硼氢化试剂在二氧化碳还原官能化反应中的研究进展
郭志强,杨博如,席婵娟
(1.山西大学大型科学仪器中心,功能分子山西省重点实验室,太原 030006;2.清华大学化学系,生命有机磷化学及化学生物学教育部重点实验室,北京 100084)
1 Introduction
Carbon dioxide(CO2)is a low-toxic,abundant,recyclable molecule,and a satisfactory C1 source for the synthesis of a variety of value-added chemicals. It can replace toxic C1 sources such as CO,iodomethane,phosgene to realize the sustainable use of limited resources. Therefore,the recycling of CO2from the most basic research to actual industrial production has aroused a lot of interest. Over the past decade,scientists have made many contributions to the reduction of CO2as renewable C1 source to synthesize value-added products[1~5].
However,due to the high kinetic and thermodynamic stability of CO2,it is necessary to design the activa⁃tion of CO2for transforming it effectively. So,many reductive functionalizations of CO2with reducing reagents in the presence of catalysts[6~16]to achieve the valuable chemicals were reported. Borohydride reagents have been found wide use as reducing agents in organic synthesis. Among them,reductive functionalization of CO2using the borohydride reagents have provided a straightforward method[17,18]. The reduction of CO2using metal borohydride could be traced back to the 1950s. Helleret al.[19]studied the reaction of14CO2with lithium boro⁃hydride in ether,and clarified the formation of lithium formate products,in combination with diborane and methanol as secondary products. Later,aminoborane and its derivatives have aroused great interest due to their characteristics of low cost,non-toxicity,easy operation,large hydrogen storage capacity and high atomic economy[20~22]. Reduction functionalization of CO2has attracted more and more attention using the other borohydride reagents. These reduction processes can enable nucleophile to achieve substrate formylation,methylene,and methylation in a controlled range(Fig.1). The oxidation state of C in CO2can be reduced to+2,0,or −2 in turn to afford the corresponding C2+,C0and C2−products,respectively. Herein,this review summarized the reductive functionalization of CO2to form high value-added chemicals using the borohydride reagents.
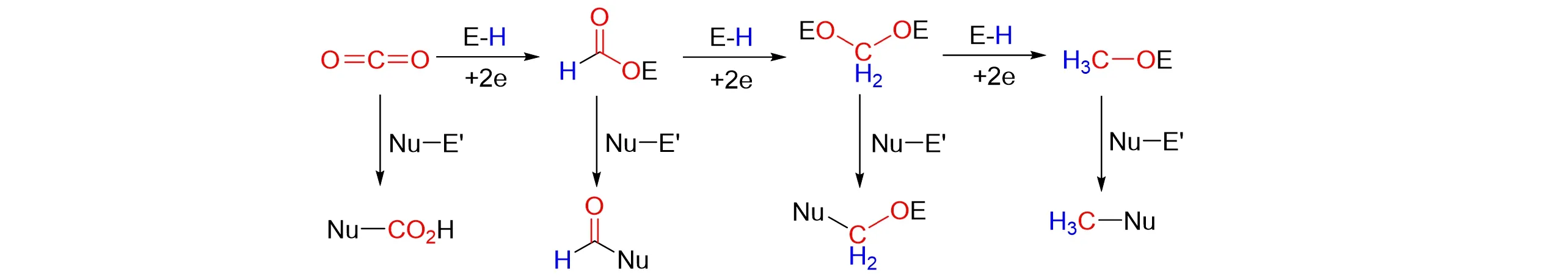
Fig.1 Hierarchical reduction functionalization of CO2
2 Reduction of CO2 with Metal Borohydride
Metal borohydrides(such as LiBH4,NaBH4,KBH4,etc.),as a type of inexpensive,easy-to-handle,commercially available,and mild reductant,have been reported in many methods of CO2reductive functionalization. They could achieve CO2reduction conversion at room temperature or under other mild conditions with or without catalysts. CO2as C1 source could be reduced and functionalized with nucleophile to generate value-added chemicals,such as formamide,methylamine,and their derivatives,etc. Among them,since the reduction of C0products(such as formaldehyde)to C2−products(such as methylamine and methanol)is faster than the reduction of C2+products(such as formamide and formic acid)to C0products,it is a challenge to isolate or capture C0products[23].
