Ecological damage of submerged macrophyte Myriophyllum spicatum by cell extracts from microcystin (MC)- and non-MC-producing cyanobacteria, Microcystis*
Yunni GAO , Hui YANG , Xiaofei GAO , Mei LI , Man ZHANG , Jing DONG ,Jingxiao ZHANG , Longfei LI , Xuejun LI ,**, Michele A BURFORD
1 Engineering Lab of Henan Province for Aquatic Animal Disease Control, Engineering Technology Research Center of Henan Province for Aquatic Animal Cultivation, College of Fisheries, Henan Normal University, Xinxiang 453007, China
2 Australian Rivers Institute, Griffi th University, Nathan, Queensland 4111, Australia
Abstract To explore how decomposed Microcystis-dominant cyanobacterial blooms aff ect submerged macrophytes, the submerged plant Myriophyllum spicatum was exposed to cell extracts from microcystin(MC)- and non-MC-producing Microcystis strains in a laboratory experiment. Results showed that both Microcystis cell extracts exerted obvious damages to plant biomass, photosynthesis, primary and secondary metabolism measures, and resistance of plant antioxidant systems, with MC-producing Microcystis having stronger eff ects due to the presence of MCs. Cyanotoxins other than MCs responsible for the negative eff ects from both Microcystis strains needs further identif ication. The Shannon diversity and Chao1 indices of epiphytic and planktonic bacteria were decreased by the cell extracts from both Microcystis strains.However, epiphytic and planktonic bacterial communities responded diff erently to Microcystis cell extracts at the genus level. The dominant genera of planktonic bacteria including Enterobacter, Pseudomonas,and Novosphingobium from phylum Proteobacteria, Chryseobacterium from phylum Bacteroidetes, and Microbacterium from Actinobacteriota in the treatments with cell extracts were previously reported to have strains with algicidal and MC-degrading capabilities. Bacterial genes associated with energy production and conversion, amino acid transport and metabolism, and inorganic ion transport and metabolism, were more abundant in both treatments than the control for planktonic bacteria, but less abundant for epiphytic bacteria.We speculate that planktonic bacterial communities have the potential to use and degrade substances derived from Microcystis cell extracts, which may be benef icial for M. spicatum to alleviate damages from Microcystis. Further research is needed to verify the structure and function dynamics of epiphytic and planktonic bacteria in the interaction between cyanobacteria and submerged macrophytes.
Keyword: microcystins; Microcystis; Myriophyllum spicatum; dissolved organic carbon; epiphytic and planktonic bacteria
1 INTRODUCTION
With the intensif ication of eutrophication and global climate change, freshwater cyanobacteria blooms appear to be increasing in eutrophic waterbodies worldwide, causing serious ecological, societal, and economic losses (Burford et al., 2020; Plaas and Paerl, 2021).Microcystisis one of the dominant nonnitrogen-f ixing genera found throughout the world in tropical, subtropical, and temperate zones (Xiao et al., 2018).Microcystisblooms have been recorded in at least 108 countries throughout the world, 79 of which also reported them to produce monocyclic heptapeptides toxins, i.e., microcystins (MCs) (Harke et al., 2016). Many MC- and non-MC-producingMicrocystisstrains have been found coexisting inMicrocystisblooms (Bormans et al., 2020; Sidelev et al., 2020; Liu et al., 2021). Although the hazards of pure MCs and MC-containingMicrocystisto organisms and public health have been studied intensively (Rastogi et al., 2014; Svirčev et al.,2017; Wang et al., 2021), the ecological eff ects ofMicrocystispopulations on aquatic organisms have not been thoroughly elucidated. More serious cellular damages to medaka f ish and signif icant lethal eff ects on crustaceans (Daphniamagna) were found to be caused by the extracts ofMicrocystisnatural blooms than pure MCs or MC-containing extracts (Smutná et al., 2014; Le Manach et al., 2016). Whether a part of the negative eff ects is from non-MC-producingMicrocystisstrains is unclear, which hinders us from fully understanding the ecotoxicity of cyanobacterial blooms.
Submerged macrophytes play important roles in maintaining clear water status, including taking up nutrients (Finkler Ferreira et al., 2018), reducing sediment resuspension (Rolland et al., 2015), providing habitats for zooplankton (Wood et al., 2017), and allelopathic inhibition on surrounding phytoplankton(Hilt et al., 2006). The reestablishment of submerged macrophytes is crucial for the restoration of structure and function of an impaired shallow aquatic ecosystem(Bai et al., 2020). However, the damage from cyanobacterial blooms on submerged macrophytes is one of the constraints in the restoration practices in shallow eutrophic waterbodies (Jiang et al., 2019a).Decomposed cells of one MC-producingMicrocystisaeruginosastrain could cause more severe damage to the submerged macrophyteVallisnerianatanscompared to fresh cyanobacteria (Zhang et al., 2021).The exudates and extracts of the same MC-producingM.aeruginosastrain during the decline phase could aff ect negatively the growth, anti-oxidative systems,and ultrastructure ofV.natans(Li et al., 2020b). MCs consistently inhibited the photosynthesis of aquatic plants under a range of exposure concentrations (Zhang et al., 2022). Treatments with 0.5 μg/L of MC-leucine(L) arginine (R) inhibited the photosynthetic oxygen production ofCeratophyllumdemersum,Elodeacanadensis, andM.spicatum(Pf lugmacher, 2002).Oxidative damage ofV.natanswas also apparent when exposed to 1.0-μg/L pure MC-LR (Jiang et al.,2011). MCs and MC-producingMicrocystisexerted negative eff ects on submerged plants, but the eff ect of non-MC-producingMicrocystisstrains on submerged plants is still being debated.
Bacteria are essential components with multiple roles in aquatic ecosystems. They have close links with submerged macrophytes. On one hand, they can settle on the surface of submerged macrophytes to form epiphytic biof ilms, respond quickly to environmental variations and interact with submerged macrophytes (Wijewardene et al., 2022). On the other hand, bacterioplankton or planktonic bacteria have been widely investigated (Teeling et al., 2012; Liu et al., 2015; Yang et al., 2020). The structure and function of bacterioplankton communities diff ered between macrophyte-dominated and phytoplanktondominated regimes in Taihu Lake in China (Wu et al., 2007; Wang et al., 2020). Epiphytic bacterial communities are inf luenced by abiotic environmental factors (Hempel et al., 2008) and cyanobacterial blooms including pure MCs (Jiang et al., 2019a, b; Li et al., 2020a, b). The structure, diversity, and activity of planktonic bacteria are shifted during formation and breakdown of cyanobacterial blooms, and involved in MC degradation (Lezcano et al., 2017; Steiner et al., 2017). However, fewer studies have compared the responses of epiphytic and planktonic bacterial communities to the stress of cyanobacterial blooms,despite it should be an important part involved in the ecological damage of submerged macrophytes from cyanobacterial blooms. It has been reported that epiphytic bacteria exhibited higher diversity and distinct community composition than the planktonic bacteria for at least two submerged macrophytes in natural waterbodies (He et al., 2014; Liu et al., 2019).However, whether they responded diff erently to the stress from cyanobacterial blooms remains unknown yet.
Myriophyllumspicatumis ranked to be the most allelopathic of the tested plant species ( Hilt and Gross,2008; Gao et al., 2017). The selective inhibition ofM.spicatumon cyanobacteria rather than eukaryotic algae was previously demonstrated in laboratory experiments and f ield conditions (Zhu et al., 2010;Švanys et al., 2014).M.spicatumhas effi cient nutrient assimilation and capacity to inhibit microalgal growth in fresh and low-salinity brackish water bodies (Liu et al., 2018). However, less attention has been paid to the eff ects of cyanobacterial blooms onM.spicatumin comparison with other submerged macrophytes, e.g.,V.natans, thereby hindering its scientif ic application in restoration of shallow eutrophic waterbodies.
