猪环状RNA IGF1R促进脂肪细胞分化
张 娜, 李 萌, 李 娇, 孟 珊, 蔡春波, 杨 阳, 高鹏飞,郭晓红, 曹果清, 李步高
(山西农业大学动物科学学院动物遗传育种与繁殖系猪种质创新实验室,山西 太谷 030801)

circRNA富含微RNA(microRNA,miRNA)结合位点,可以充当与miRNA结合的竞争性内源RNA(competing endogenous RNAs, ceRNA),从而调控靶基因的表达。第1个被揭示具有调控功能的circRNA为ciRS7,它含有470个可与miR-7的结合的位点,可通过竞争性结合miR-7来提高其靶基因的表达,进而发挥作用[16]。另外,circARF3可通过吸附miR-103调节NF-κB信号通路,进一步促进线粒体自噬,从而抑制脂肪炎症[17]。郭行雅[18]研究发现,小鼠circRNA-0046367竞争性结合miR-34a可消除其对PPARα的抑制,从而降低脂代谢相关基因的表达,减轻肝的脂肪变性。miR-34a还能靶向PDGFRα抑制其表达,从而抑制脂肪生成[19]。Circ11897通过吸附miR-27a和miR-27调节猪皮下脂肪组织中脂肪细胞的脂滴生成和脂质代谢[20]。miR-125a可促进3T3-L1前脂肪细胞增殖,并且通过负调节STAT3抑制3T3-L1前脂肪细胞分化[21]。circRNA在脂肪代谢过程中具有重要的调控作用,但其对猪成脂分化的作用及机制还鲜有研究。
基于本课题组前期测序[22],发现circIGF1R来源胰岛素样生长因子1受体(insulin-like growth factor 1 receptor, IGF1R)基因的第2外显子,属于外显子circRNA。本研究对猪circIGF1R进行鉴定及分析,探明其表达规律,绘制circIGF1R相关的ceRNA调控网络,并构建circIGF1R过表达载体,在间充质干细胞C3H10T1/2中异位表达,研究其对脂肪细胞分化的作用,以期为探究circIGF1R在猪脂肪代谢中的作用机制奠定基础。
1 材料与方法
1.1 试验材料
1.1.1 试验样品采集 以刚出生的大白猪仔猪为试验动物,分离肌内前体脂肪细胞;采集90 d杜长大猪的心、肝、脾、肺、肾、背最长肌、背部皮下脂肪等组织;采集1 d、90 d、180 d杜长大猪的皮下脂肪。
1.1.2 试剂 间充质干细胞C3H10T1/2为山西农业大学动物遗传育种实验室保存;Trizol购自Invitrogen公司;TB Green® Premix Ex TaqTM和PrimeScript RT reagent Kit with gDNA Eraser购自TaKaRa公司;Opti-MEM、FBS购自Gibco公司;Lipofectamine 3000购自Thermo Fisher公司;DMEM/HIGH GLUCOSE、Phosphate Buffer Saline购自HyClone公司;油红O(Oil Red O)购自Solarbio公司;RNase R酶购自Lucigen公司;核质分离试剂盒PARISTM Kit购自Life Technologies公司;miRNA 1st Strand cDNA Synthesis Kit (by stem-loop) 、miRNA Universal SYBR qPCR Master Mix和Duo-Lite Luciferase Assay System购自诺唯赞公司;4%多聚甲醛购自Solaibio公司;3-异丁基-1-甲基黄嘌呤(IBMX)、胰岛素、地塞米松(DEX)、吲哚美辛购自Sigma公司。
1.1.3 仪器 核酸蛋白质测定(ND-1000,美国)、实时荧光定量PCR仪(7500 Real Time,美国)、全功能微孔板检测仪(SynerGyH1,美国)、细胞成像系统(EVOS FL Auto,美国)等。
1.2 细胞培养及转染