2.1 Reduction of CO2 with NaBH4
In 2014,tetrahydrofuran(THF)solution containing 0.5%(molar fraction)NaBH4as a stabilizer for BH3‧THF was used to reduce CO2to trimethoxyboroxine(MeOBO)3at ambient temperature and atmospheric pressure 1 atm(1 atm=1.01×105Pa)was reported by Mizuta’s group[24],as shown in Fig.2. They found that the reaction did not require any complex additives. More importantly,this reaction did not take place in the“purified”BH3‧THF solution(without NaBH4),confirming that the reduction of CO2in solution of THF demands NaBH4as initiator. When NaBH4was replaced by HCOONa,CO2could be successfully reduced to(MeOBO)3with similar yield.
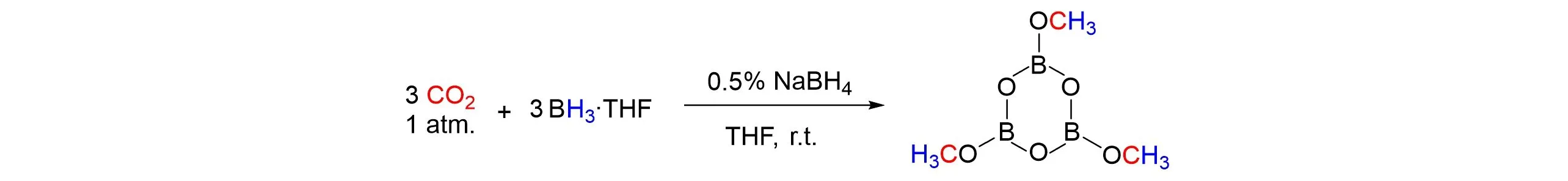
Fig.2 Reduction of CO2 to trimethoxyboroxine(MeOBO)3 with NaBH4
Then,Cummins’s group[25]used 1 mol NaBH4to absorb 3 mol CO2to afford Na[HB(OCHO)3]in CH3CN and at 300 psi(≈2 MPa)pressure,without expensive borane or catalyst. The product Na[HB(OCHO)3]was characterized by full spectrum and X-ray crystallography. NaBH4reacted with CO2without strictly drying solvent and the mixture was quenched to form formic acid even at an atmospheric pressure. It shows that the inherent reactivity of borohydride anions should be considered and strictly controlled when studying the reduc⁃tion of substrates containing borohydride,as shown in Fig.3. In addition,Williamset al.[26]described that di(carbene)-supported nickel species is an efficient catalyst for the reduction of CO2to methanol at room temperature using sodium borohydride as reductant in 2016. In this report,two di(carbene)-supported nickel catalysts feature the unusual stability,and enable>1.1 million turnover numbers of CO2. At the same year,the theoretical studies have been performed on the mechanism aspects of the CO2conversion to methanol using borane molecules and borohydride salts of M[RBH3](M=Li,Na and R=Ph,CH3,CN,H)as the reductive re⁃agent. The results indicated the Li[MeBH3]and Li[PhBH3]are the best reductive agents for the reduction of CO2to methanol,the charge density of the boron atom and the electronic interactions of the substituted groups affect the Gibbs free energy of activations in the process[27].

Fig.3 Reduction of CO2 with NaBH4 in CH3CN
Furthermore,CO2as green and sustainable C1 source that can be converted to high value-added chemical have significance of prominence. In 2017,Liuet al.[28]reported a novel route that use NaBH4as the reducing reagent,the reductive formylation of amines with CO2was realized at 100 °C under catalyst-free conditions,and a series of formylated products was obtained in excellent yields,as shown in Fig.4(A). The reaction mechanism investigation demonstrated that NaBH4could react with CO2to form intermediate,which further reacted with amines,producing the formylated compounds. In addition,NaBH4was also very efficient for the reductive cyclization ofo-phenylenediamine with CO2to synthesize benzimidazoles.