Hence, this study aimed to assess the ecological damage to the submerged macrophyteM.spicatumby cell extracts from MC- and non-MC-producingMicrocystisstrains during the decline phase. The specif ic tasks were: (1) to compare the eff ects of both strains on the growth, physiology, and metabolism ofM.spicatum, (2) to determine the responses of epiphytic and planktonic bacterial community to the cell extracts from bothMicrocystisstrains, (3) to measure changes in the abiotic environment during the experiment, and (4) to analyze the chemical prof iles of the cell extracts from both strains.
2 MATERIAL AND METHOD
2.1 Cultivation of plants and two Microcystis strains
FreshM.spicatumplants were obtained from the Honghu Lake (29.827°N, 113.476°E) in Hubei Province, China, and cultivated in aquariums in 10-cm deep sediments under a greenhouse until new roots grew. Healthy apical shoots were cleaned and pre-cultured in 1/10 diluted BG11 medium under climate-controlled conditions at a temperature of 22±3 °C and a light intensity of 25 μmol/(m2·s) with 12-h light/12-h dark cycles for subsequent laboratory experiments. The 1/10 diluted BG11 medium was suitable for the cultivation ofM.spicatumas indicated in our preliminary experiments. The plants were cleaned with deionized water and rinsed with sterile water before the experiments.
One MC-producing (FACHB 915) and one non-MC-producingMicrocystisstrain (FACHB 1005)were obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB),the Chinese Academy of Sciences. They were cultured in BG11 medium in 1-L Erlenmeyer f lasks under the same conditions as outlined above. Cells in decline phase after 14 days of culture were prepared for the experiments.
Microcystiscell extracts were prepared. The cell densities of theMicrocystiscultures were counted with a hemocytometer under a microscope (BA210,MOTIC, Xiamen, China) at 400× magnif ication. The uniform cultures were centrifuged at 6 000 r/min for 15 min. The obtained cell pellets were mixed with 1/10 diluted BG11 medium, frozen at -80 ℃ for 8 h and thawed at 20 ℃ f ive times until all the cell walls were observed to be ruptured under a microscope.The crude extracts were subsequently centrifuged at 6 000 r/min for 15 min. The supernatant was f iltered through a f ilter membrane (0.45-μm pore size) and used as extracts for subsequent exposure experiment and chemical composition analysis.
2.2 Experimental design
The cleaned plant apical shoots ofM.spicatumof about 12-cm length were cultivated with 1/10 diluted BG11 medium in beakers at 2.5±0.17-g fresh weight per liter. The bottom was secured with sterile glass beads. The prepared cell extracts of MC- and non-MCproducingMicrocystisstrains were then added to the beakers in the MC+ and MC- treatments respectively.The f inal concentrations of the extracts used in the treatments were equivalent to those from a cell density of 5.0×106cells/mL, which is in the concentration range of dense bloom population in eutrophic waters(Wu et al., 2014; Liu et al., 2016). Beakers without the extracts were prepared as control (CT). Each treatment was performed in quintuplicate. All the beakers were covered with breathable sealing membranes and cultured under the conditions described above for 14 days. The biof ilm formed rapidly on the surface of plant leaves and remained stable within 10 days(Zhang et al., 2010). After 14 days of the cultivation,the plants were harvested to collect epiphytic bacteria samples immediately, and growth and physiological parameters were measured. The culture medium was also collected for the analysis of bacterioplankton,physicochemical indices, and MC concentration.
2.3 Plant growth
The biomass and length of the plant shoots were measured at the beginning (day 0) and the end (day 14)of the experiment to evaluate the growth ofM.spicatum.
2.4 Photosynthetic pigment content
The fresh plant shoots (0.1 g) were ground in a mortar and a pestle with 5 mL of 80% acetone, and the homogenate was kept in the dark condition at 4 ℃ for 24 h. The homogenate was centrifuged at 11 180×gfor 10 min. The absorbance of supernatant was determined spectrophotometrically at 647 and 663 nm for chlorophylla(chla) andb(chlb), and at 470 nm for carotenoids (Amorim et al., 2017).
2.5 Determination of plant physiological indicators
The fresh plant shoots (0.2 g) were homogenized in a mortar and a pestle with liquid nitrogen for the determination of plant soluble proteins, soluble sugars, total phenols, malondialdehyde (MDA),superoxide dismutase (SOD), peroxidase (POD),catalase (CAT), glutathione S-transferase (GST), and glutathione (GSH), through assay kits from Nanjing Jiancheng Company, China.
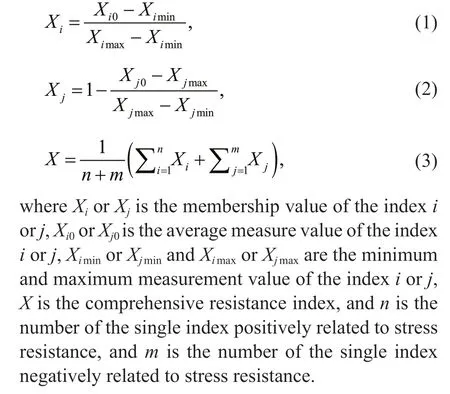
The comprehensive resistance of the plant antioxidant systems was calculated using the membership function method in fuzzy mathematics(Zhang et al., 2021). The indicators SOD, CAT, GST,and GSH are positively related to stress resistance,which was calculated using Eq.1. The indicators MDA and POD are negatively related to stress resistance using Eq.2. Comprehensive resistance index was calculated using Eq.3.
2.6 Microbial characterization
All harvested fresh plant shoots were placed in sterile centrifuge tubes with 10 mL of 0.1-mol/L phosphate buff ered saline (PBS), and subjected to ultrasonic washing for 1 min, and the process was repeated three times (Li et al., 2020b). The washing water was collected together and f iltered through a 0.22-μm pore size f iber f ilter. The f ilter membranes with epiphytic bacteria were frozen quickly by immersing into liquid nitrogen and then stored at -80 ℃ for further DNA extraction and analysis of epiphytic bacteria.
The plant culture solution (500 mL) for each treatment was f iltered through a 0.22-μm f iber f ilter.The f ilter membranes with planktonic bacteria were collected and stored at -80 ℃ for further DNA extraction and analysis of bacterioplankton.
DNA extraction and high-throughput sequencing were carried out by Majorbio BioPharm Technology Co. Ltd. (Shanghai, China). DNA was extracted from the pre-treatment samples by using the Fast DNATMSPIN Kit. The V3-V4 hypervariable region of 16S rRNA was amplif ied using a barcoded universal primer set, including 338F(5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R(5′-GGACTACHVGGGTWTCTAAT-3′). Highthroughput sequencing was conducted using the Illumina MiSeq platform. Bioinformatics analysis was used to describe the diversity of microbial communities and to verify microbial species at the phylum and genus levels (Fadrosh et al., 2014).
2.7 Physiochemical indices and MC concentration
The culture solution was f iltered with a 0.45-μm f ilter membrane, and the f iltrate was collected for subsequent determination. Dissolved total nitrogen(TDN) and dissolved total phosphorus (TDP) contents were determined by digestion method according to the Chinese National Standards for Water Quality.Dissolved organic carbon (DOC), and dissolved inorganic carbon (DIC) contents were determined by total organic carbon analyzer (Multi N/C 3100,Analytik Jena, Germany). The concentration of MCs in the solutions was determined with a MC-LR ELISA kit (Institute of Hydrobiology, Chinese Academy of Sciences).