刚出生的仔猪放血处死后,75%酒精清洗全身,取背最长肌放入75%酒精中清洗后放入含4%双抗的PBS中备用。使用含1%双抗PBS反复冲洗肌肉组织,并在冲洗过程中剪除肌肉中的血管与筋膜,将处理干净的肌肉组织放入装有少量PBS的小烧杯中,将肌肉尽量剪碎,再将组织糜与Ⅱ型胶原酶按照1∶2混合后装入50 mL离心管中,至于恒温震荡仪中37 ℃,120 r/min震荡消化90 min左右,消化完成后加入等量完全培养基终止消化,依次过100 μm、70 μm、40 μm过滤筛,每次过滤之后1 500 r/min离心10 min,弃上清液,用完全培养基对细胞混合物沉淀重悬清洗1次,1 500 r/min离心10 min,弃上清,加入完全培养基后均匀铺在10 cm细胞培养皿中,置于37 ℃,5% CO2恒温细胞培养箱中培养。培养的原代肌内前体脂肪细胞传代1~2次后可用于试验。
将C3H10T1/2铺板后待细胞密度达70%左右进行转染,使用lipo3000转染试剂转染OE-circIGF1R及OE-NC,转染6 h后更换完全培养基,转染24 h ~ 48 h后检测过表达效率。转染后待细胞密度达90%,更换诱导培养基诱导细胞分化,诱导分化7 d后进行qRT-PCR及油红O染色。
1.3 RNA提取及qRT-PCR
细胞RNA的提取操作步骤参考Trizol试剂说明书,并使用核酸检测仪测定RNA浓度,将RNA浓度归一为1 000 ng/μL,使用反转录试剂盒将RNA反转录成cDNA。以cDNA为模板,使用SYBR Select Master Mix和ABI Prism 7900监测系统进行实时荧光定量PCR(qRT-PCR)。引物委托生工生物工程(上海)股份有限公司合成。引物序列等见Table 1。以18S rRNA为内参,采用2-ΔΔCt法计算相对表达量。
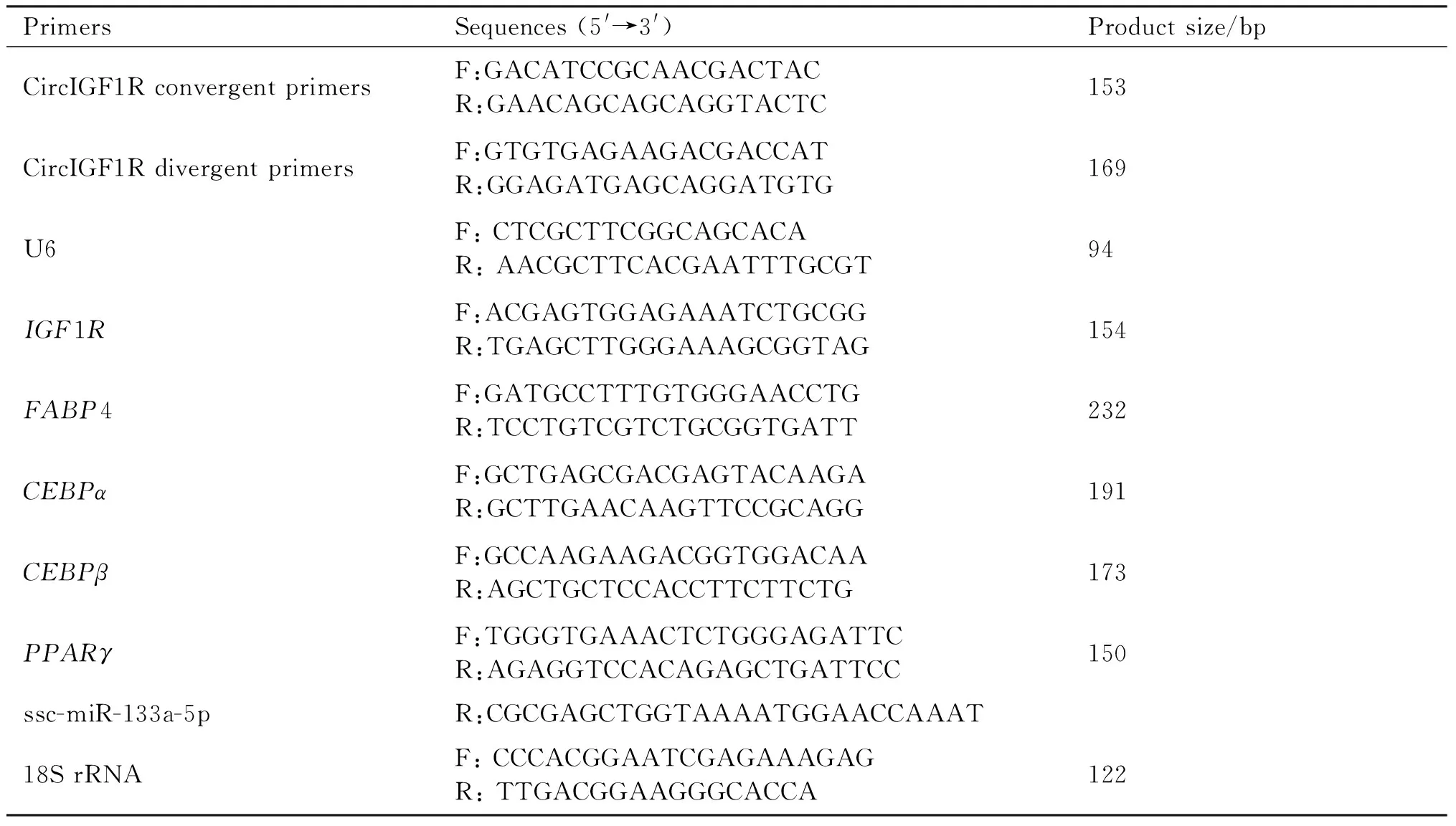
Table 1 Primer sequences
1.4 RNase R消化试验
将RNA分成均等的2份,RNase R酶处理组:RNA 4 μL、10×反应缓冲液2 μL、RNase R 0.5 μL、无RNase水 13.5 μL;对照组:RNA 4 μL、无RNase水 16 μL,进行RNase R(20 U/μL)消化抗性试验,37 ℃水浴消化30 min。使用反转录试剂盒将RNA反转录成cDNA。以cDNA为模板进行实时荧光定量PCR(qRT-PCR)。
1.5 核质定位
当肌内前体脂肪细胞密度达90%时,用胰酶将细胞消化后收集细胞,提取细胞核、细胞质RNA,提取步骤参考核质分离试剂盒说明书,并使用核酸检测仪测定RNA浓度,将RNA反转录成cDNA。以cDNA为模板qRT-PCR。以U6为内参,采用2-△△Ct法计算相对表达量。
1.6 双荧光素酶报告基因分析
构建circRNA及FABP4的野生型及突变型psiCHECK-2载体,待24孔板内293 T细胞长至50%时转染,分组为mimics+circIGF1R-Mut、mimics+ circIGF1R-WT、miR NC+circIGF1R-WT;mimics+FABP4-Mut、mimics+FABP4-WT、miR NC+FABP4-WT,每组做3个重复,转染48 h,弃培养基,1×PBS清洗细胞2次,操作步骤参考Duo-Lite Luciferase Assay System试剂盒。
1.7 油红O染色
待C3H10T1/2诱导分化7 d后移除培养基,1×PBS清洗细胞2次,操作步骤参考油红O说明书,染色完成后使用倒置显微镜拍照。
1.8 统计学方法
试验均设置3个生物学重复,利用SPSS Statistics 22.0软件进行显著性分析,采用Duncan’s法进行多重比较,使用Graphpad Prism 5.0作图,P<0.05表示差异显著,P<0.01表示差异极显著。
2 结 果
2.1 猪circIGF1R鉴定及亚细胞定位
