the investigation period (Fig.7a).The high content of organic matter in the surface sediment (Fig.6) would be expected to lead to consumption of oxygen across the SWI in summer and autumn due to microbial decomposition of the organic matter (Kristensen, 2000). The consumption of oxygen may accelerate the formation of anoxic conditions across the SWI and thereby accelerate the dissolution of NH4+-N from the sediment (Brandes and Devol, 1997) and increase diff usion rates. However,we found that the rates of SRP diff usion were higher during spring and summer than during autumn and winter (Fig.7b). A possible explanation is that the sedimentary P release was induced by low levels or depletion of DO during algal decomposition (Gao et al., 2013; Zhu et al., 2013). Furthermore, we also found that the diff usive f luxes of NH4+-N and SRP were lower in the littoral zones than in the open regions of many eutrophic lakes (Lewandowski et al.,2007; Liu et al., 2016). The reason may be the lower water content and porosity of the sediments in the littoral zones of Chaohu Lake (Fig.6). Liu et al. (2019)also found that reduced water content and porosity of sediment might also suppress the diff usion of NH4+-N and SRP from sediment. In addition, we observed that the accumulation of algae had a more serious impact on the diff usive f luxes of NH4+-N and SRP in the RCLZ than in the ULZ. The RCLZ would be favorable for the formation of anaerobic environments due to their higher levels of algal accumulation.
4.3 Management insights
The current study demonstrated that reed wetlands were able to capture and accumulate algae in the littoral zones of Chaohu Lake. These results are important for ecological restoration methodology,such as planting reeds in the littoral zones can more eff ectively retain accumulated algae to mitigate algal nuisance in the open regions. Considering the limited ability of reed wetlands to degrade algae as well as the partial deterioration of the ecological environment in the littoral zones, physical measures such as mechanical salvage are suggested to remove massive algal blooms trapped by reed wetlands in the warm seasons or the downwind region of Chaohu Lake.Fundamentally speaking, the main methods for preventing and controlling algal blooms involve reductions of nutrient levels in Chaohu Lake (e.g.,controlling pollution sources in upstream basins,treating the water in the river, treatment of heavily polluted sediments and removal of algal sources from sediment).
5 CONCLUSION
After studying the impact of the accumulation of algal blooms on reed wetlands in the littoral zones of Chaohu Lake, we conclude that more algal biomass was trapped in the RCLZ than in the ULZ, indicating that reed wetlands could obviously retain accumulated algae in the littoral zones. The accumulation of algae in the littoral zones exhibited obvious spatiotemporal characteristics. Algal accumulation levels were highest in summer due to high water temperatures and algal biomasses. Likewise, the northern littoral zones were conducive to the development of large algal blooms because of the wind pattern. The nutrient levels and organic matter content in surface sediment in autumn showed signif icantly responses to algal accumulation in the littoral zones. The values of TN,TP, Chla, and LOI in surface sediments were higher in the RCLZ than in the ULZ due to a high rate of primary production and a reduced rate of decomposition under anaerobic conditions. Moreover,diff erent seasons showed diff erent responses to algal accumulation in the littoral zones in terms of the rates of NH4+-N and SRP diff usion. The diff usive f luxes of NH4+-N and SRP were lower in the littoral zones than in the open regions due to the lower water content and porosity of the sediments. The accumulation of algae had a more serious impact on the diff usive f luxes of NH4+-N and SRP in the RCLZ than in the ULZ due to the higher levels of algal accumulation. Thus,considering the limited ability of reed wetlands to degrade algae as well as the partial deterioration of the ecological environment in the littoral zones, we need to combine mechanical salvage with other physical methods to eliminate blooms when algae accumulate massively in the RCLZ. Our study may provide a scientif ic basis for the prevention and control of algal blooms in the littoral zones of Chaohu Lake and other similar large shallow eutrophic lakes.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.
7 ACKNOWLEDGMENT
We thank Lei JIANG and Chengfei TONG from Nanjing Institute of Geography and Limnology,Chinese Academy of Sciences for their assistance in the sampling and parameters setting.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