2.8 Chemical prof ile analysis of cell extracts
Thermo ultra performance liquid chromatography(UPLC-Q) exactive quadrupolerod-electrostatic f ield Orbitrap high-resolution mass spectrometry(MS) system (Thermo, USA) was used to analyze the subsamples of the cell extracts from bothMicrocystisstrains. The chromatography separation was performed on Hypersil Gold Vanquish column(100 mm×2.1 mm, 1.9 μm) with 5 mmol/L of ammonium acetate solution (A)-acetonitrile (B) as the mobile phase. The gradient elution was 0-15 min,5%-50% B; 15-35 min, 5%-98% B; 35-42 min,98% B; 42-43 min, 98%-5% B; 43-47 min, 5% B.The f low rate was set at 0.3 mL/min, and the column temperature was 40 ℃. Electrospray ionization(ESI) source was applied and operated in Full-scan/Targeted-dd MS2/negative ion mode.
2.9 Data analysis
Data were presented as the means±standard deviations of quintuplicate samples. After determining the normality using Shapiro-Wilks test and homogeneity using Bartlett’s test, one-way analysis of variances(ANOVA) was performed amongst the experimental groups. Multiple comparisons were performed using(f ishers least signif icant diff erence) LSD test atP<0.05(Bates et al., 2012). R studio (Version 3.6.2) was used for statistical analysis of bacteria data (R Development Core Team, 2011). Microsoft Excel 2019 was used for analysis of the other data.
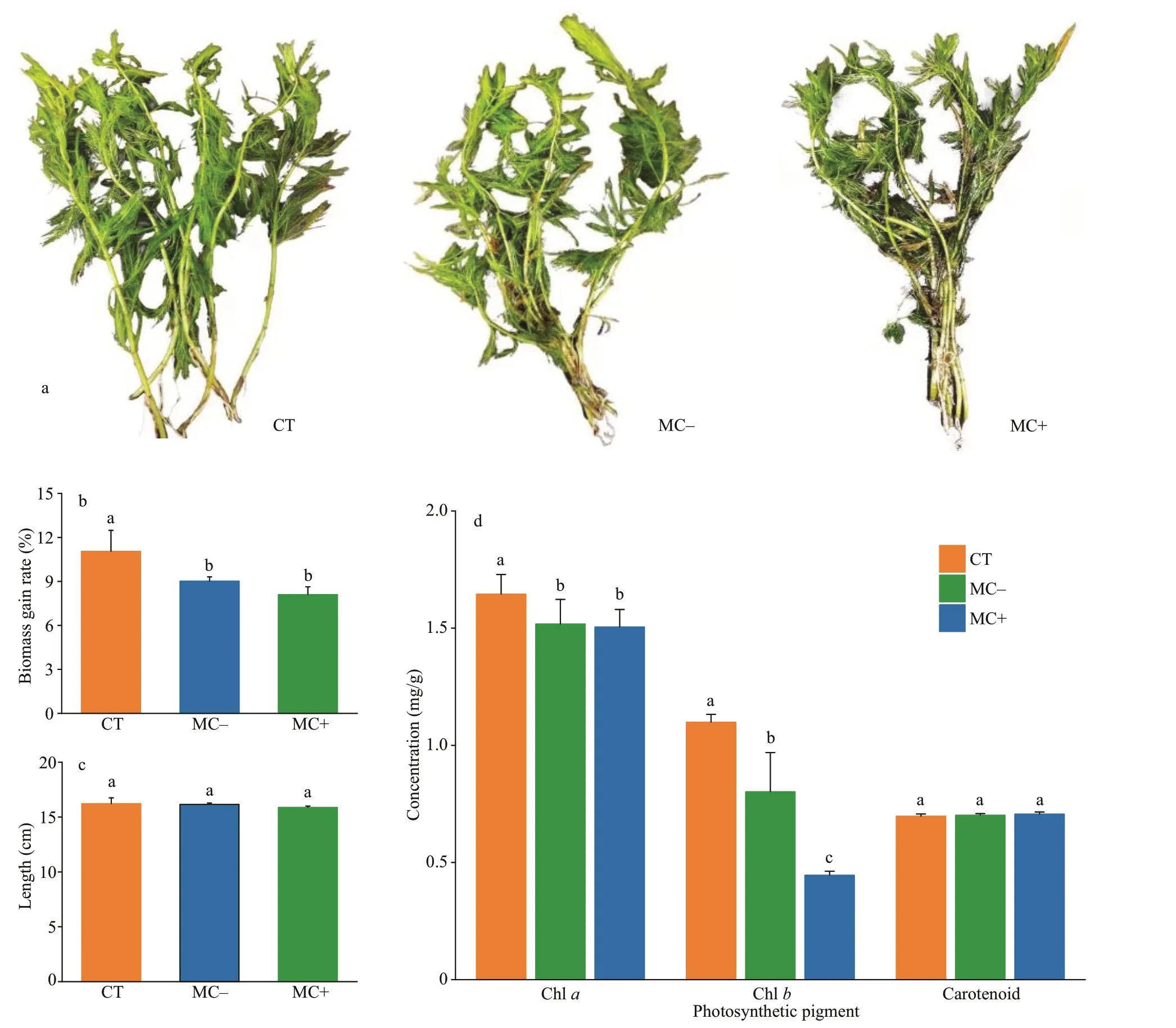
Fig.1 Eff ects of the cell extracts from MC-producing (MC+) and non-MC-producing (MC-) Microcystis strains on the growth morphology (a), biomass gain rate (b), shoot length (c), and photosynthetic pigments (d) of Myriophyllum spicatum
3 RESULT
3.1 Plant growth and pigment responses
New white roots of plants in all groups were observed on day 14. However, a few leaves ofM.spicatumturned yellow and rotted in both treatment groups. The proportion of the rotted leaves was higher in the MC+ treatments (Fig.1a). The plant biomass was increased by 9.02% and 8.10% in the MC- and MC+ treatments, respectively, during the experiment, and was signif icantly lower than that in the CT group (11.05%;P<0.05; Fig.1b). The average length of the plant shoot increased by 4 cm, after the 14-day exposure toMicrocystisextracts, and was not signif icantly diff erent between the treatment groups and the control (P>0.05; Fig.1c). The chl-aand chl-bcontents in the plant apical shoots in the MC- and MC+ treatments were reduced signif icantly in comparison with those in the CT group (P<0.05;Fig.1d). No signif icant diff erence in carotenoid contents was found between the CT group and the treatments (P>0.05).
3.2 Changes in plant metabolites
The contents of soluble proteins, soluble sugars and total phenols inM.spicatumin the treatments decreased signif icantly after 14 days of exposure toMicrocystiscell extracts (Fig.2;P<0.05). Theinhibitory eff ects of the cell extracts from the MCproducingMicrocystison the soluble sugar and total phenols ofM.spicatumwere greater than those from the non-MC-producingMicrocystis(P<0.05).