前期课题组进行了1 d、90 d与180 d大白猪的背最长肌全转录物组测序,经筛选发现一条来源于IGF1R第二外显子的环状RNA,全长546 bp,将其命名为circIGF1R(Fig.1)。
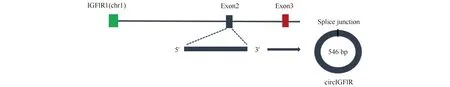
Fig.1 Schematic diagram of circIGF1R ring formation circIGF1R is formed by back-splicing of the second exon of its parental gene. The chr1 represents chromosome 1. Splice junction is the end-to-end position of the circRNA
据PCR发现,聚合引物(convergent primers)在以gDNA和cDNA为模板时均能扩增出单一条带;发散引物(divergent primers)只在以cDNA为模板时扩增出单一条带,以gDNA为模板时未见条带(Fig.2A),表明circIGF1R是猪基因组的反向剪接产物。对发散引物产物菌液进行Sanger测序,结果发现,确实存在反向剪接位点(Fig.2B)。RNase R酶处理结果显示,RNase R酶消化后,circIGF1R的表达量无显著变化(P>0.05),而IGF1R和18S rRNA的表达量极显著下降(P<0.01)(Fig.2C)。由此证明,circIGF1R是来源于IGF1R基因的第2外显子的外显子circRNA。核质分离结果显示,circIGF1R 61%位于细胞质中(Fig.2D)。
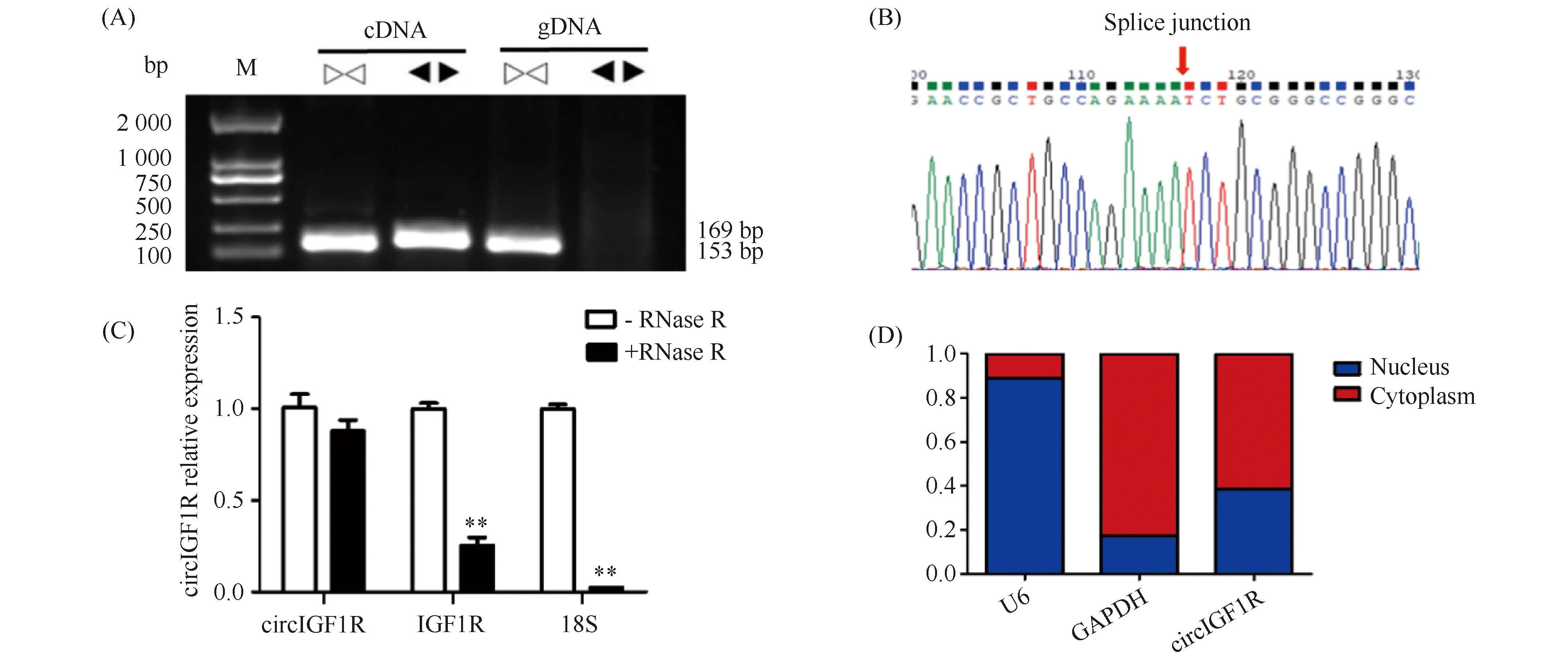
Fig.2 Identification and subcellular localization of circIGF1 (A) circIGF1R agarose gel electrophoresis. (B) Sanger sequencing to verify the circIGF1R splicing site. (C) RNase R digestion tests to examine circIGF1R and linear genes. (D) circIGF1R subcellular location. The values are presented as the means ± SEM (n=3),* represents significant difference (P<0.05),** represents extremely significant difference (P<0.01), the same as below.▷◁represents divergent primers, ◀▶ represents convergent primers
上述结果证明,circIGF1R是由胰岛素样生长因子1受体(insulin-like growth factor 1 receptor, IGF1R)第二外显子形成的circRNA。
2.2 猪circIGF1R表达分析
组织表达分析显示,circIGF1R在心血管组织中表达量最高,其次是背部皮下脂肪与背最长肌等(Fig.3A)。circIGF1R在不同发育阶段脂肪组织中差异表达,其表达量随着日龄增加呈上升趋势(Fig.3B)。circIGF1R在肌内前体脂肪细胞诱导分化不同时期呈现先升高后降低的趋势,其表达量在诱导分化第5 d最高(Fig.3C)。