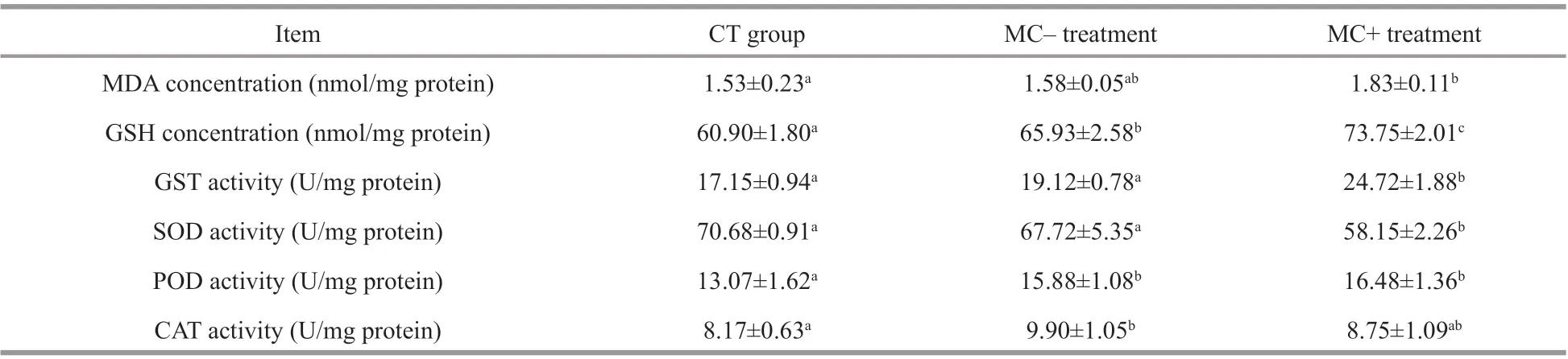
Table 1 Physiological measures on the antioxidant defense systems of M. spicatum in the MC- and MC+ treatments and in the CT group
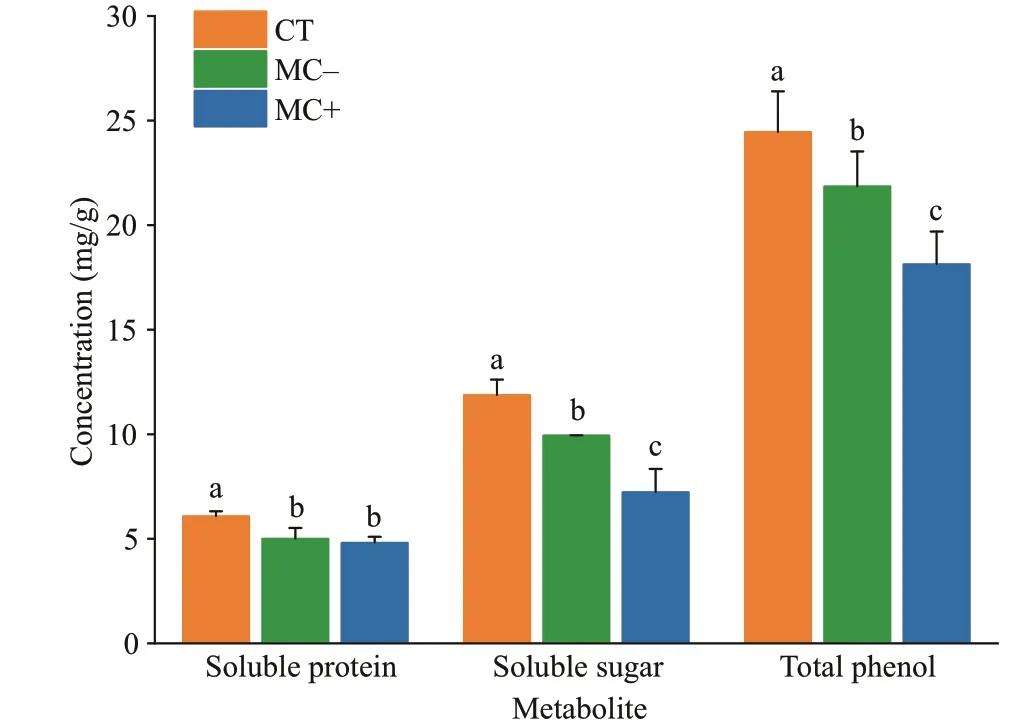
Fig.2 Contents of soluble proteins, soluble sugars, and total phenols in M. spicatum in the MC- and MC+treatments and the CT group
3.3 Plant oxidative response
The MDA concentrations were signif icantly higher in the MC+ treatments (P<0.05) than those in the control after 14 days of exposure, and were slightly higher in the MC- treatments than in the control(P>0.05; Table 1). The GSH concentrations and GST activity increased in both treatments, and were 21.10%and 44.08% higher in the MC+ treatments compared with those in the control (P<0.05). Meanwhile, the SOD activity decreased in both treatments, and was the lowest in MC+ treatments. The POD activity levels in the MC- and MC+ treatments were 21.45% and 26.03% higher than those in the CT group (P<0.05).The CAT activity in the MC- treatments was 21.19%higher than that in the CT group (P<0.05) and only7.09% higher in the MC+ treatments (P>0.05). The comprehensive resistance index ofM.spicatum,calculated based on the six indices of the antioxidant defense systems, was lower in the treatment groups than in the CT group. The plant resistance index to the cell extracts of MC-producingMicrocystiswas the lowest (Table 2).
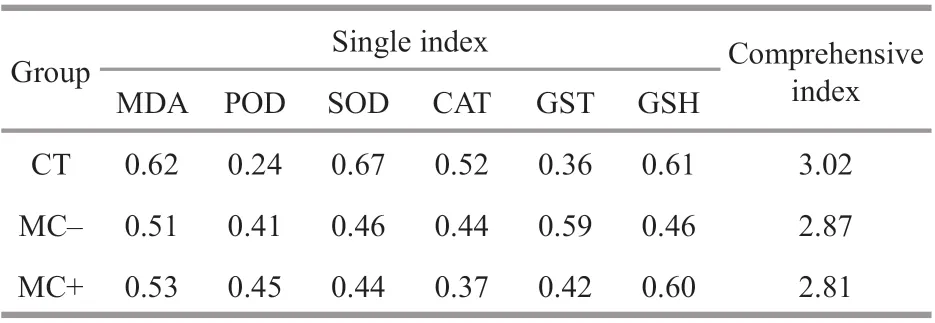
Table 2 Comprehensive stress resistance evaluation of the physiological indices of plant M. spicatum in MCand MC+ treatments and the CT group
3.4 Plant-growth environment
3.4.1 Epiphytic and planktonic bacteria
Average number of quality sequences obtained from all samples ranged from 44 016 to 50 468,with an average read length between 414 and 420 bp(Table 3). These sequences were classif ied into 1 047 OTUs, with a coverage of 1. All OTUs were assigned to 24 phyla and 436 genera. The Chao1 and Shannon indices of epiphytic bacterial communities were signif icantly lower (P<0.05) in the treatments withMicrocystscell extracts (MC+, MC-) compared with those in the CT. For planktonic bacteria, the Chao1 indices in the MC+ and MC- treatments were signif icantly lower than those in the CT group(P<0.05). The Shannon indices in the MC+ and MCtreatments were also lower than those in the CT group but without signif icant diff erences. The number of OTUs, Chao1 and Shannon indices were higher for epiphytic bacteria than for planktonic bacteria in the CT group.
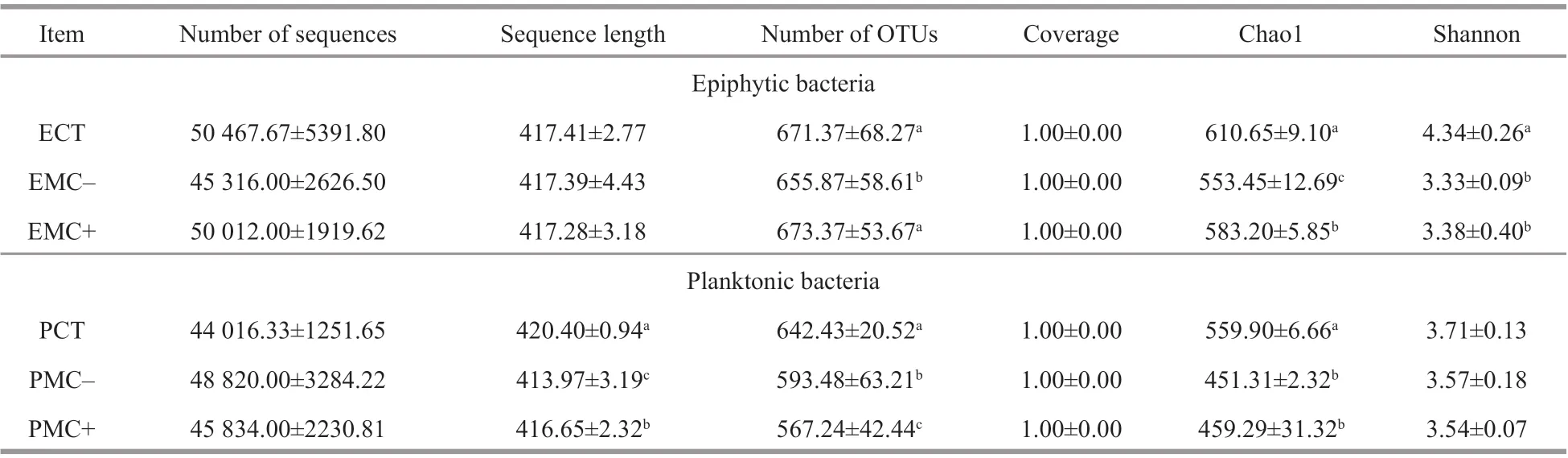
Table 3 Number and length of sequences, number of OTUs, Chao1, and Shannon indices of epiphytic (E) and planktonic bacteria (P) in the CT, MC-, and MC+ groups
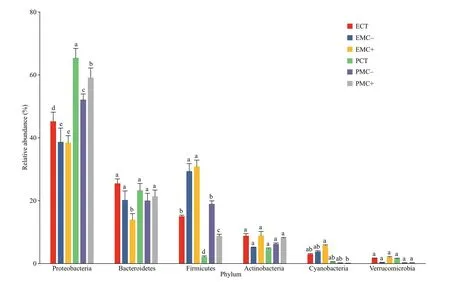
Fig.3 Six most dominant phyla of epiphytic (E) and planktonic bacteria (P) in the CT, MC-, and MC+ groups
Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria were the four most dominant phyla of epiphytic and planktonic bacterial communities across all experimental groups (Fig.3). The relative abundance of epiphytic Proteobacteria was signif icantly lower than that of planktonic Proteobacteria. The relative abundance of Proteobacteria and Bacteroidetes in the CT group was higher than that in the MC+ and MC- treatments, with signif icant diff erences only for Proteobacteria (P<0.05). However, the relative abundance of Firmicutes in the MC+ and MCtreatments was higher than that in the CT group for epiphytic and planktonic bacteria (P<0.05).Its relative abundance was signif icantly higher for epiphytic bacteria than for planktonic bacteria in the same treatments (P<0.05).
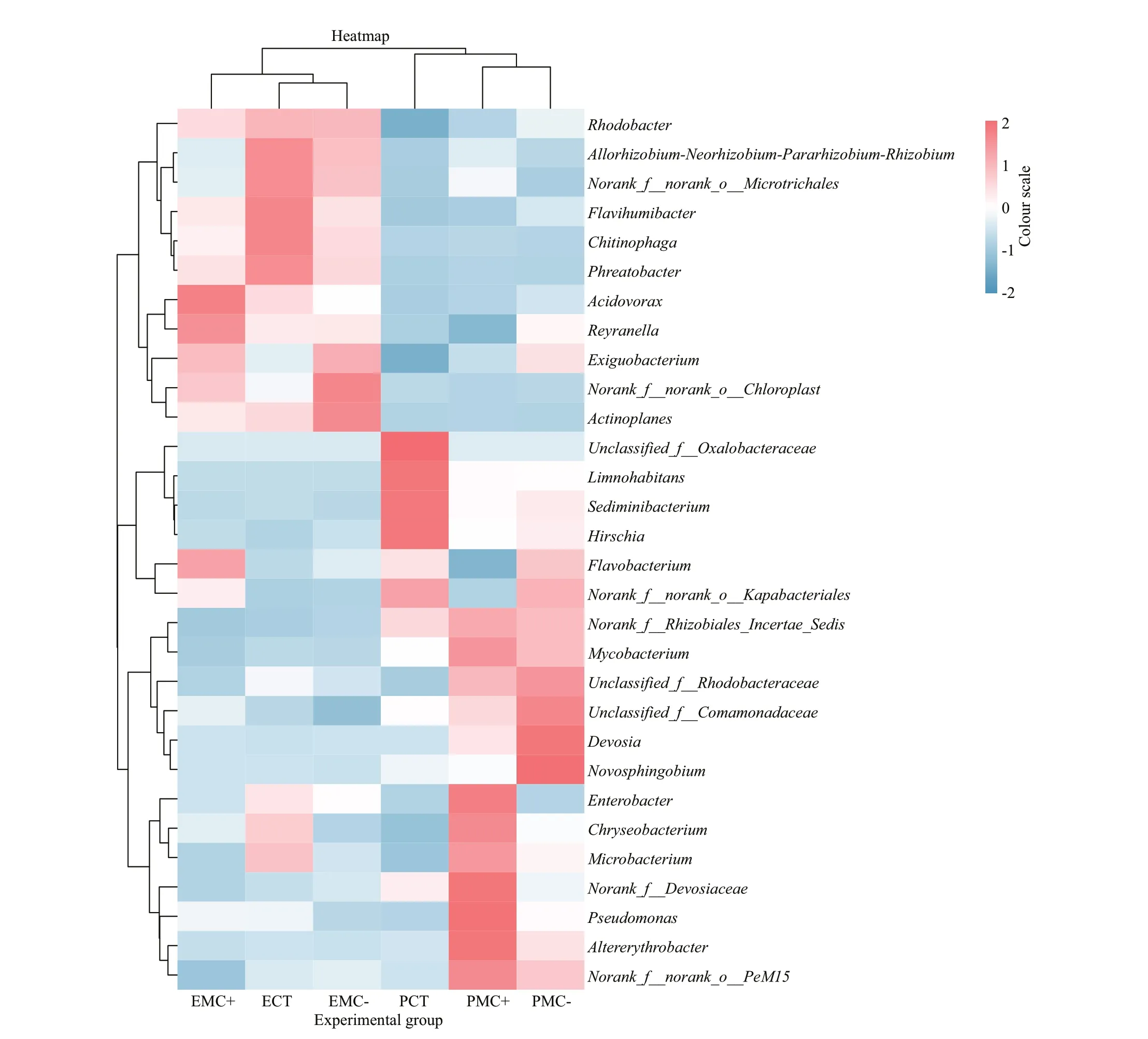
Fig.4 Hierarchical cluster heatmap of the 30 most dominant genera of epiphytic (E) and planktonic bacteria (P) in the CT,MC-, and MC+ groups
The main genera of epiphytic bacteria were clustered together, clearly diff erent from those of planktonic genera based on the hierarchical cluster heatmap of the 30 most dominant genera (Fig.4).For epiphytic bacteria, f ive genera includingFlavihumibacter,Chitinophaga, andPhreatobacterin the CT group, three genera, i.e.,Exiguobacterium,norank_f_norank_o_Chloroplast(belonging to Cyanobacteria) andActinoplanesin the MCtreatments, and two genera, i.e.,AcidovoraxandReyranellain the MC+ treatments showed the highest relative abundance. For planktonic bacteria, four genera includingLimnohabitans,Sediminibacterium,andHirschiashowed the highest relative abundance in the CT group. Four genera includingDevosiaandNovosphingobiumshowed the highest relative abundance in the MC- treatments, whereas eight genera includingEnterobacter,Pseudomonas, andAltererythrobactershowed the highest relative abundance in the MC+ treatments.