Fig.3 Expression analysis of circIGF1R The RNA of heart, liver, spleen, lung, kidney, skeletal muscle and subcutaneous fat of three Duroc-landrace-yorkshire (DLY) pigs were extracted for the following analysis. The subcutaneous fat RNA of three DLY pigs at 1 day, 90 days, and 180 days were extracted for expression profiles at different developmental stages. Porcine intramuscular preadipocyte cells were harvested for the following analysis at 0, 1, 3, 5 and 7 days at differentiation. (A) qRT-PCR analysis showing the expression of circIGF1R in various tissues. (B) qRT-PCR analysis showing the expression of circIGF1R in different developmental stages of pigs. (C) qRT-PCR analysis showing the expression of circIGF1R in different stages of intramuscular preadipocyte differentiation. The values are presented as the means ± SEM (n=3). Different letters indicate significant differences (P<0.05). 18S rRNA was used as an internal control
上述结果表明,circIGF1R在猪的各个组织中均有表达,且其表达量在脂肪组织中随日龄增加呈上升趋势。
2.3 猪circIGF1R功能预测
通过RNAhybird软件预测发现,circIGF1R可靶向结合多个miRNA:ssc-miR-15a、ssc-miR-16、ssc-miR-133a-5p、ssc-miR-214-3p、ssc-miR-885-3p等,miRDB、Targetscan、miRWalk在线软件预测发现,这些miRNA存在多个潜在靶基因,利用Cytoscape构建circIGF1R-miRNA-mRNA调控网络(Fig.4)。

Fig.4 CircIGF1R-miRNA-mRNA regulatory network The blue circle represents circRNA. The yellow circle represents miRNA. The green circle represents the target gene. The pink circle indicates the selected target gene
通过DAVID数据库对ssc-miR-133a-5p靶基因进行GO和KEGG分析。GO分析发现,circIGF1R的靶标基因的作用主要有转录(transcription)、RNA聚合酶II转录因子活性(RNA polymerase II transcription factor activity)、核(nucleus)等(Fig.5A)。KEGG通路富集分析表明,circIGF1R的靶基因主要富集在胰岛素抵抗(insulin resistance)、AMPK信号通路(AMPK signaling pathway)、吗啡成瘾(morphine addiction)cGMP-PKG信号通路(cGMP-PKG signaling pathway)、肿瘤坏死因子信号通路(TNF signaling pathway)(Fig.5B)。