Eight genera showed signif icant diff erences between epiphytic and planktonic forms amongst diff erent treatments (Fig.5;P<0.05). The genusnorank_f__Rhizobiales_Incertae_Sedisbelonging to phylum Proteobacteria showed the highest abundance in the MC+ treatments and the lowest abundance in the CT group for epiphytic and planktonic bacteria. The genusDevosiabelonging to phylum Proteobacteria showed higher abundance in planktonic forms than in epiphytic forms, with the highest abundance in the MCtreatments.AltererythrobacterandLimnohabitansbelonging to phylum Proteobacteria showed higher abundance in planktonic forms than in epiphytic forms,with the highest abundance in the MC+ treatments and CT group, respectively. The abundance ofAcidovoraxbelonging to phylum Bacteroidia andnorank_f__norank_o__Chloroplastbelonging to Cyanobacteria was higher in epiphytic forms than in planktonic forms,with the highest abundance in the CT group and MC+treatments, respectively.Pseudomonasbelonging to phylum Proteobacteria andnorank_f__Devosiaceaebelonging to Actinobacteria showed higher abundance in planktonic forms than in epiphytic forms especially in the MC+ treatments.
PICRUSt-prediction revealed a signif icantly higher abundance of clusters of orthologous groups in planktonic forms than in epiphytic forms associated with energy production and conversion, amino acid transport and metabolism, transcription, inorganic ion transport and metabolism, general function prediction only, and function unknown, especially for the MC+and MC- treatments (Fig.6;P<0.05). No signif icant diff erence was found for the remaining clusters of orthologous groups (P>0.05).
3.4.2 Abiotic environment
The DOC concentrations in the culture solution increased 6.9 and 10.5 times immediately after adding cell extracts from MC- and non-MC-producingMicrocystisstrains compared with those in the CT(Fig.7a). DIC concentrations also increased 2.2- and 1.8-fold, respectively (Fig.7b). The TDN and TDP concentrations increased about 1.2 and 1.5 times in both treatments compared with those in the CT(Fig.7c-d).
The initial DOC concentrations were the highest in the MC- treatments, and the lowest in the CT group. Their concentrations showed no signif icant diff erences between the treatments and the CT group at the end of the experiment (Fig.7a;P>0.05). The DOC concentrations in the MC- and MC+ treatments decreased, whereas those in the CT group increased over time. The initial DIC concentrations were signif icantly higher in the MC- and MC+ treatments than in the CT group (Fig.7b;P<0.05), and the DIC concentrations increased more than threefold at the end of the experiment for all experimental groups.TDN concentrations slightly increased in the CT but decreased over time in the two treatments (Fig.7c).DOC, DIC, and TDN concentrations at the end of the experiment did not signif icantly diff er between the CT and the two treatments (P>0.05). TDP concentrations in the control and the two treatments decreased with time (Fig.7d). TDP concentrations were signif icantly higher in both treatments than in the CT group (P<0.05).
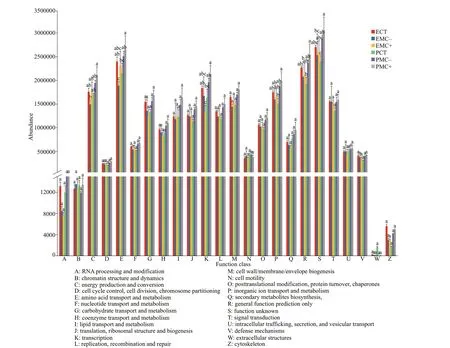
Fig.6 Clusters of orthologous groups of epiphytic (E) and planktonic bacteria (P) and their abundance in the CT, MC-, and MC+ groups by PICRUSt
3.5 Chemical prof iles of cell extracts from both Microcystis strains
More than 400 chemicals were determined in the cell extracts of both strains by using UPLC-Oribitrap MS/MS analysis. The dominant forms of microcystin in the cell extracts of MC-producing strains were MCLR and [Dha7]-microcystin-LR, and their relative abundance was 5%. They were undetected in the cell extracts of non-MC-producing strains. The relative abundance of the 20 most dominant chemical genera accounted for more than 60% (Fig.8a). The main chemical classes were amino acids, antibiotics, fatty acids, esters, and amide. The relative abundance of amino acids in MC-producing cell extracts was 24%,much higher than the 8% of non-MC-producing cell extracts. However, the relative abundance of esters in non-MC-producing cell extracts was 24%, much higher than the 7% of MC-producing cell extracts.MC-LR concentrations in the MC+ treatment were 0.85 μg/L in the beginning and 0.03 μg/L at the end of the experiment (Fig.8b).
4 DISCUSSION
4.1 Damage to M. spicatum by cell extracts from both Microcystis strains
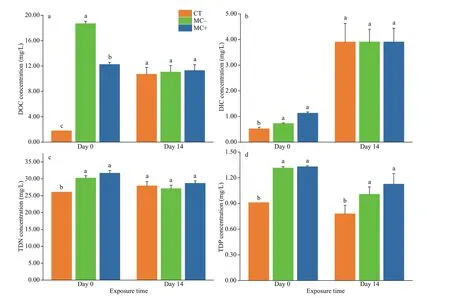
Fig.7 Concentrations of dissolved organic carbon (DOC) (a), dissolved inorganic carbon (DIC) (b), dissolved total nitrogen(TDN) (c), and dissolved total phosphorus (TDP) (d) in plant culture solutions of all experiment groups on days 0 and 14
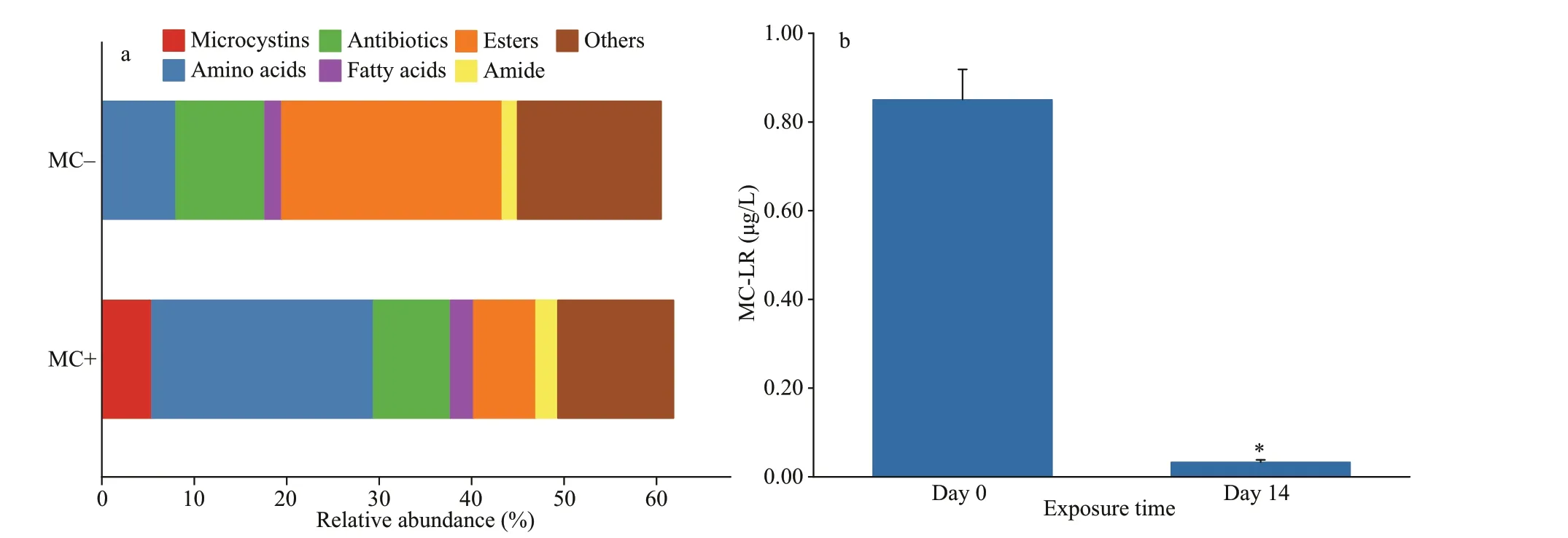
Fig.8 Relative abundance of the 20 most dominant chemicals at class level (a) in the cell extracts of MC-producing (MC+)and non-MC-producing Microcystis (MC-), and changes in MC-LR concentrations in the MC+ treatments on days 0 and 14 (b)
The present study provided multiple lines of evidence that cell extracts from MC- and non-MC-producingMicrocystisstrains during the decline phase negatively aff ected the growth, photosynthesis, antioxidant systems, and primary and secondary metabolism of the submerged macrophyteM.spicatum. Damage toM.spicatumby MC-producingMicrocystiswas more extensive than that by non-MC-producing strains.Several studies have shown that pure MCs or MCproducingMicrocystispopulations aff ect the growth and physiological processes of submerged plants,includingM.spicatum,C.demersum,E.canadensis,andV.natans(Pf lugmacher, 2002; Jiang et al., 2011;Li et al., 2020a). However, the negative eff ects ofMicrocystisblooms during the decline phase on submerged macrophytes were previously found to be stronger than those during the formation phase (Li et al., 2020b; Zhang et al., 2021). The eff ects of non-MCproducingMicrocystison submerged macrophytes are not yet attracting considerable attention. A previous study has found that the physiological processes of another submerged macrophyteEgeriadensawas aff ected by MC-producingMicrocystisbut not by non-MC-producingMicrocystis(Amorim et al.,2017). Our study provided growth and physiological evidence for the f irst time thatM.spicatumcan be aff ected by cell extracts from MC- and non-MCproducingMicrocystisstrains during the decline phase, thereby improving our understanding of the overall impacts ofMicrocystisblooms on submerged macrophytes.