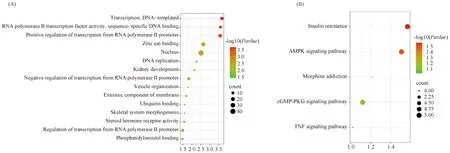
Fig.5 Enrichment analysis of GO (A) and KEGG (B) of ssc-miR-133a-5p target genes The horizontal axis represents the enrichment factor, and the vertical axis represents the path. Different colors represent different adjusted p-values, from blue to red, indicating that the adjusted p-value is increasing from large to small, and the degree of enrichment is becoming more and more significant. The size of the dot represents the number of genes enriched in this pathway
RNAhybird预测发现,circIGF1R可与ssc-miR-133a-5p种子序列结合(Fig.6A),FABP4可与ssc-miR-133a-5种子序列结合,是ssc-miR-133a-5p潜在的靶基因(Fig.6B)。
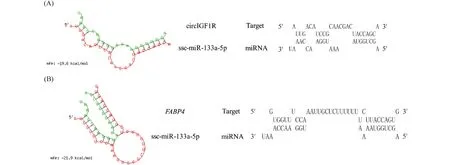
Fig.6 Binding site prediction (A) RNAhybird predicts the binding site of circIG1R and ssc-miR-133a-5p (B) the binding site of ssc-miR-133a-5p and the target gene FABP4
以上,基于基因表达相关性和预测的靶标关系,绘制了GO和KEGG富集分析、构建了ceRNA网络及预测了circIGF1R-ssc-miR-133a-5p-FABP4之间的靶向序列。
2.4 ssc-miR-133a-5p及FABP4表达分析
组织表达分析显示,ssc-miR-133a-5p在肝中表达量最高,其次是心血管、肾等组织(Fig.7A);ssc-miR-133a-5p在不同发育阶段脂肪组织中差异表达,其表达量随着日龄增加呈下降趋势(Fig.7B);在肌内前体脂肪细胞诱导分化不同时期呈现先降低后升高的趋势,其表达量在诱导分化第3 d最低(Fig.7C),其表达趋势与circIGF1R相反。
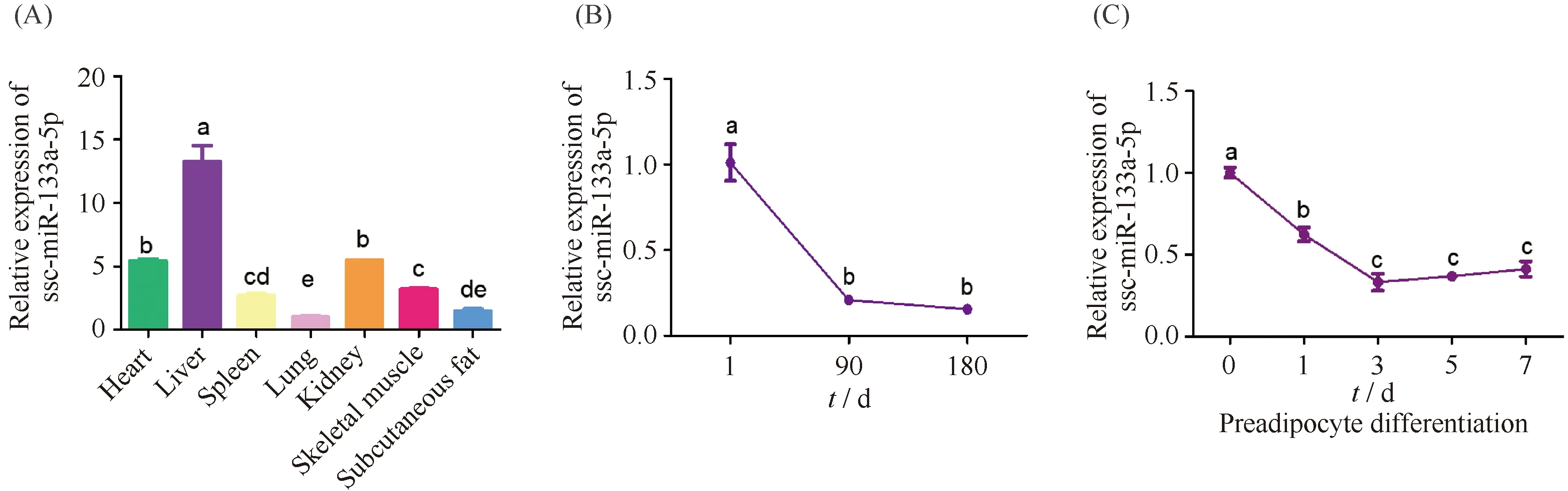
Fig.7 Expression analysis of ssc-miR-133a-5p The RNA of heart, liver, spleen, lung, kidney, skeletal muscle and subcutaneous fat of three Duroc-landrace-yorkshire (DLY) pigs was extracted for the following analysis. The subcutaneous fat RNA of three DLY pigs at 1 days, 90 days, and 180 days was extracted for expression profiles at different developmental stages. Porcine intramuscular preadipocyte cells were harvested for the following analysis at 0, 1, 3, 5 and 7 days