The chemical prof iles of cell extracts from both strains diff ered, which may explain the diff erent extents of their inhibition eff ects onM.spicatum. The presence of MC-LR and [Dha7]-microcystin-LR with a relative abundance of 5% and an initial concentration of 0.85 μg/L in the MC+ treatment may be the main reason why the cell extracts of MC-producingMicrocystisexerted stronger inhibition eff ects onM.spicatumcompared to the MC- treatment. The photosynthesis of aquatic plants has been found to be consistently inhibited by MCs within a range of exposure concentrations (Zhang et al., 2022). The photosynthetic oxygen production ofM.spicatumcould be clearly inhibited by 0.5-μg/L MC-LR(Pf lugmacher, 2002). Apart from MC,Microcystiswas reported previously to produce other cyanopeptide compounds, primarily including aeruginosins,anabaenopeptins, cyanobactins, cyanopeptolins,microginins, and microviridins (Janssen, 2019).Non-MC-producingMicrocystismutant reportedly produces higher concentrations of cyanopeptolins,aerucyclamides, and aeruginosins than the wild MCproducing strain (Briand et al., 2016). Herein, we did not detect these compounds in the cell extracts, but the species and relative abundance of amino acids were found to diff er between MC- and non-MC-producingMicrocystis. Amino acids play various roles in the production of MCs and other cyanotoxins (Dai et al., 2009). The ecotoxicity of cyanopeptides against grazing crustacean, fungi, and bacteria rather than aquatic plants has been previously reported (Janssen,2019). To answer whether and which cyanopeptides are responsible for the inhibition onM.spicatumby cell extracts from non-MC-producingMicrocystis,further research using higher analytical techniques for identif ication and quantif ication, as well as ecotoxicity assessment with reference standards, is needed.
Measures related to the photosynthesis and the antioxidant system of plants are frequently selected as biomarkers for the impacts of pure MCs and MCproducingMicrocystiscells (Pf lugmacher, 2002;Jiang et al., 2011; Zhang et al., 2021). In the current study, the reduced plant growth because of exposure to cell extracts from bothMicrocystisstrains was due to the damage to photosynthesis processes, changes in primary and secondary metabolism measures and eff ects on the plant antioxidant defense systems.The concentrations of the photosynthesis pigments,chlaand chlbmarkedly decreased, which may indicate damage to photosynthesis structures and function. Additionally, the reduced contents of soluble proteins and sugars in the plants indicated that nutrient biosynthesis pathways were damaged byMicrocystiscell extracts. Plant phenols are the typical allelochemicals produced byM.spicatumfor self-defense against cyanobacteria (Zhu et al., 2010).Therefore, the measured decline in total phenol contents ref lected a decrease in plant defense to theMicrocystiscell extracts.
Changes in the indicators related to plant antioxidant systems, including GSH, GST, SOD, and CAT were observed. Their roles are to limit the formation of new reactive oxygen species and accelerate the scavenging of generated free radicals (Wu et al., 2009). Studies have shown that the MCLR-GSH conjugate can be formed to detoxify MC-LR (Jiang et al., 2011),and MC-LR can be transformed into a glutathione conjugate by GST, which has the ability to detoxify the metabolites of lipid peroxidation (Stüven and Pf lugmacher, 2007). This f inding may explain the signif icant decrease in MC-LR concentration in the MC+ treatments during the experiment. Additionally,the increase in POD and MDA contents indicated the accumulation of reactive oxygen species and lipid peroxidation, and a decrease in plant defense capacity (Zhang et al., 2021). Lower comprehensive resistance indices of the plant antioxidant systems in the MC+ and MC- treatments indicated that the damage inf licted byMicrocystiscell extracts reduced the comprehensive defense capability of the plantM.spicatum.
4.2 Responses of epiphytic and planktonic bacteria
A previous investigation in Taihu Lake, China,has shown thatMicrocystisblooms result in low bacterioplankton diversity, whereas Bacillariophyta and Cryptophyta blooms lead to more diverse bacterioplankton communities (Niu et al., 2011). The present study provided evidence that the richness expressed as Chao1 indices and diversity expressed as Shannon indices of epiphytic and planktonic bacteria decreased after adding cell extracts of bothMicrocystisstrains.
Bacterial community composition was also altered at the phylum and genus levels with the addition ofMicrocystiscell extracts. At the phylum level, the relative abundance of Proteobacteria and Bacteroidetes was lower but that of Firmicutes was signif icantly higher in the MC+ and MCtreatments than in the CT group. Previous studies have found that Proteobacteria was dominant in epiphytic bacteria attached to macrophytes, and bacterioplankton in adjacent waters (Hempel et al.,2008; Burke et al., 2011; Gong et al., 2018; Jiang et al., 2019a). Bacteroidetes was previously found to be abundant in epiphytic bacterial communities of several submerged macrophytes includingM.spicatumin natural water bodies (Hempel et al.,2008). Firmicutes was the main dominant phylum in various anaerobic habitats, i.e., periphytic biof ilm of pollutant-removal systems and sediments during the occurrence of MC-LR biodegradation and adsorption in Meiliang Bay, Taihu Lake (Li et al., 2016). The abundance of Firmicutes is always higher than that of Proteobacteria in sludge samples (Li et al., 2015).Bacteria within Firmicutes have the ability to degrade various organic compounds, including proteins and carbohydrates (Levén et al., 2007). We speculate that the increased initial DOC concentrations in the MC+ and MC- treatments after addingMicrocystiscell extracts may benef it the increased abundance of Firmicutes, which may degrade these organic compounds and cause the decrease in DOC concentrations in the MC- and MC+ treatments at the end of the experiment.
At the genus level, variations in the community structure of epiphytic bacteria in diff erent treatments indicated the inf luences fromMicrocystiscell extracts on epiphytic bacteria. Five genera with the highest abundance in the CT group, were reduced byMicrocystiscell extracts in the MC- and MC+treatments. The genusPhreatobacter, belonging to phylum Proteobacteria, has been documented only in ultrapure-water purif ication systems and drinking-water distribution systems with low chlorine concentrations (Perrin et al., 2019). We speculated that the growth of this genus needs a clear-water state without environmental stress. Another dominant genus,Chitinophagafrom phylum Bacteroidota in the CT group, has been found previously in effi cient dissolved cyanobacterial solutions as one of the dominant genera, indicating its potential role to help the host plants compete with cyanobacteria (Berg et al., 2009). These bacteria may originate from the plantM.spicatumitself in epiphytic forms, despite the strict washing steps including the use of sterile water to remove as many bacteria as possible. The good growth performance ofM.spicatumfurther indicates that these dominant genera in the CT group may have mutualistic interactions with the host plants(Wijewardene et al., 2022).