of differentiation. (A) qRT-PCR analysis showing the expression of ssc-miR-133a-5p in various organizations. (B) qRT-PCR analysis showing the expression of ssc-miR-133a-5p in different developmental stages of pigs. (C) qRT-PCR analysis showing the expression of ssc-miR-133a-5p in different stages of intramuscular preadipocyte differentiation. The values are presented as the means ± SEM (n=3). Different letters indicate significant differences (P<0.05). 18S rRNA was used as an internal control
FABP4在皮下脂肪中表达量极显著高于其他组织(Fig.8A);FABP4表达量随着日龄增加在脂肪组织中升高(Fig.8B);在肌内前体脂肪细胞诱导分化不同时期从第3 d开始极显著升高(Fig.8C),其表达趋势与circIGF1R相似。
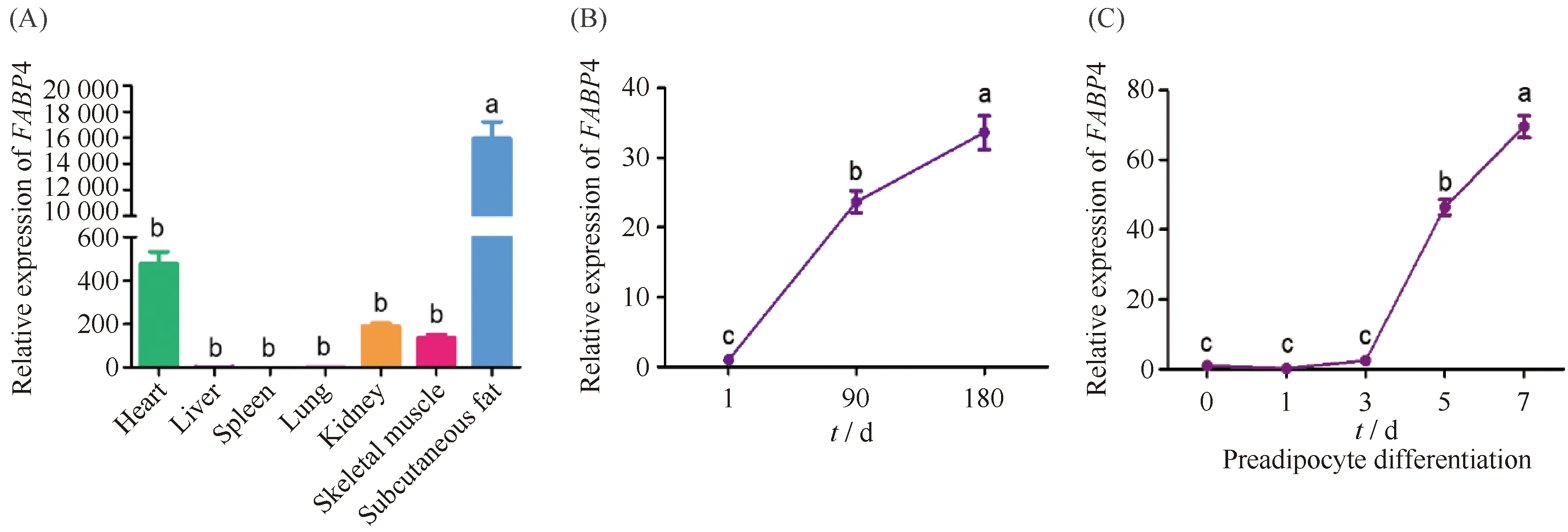
Fig.8 Expression analysis of FABP4 The RNA of heart, liver, spleen, lung, kidney, skeletal muscle and subcutaneous fat of three Duroc-landrace-yorkshire (DLY) pigs was extracted for the following analysis. The subcutaneous fat RNA of three DLY pigs at 1 days, 90 days, and 180 days were extracted for expression profiles at different developmental stages. Porcine intramuscular preadipocyte cells were harvested for the following analysis at 0, 1, 3, 5 and 7 days of differentiation. (A) qRT-PCR analysis of expression of FABP4 in various tissues. (B) qRT-PCR analysis of expression of FABP4 in different developmental stages of pigs. (C) qRT-PCR analysis showing the expression of FABP4 in different stages of intramuscular preadipocyte differentiation. The values are presented as the means ± SEM (n=3). Different letters indicate significant differences (P<0.05). 18S rRNA was used as an internal control
过表达circIGF1R后,检测到ssc-miR-133a-5p表达量极显著降低(P<0.01)(Fig.9A),FABP4表达量极显著升高(P<0.01)(Fig.9B)。