The genera with the highest abundance in the MCtreatment wereExiguobacteriumbelonging to phylum Firmicutes,norank_f_norank_o_Chloroplastwithin phylum Cyanobacteria andActinoplaneswithin phylum Actinobacteriota, whereas the dominant genera in the MC+ treatments wereAcidovoraxwithin phylum Bacteroidota,ReyanellaandFlavobacteriumboth belonging to phylum Proteobacteria. The growth of these genera was promoted in the MC+ and MCtreatments. Most of them have been previously reported in water bodies withMicrocystisblooms, e.g.,Acidovorax,Exiguobacterium, andFlavobacteriumin Taihu Lake (Fan et al., 2016). They contribute to nutrient exchange and interact with bloom formers likeMicrocysits(Hoke et al., 2021), enhance the growth of cyanobacteria includingMicrocysits(Berg et al., 2009), and contain strains those are important pathogens of aquatic animals (Zheng et al., 2020).However,Flavobacteriumincludes strains those can degrade cyanobacterial toxins or other recalcitrant and problematic organic compounds (Berg et al., 2009).Hence, further research is needed to determine the interactions of epiphytic bacteria withM.spicatumin the face of cyanobacteria stress.
For planktonic bacteria, the four genera belonging to phylum Proteobacteria includingLimnohabitansshowed the highest relative abundance in the CT group.Limnohabitansis a typical bacterioplankton taxon in freshwater bodies, and it lacks the capacity to live on the leaf surfaces of submerged macrophytes (He et al.,2014). Its abundance is signif icantly reduced in the MC+ and MC- treatments, indicating its sensitivity to stress fromMicrocystiscell extracts. Four genera includingDevosiaandNovosphingobiumshowed the highest abundance in the MC- treatments, whereas eight genera includingEnterobacter,Pseudomonas,Chryseobacterium, andMicrobacteriumshowed the highest abundance in the MC+ treatments. The presence of the generaDevosia,Novosphingobium,Enterobacter,Pseudomonas, andChryseobacteriumin eutrophic water bodies with cyanobacterial blooms such as Taihu Lake has been previously documented(Guo et al., 2015; Fan et al., 2016; Chen et al., 2018).Multiple strains within the generaEnterobacter,Pseudomonas,Chryseobacterium,Microbacterium,andNovosphingobiumhave been reported their MCdegradation capabilities (Dziga et al., 2013; Kumar et al., 2018; Wang et al., 2018; Hoke et al., 2021).Their degradation capacity varied from 9.4 μg/(L·d)to 2 548 μg/(L·d) (Dziga et al., 2013). The MCs concentrations decreased by 97% at the end of the experiment, these dominant genera may also contribute to this process together with the plant itself. In addition to bioactive secondary metabolites,Microcystishave been shown to produce and release diff ering concentrations of various forms of organic substances (Pivokonsky et al., 2014; Villacorte et al., 2015). These substances may be consumed and degraded by dominant genera in the MC+ and MCtreatments.
The richness and diversity of epiphytic bacteria were higher than those of surrounding bacterioplankton,and the bacterial composition also diff ered at the genus level in our study. This f inding was consistent with previous f ield investigations on submerged macrophytesPotamogetoncrispusandM.spicatum,in which epiphytic bacteria possess higher diversity and more distinct community composition than the surrounding bacterioplankton (He et al., 2014; Liu et al., 2019). Bacteria may prefer to grow attached to a substrate, and most bacteria may originate from plant leaves and stems based on our experimental procedures in the present study. Diff erent bacterial compositions lead to diff erent bacterial functions. A previous study using PICRUSt analyses suggested the distinct nutrient-source utilization of epiphytic bacteria and bacterioplankton (Shi et al., 2017).In the MC+ and MC- treatments, bacterial genes associated with energy production and conversion,amino acid transport and metabolism, and inorganic ion transport and metabolism were more abundant for bacterioplankton than for epiphytic bacteria.This f inding indicated that bacterioplankton might have strong capability to consume and degrade substances derived fromMicrocystiscell extracts. The dominant genera with algicidal and MC degradation capabilities in bacterioplankton community may be the main contributor to this process. Conversely, the nutrient-source utilization of epiphytic bacteria may be aff ected by the cell extracts. PICRUSt analyses revealed that bacterioplankton and epiphytic bacteria responded diff erently to cell extracts fromMicrocystisat the end of the experiment. Bacterioplankton may play a role in alleviating damage toM.spicatumby decomposing substances fromMicrocystisblooms if they can consume and degrade toxic substances derived fromMicrocystis. The structure and function of epiphytic bacteria may be aff ected negatively by toxic substances fromMicrocystis. Whether this process enhances or alleviates damage toM.spicatumremains unclear.
Based on the experimental results, we speculated on the ecological damage toM.spicatuminf licted by decomposing substances fromMicrocystisblooms,as shown in the schematic in Fig.9. Decomposing substances from MC- and non-MC-producingMicrocystispopulations aff ected the photosynthesis,primary and secondary metabolism, and defense capacity of the antioxidant systems ofM.spicatum.MC-producingMicrocystisinf licted stronger damage to the plant due to the presence of MCs. Meanwhile,epiphytic and planktonic bacteria responded diff erently to the decomposing substances fromMicrocystisblooms. The nutrient utilization capability of bacterioplankton was promoted by decomposing substances fromMicrocystis, and several dominant genera had the potential to degrade MCs. Conversely,the nutrient utilization capability of epiphytic bacteria was inhibited. The responses of planktonic bacteria may be benef icial forM.spicatumto alleviate damage fromMicrocystis, but the interactions between epiphytic bacteria andM.spicatumfacing stress fromMicrocystisremains unclear. Further research should focus on the structure and function dynamics of bacteria in various microhabitats to reveal the ecological mechanisms of the interactions between cyanobacteria and submerged macrophytes.
5 CONCLUSION
This study compared multiple physiological responses ofM. spicatumto cell extracts of MC- and non-MC-producingMicrocystisstrains. The growth,photosynthesis, antioxidant systems, and primary and secondary metabolism of the submerged macrophyteM.spicatumwere signif icantly aff ected by cell extracts from bothMicrocystisstrains. Damage inf licted by MC-producing strains was greater than that by non-MC-producing strains due to the presence of MCs.
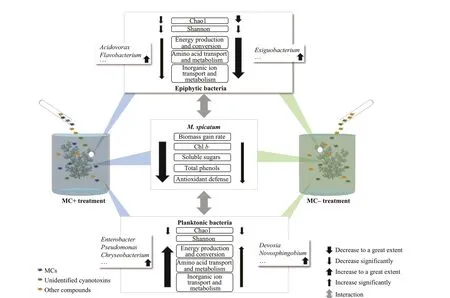
Fig.9 Schematic diagram of the ecological damage to the submerged macrophyte M. spicatum by the decomposing substances from MC- and non-MC-producing Microcystis populations
Epiphytic and planktonic bacterial communities responded diff erently toMicrocystiscell extracts.Bacterial genes associated with energy production and conversion, amino acid transport and metabolism,inorganic ion transport and metabolism were more abundant in the MC+ and MC- treatments than in the CT group for planktonic bacteria but less abundant for epiphytic bacteria. Planktonic bacteria seemed to utilize and degrade substances derived fromMicrocystiscell extracts, which may be benef icial forM.spicatumto alleviate damage fromMicrocystis.
6 DATA AVAILABILITY STATEMENT
All datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We thank four anonymous reviewers for their helpful comments and suggestions to improve this manuscript.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