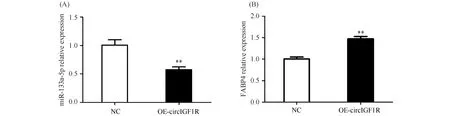
Fig.9 Expression of ssc-miR-133a-5p and FABP4 after overexpression of circIGF1R (A) Expression of ssc-miR-133a-5p after overexpression of circIGF1R. (B) Expression of FABP4 after overexpression of circIGF1R. The values are presented as the means ± SEM (n=3). 18S rRNA was used as an internal control.**P<0.01
以上结果表明,ssc-miR-133a-5p和FABP4在猪的各个组织中均有表达,且两者表达量在脂肪组织中趋势相反。其中,ssc-miR-133a-5p与circIGF1R表达量负相关,FABP4与circIGF1R表达量正相关,两者与circIGF1R的表达量趋势关系符合ceRNA假说。
2.5 双荧光素酶报告检测验证circIGF1R及FABP4与ssc-miR-133a-5p结合
根据双荧光素酶报告检测结果,mimics+circIGF1R-WT组的荧光值显著低于mimics+circIGF1R-Mut组及miR NC+circIGF1R-WT组,可知circIGF1R与ssc-miR-133a-5p可以结合(Fig.10A)。mimics+FABP4-WT组的荧光值显著低于mimics+FABP4-Mut组及miR NC+FABP4-WT组,可知FABP4与ssc-miR-133a-5p可以结合(Fig.10B)。上述结果表明,circIGF1R-ssc-miR-133a-5p-FABP4的ceRNA关系成立。

Fig.10 Dual luciferase report tests (A) Analysis results of circIGF1R and ssc-miR-133a-5p dual luciferase reporter enzymes. (B) Analysis results of FABP4 and ssc-miR-133a-5p dual luciferase reporter enzymes. The values are presented as the means ± SEM (n=3).*P<0.05
2.6 过表达circIGF1R促进C3H10T1/2细胞成脂分化
构建circIGF1R过表达载体,在小鼠间充质干细胞(C3H10T1/2)中异位表达,转染后诱导分化7 d后,进行qRT-PCR及油红O染色。qRT-PCR结果发现,过表达circIGF1R后,关键成脂调控因子CEBPα、CEBPβ、FABP4和PPARγ极显著升高(P<0.01)(Fig.11A,B);油红O染色结果发现,过表达circIGF1R后脂滴沉积明显增多(Fig.11C)。上述结果表明,过表达circIGF1R促进C3H10T1/2成脂分化。
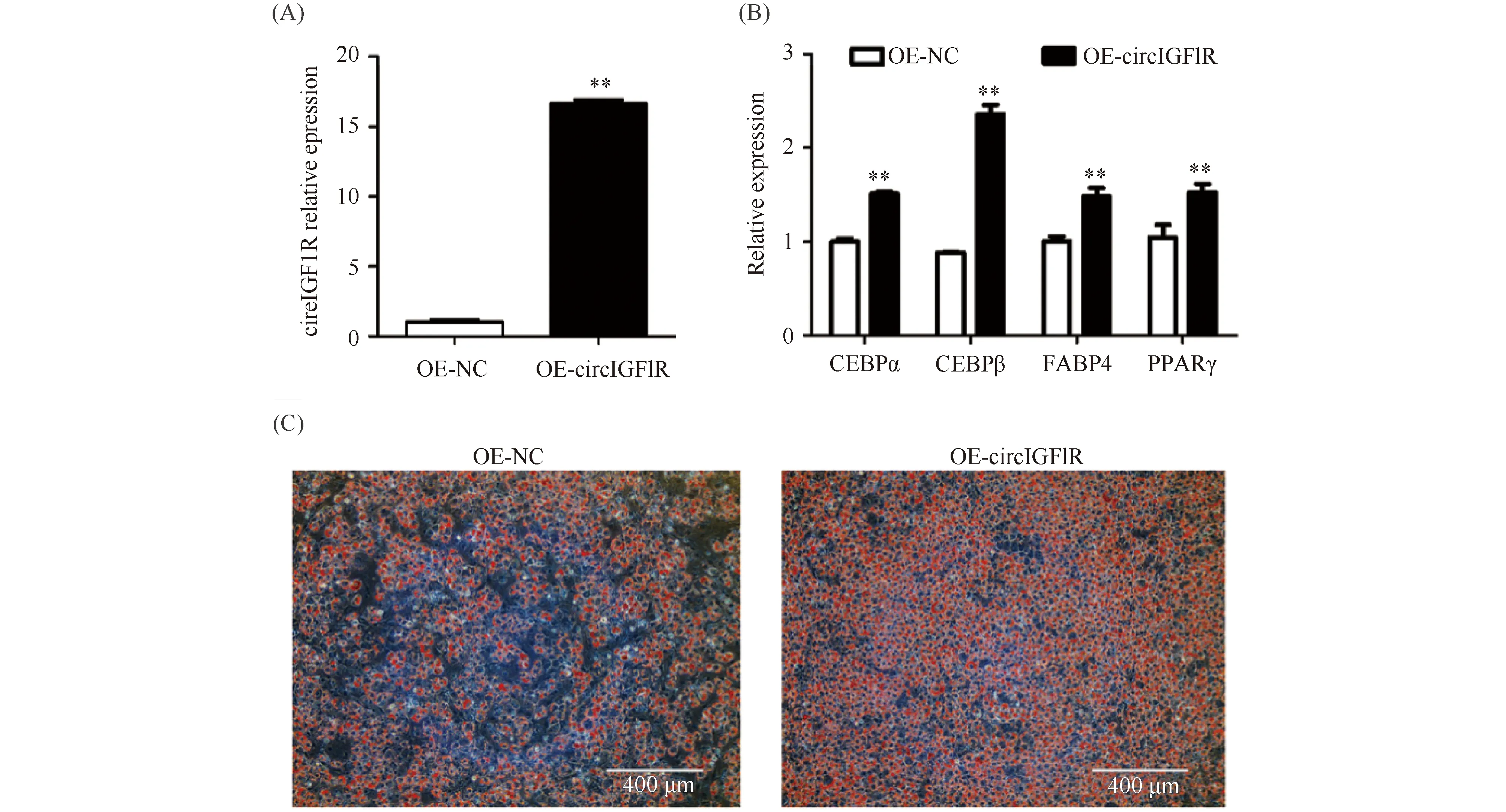
Fig.11 Overexpression of circIGF1R qRT-PCR and oil red O staining (A) The expression level of circIGF1R, detected by qRT-PCR, in C3H10T1/2 cells transfected with OE-NC and OE-circIGF1R. (B) The expression of key genes of adipogenic differentiation after transfecting with OE-NC and OE-circIGF1R plasmids. (C) The results of Oil red O staining after transfecting the OE-NC and OE-circIGF1R plasmids in C3H10T1/2 cells. Scale bar is 400 μm. The values are presented as the means ± SEM (n=3). 18S rRNA was used as an internal control.**P<0.01
3 讨论
随着生活水平的不断提高,人们对猪肉品质要求越来越高。肌内脂肪含量是影响猪肉品质的重要因素之一,是决定肉质嫩度和风味等的重要指标[23]。大量研究报道,circRNA在调控哺乳动物脂肪代谢方面发挥重要作用[12, 14, 24],但其在猪方面研究仍处于初级阶段。2019年,Liu等[25]对二花脸猪背最长肌内脂肪、背皮下脂肪、腹膜脂肪和肠系膜脂肪组织进行测序,共鉴定出13 746个circRNA,筛选出了大量与脂质代谢有关的circRNA。目前,由于测序技术的飞速发展,越来越多的circRNA被发现[20, 26],但关于circRNA的功能研究仍比较匮乏,尤其是其对猪脂肪代谢的影响。
胰岛素样生长因子(insulin-like growth factor,IGF)可通过与胰岛素样生长因子受体(insulin-like growth factor receptor,IGFR)结合,进而调节脂类代谢和影响脂肪组织发育[27]。在胰岛素样生长因子家族成员中,胰岛素样生长因子1受体(insulin-like growth factor 1 receptor,IGF1R)能够结合胰岛素样生长因子(IGF1和IGF2)配体,并通过自身磷酸化,激活PI3K和MAPK信号通路,从而控制细胞的增殖、分化和凋亡[28]。在禽类的研究中发现,IGF1R基因与控制禽类体重及骨骼生长发育的主要基因紧密连锁,说明IGF1R可能参与调控禽类生长发育[29]。在对猪的研究中发现,miRNA-455-5p可通过靶向IGF1R抑制猪前体脂肪细胞的增殖分化[30]。本研究发现,circIGF1R是来源于IGF1R第二外显子的circRNA。由此推测,circIGF1R可能参与调控猪前体脂肪细胞的成脂分化。
有研究发现,过表达miR-133a可抑制Prdm16表达进而阻止白色脂肪细胞褐变[31]。另外,Zhang等证明,上调miR-133a会诱导线粒体生物发生和C2C12成肌细胞分化[32]。本研究发现,circIGF1R与ssc-miR-133a-5p表达量呈负相关。双荧光素酶报告基因结果进一步证明,circIGF1R可与ssc-miR-133a-5p结合。由此推测,circIGF1R可作为ssc-miR-133a-5p海绵,参与调控猪成脂分化。FABP4在脂肪细胞中分化依赖性表达,是调节脂肪细胞生物功能的重要基因。FABP4正调控脂肪酸信号转导通路,可以直接靶向并将脂肪酸代谢产物传递到脂质信号转导通路中[33]。有研究表明,小鼠给予高脂饮食后,敲除FABP4基因小鼠体重增加,血浆游离脂肪酸的浓度升高,胆固醇和甘油三酯的水平降低,显示FABP4基因缺失的肥胖小鼠脂肪细胞的生理功能和游离脂肪酸代谢发生改变[34]。本研究发现,FABP4是ssc-miR-133a-5p的一个靶基因,由此说明circIGF1R可能通过与FABP4竞争性结合ssc-miR-133a-5p参与调控猪肌内脂肪代谢。异位表达circIGF1R证明,circIGF1R会促进C3H10T1/2的成脂分化,该结果与上述结果一致,更进一步说明circIGF1R可参与调控成脂分化。本研究成功鉴定了猪的一个新circRNA-circIGFIR,并且初步证明其可通过ceRNA机制调控成脂分化。为进一步研究circIGF1R调控猪肌内前体脂肪细胞分化机制奠定基础。