Methylamine is an important chemical substance in medicine,natural products,and other fields. It re⁃quires a green,sustainable method to form methylamine,and reducing CO2to methylamine is a good alterna⁃tive method. In 2018,Xi’s group[29]reported 1,4-dioxane-tuned catalyst-free reduction functionalization of CO2using amine and NaBH4,as shown in Fig.4(B). This protocol realized theN-methylation of amines using CO2as a C1-building block at 100 ℃and 1,4-dioxane as solvent. Notably,the six-electron reduction of CO2to the methyl with formation of C—N bond was attained simultaneously. It is worth noting that with the increase of NaBH4dosage,the yield and selectivity increased significantly,indicating that the ratio of CO2to NaBH4affected the selectivity. Hu and co-workers[30]reported the catalyst-free selectiveN-formylation andN-methylation of amines using CO2as a sustainable C1 source again in 2020. The selective synthesis of formamide and methylamine was realized using NaBH4as reducing agent by adjusting the reaction solvent and temperature. It was concluded that both aromatic secondary amines and aliphatic secondary amines could obtain formamide andN-methylanilines with both electron-absorbing and electron-donating groups on benzene ring,the corresponding yield could exceed 90%. In 2021,Mahajanet al.[31]also reported a catalyst-free and sustainableN-formylation approach of aromatic as well as aliphatic amines using NaBH4and CO2gas. The formation of formoxy borohydride species is critical for the success ofN-formylation reactions. And the formoxy borohydride species can promote transamidation reactions of amines using formamide and DMF as a formyl source under mild conditions. In addition,Lee’s group[32]reported the utilization of NaBH(OCHO)3generatedin situfrom the reaction of NaBH4with CO2for the reductive amination reactions. And Xiet al.[33]described the formation of NaBH4−m(OCHO)mas a reducing reagent was critical to achieve highly selective reduction of formamides to the corresponding methylamines using NaBH4with CO2.
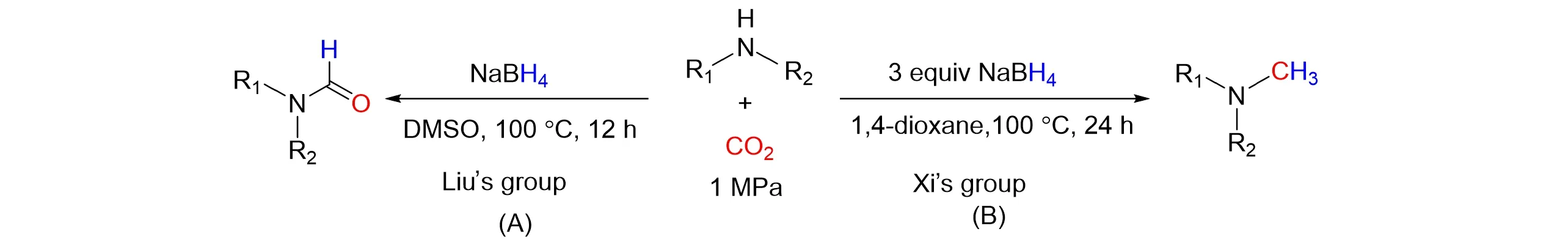
Fig.4 N⁃formylation and N⁃methylation of amines with CO2 with NaBH4
Then,Xi’s group[34]reported the first selective four-electron reduction of CO2with thiophenol using mild NaBH4as a reductant to generate dithioacetals at atmospheric pressure. This reaction provides a novel synthetic method for the highly selective conversion of CO2into methylene,and a new access to molecular structuresviathe formation of C—S bonds using CO2as the C1 source. Under the optimized reaction condi⁃tions,thep-substituted thiocresol with electron-donating groups has a higher reactivity than the substituted thiocresol with electron-withdrawing groups,and the corresponding dithioacetal yield exceeds 95%,as shown in Fig.5(A). At the same time,they also came up with an approach that the reduction of CO2and thiophenol was realized through series reaction to generate aryl methyl sulfide in NaBH4/I2system with 18-crow-6 as solvent[35],as shown in Fig.5(B). The reaction has a high reduction selectivity and a wide range of functional group tolerance. Thiophenol containing electron-donating groups and electron-withdrawing groups is compati⁃ble in this process,and the reaction can be carried out by one-pot reaction. This work not only provides a sus⁃tainable alternative for the synthesis of methyl aryl sulfide,but also can reduce greenhouse gases and provide guidance for the selective reduction of CO2.
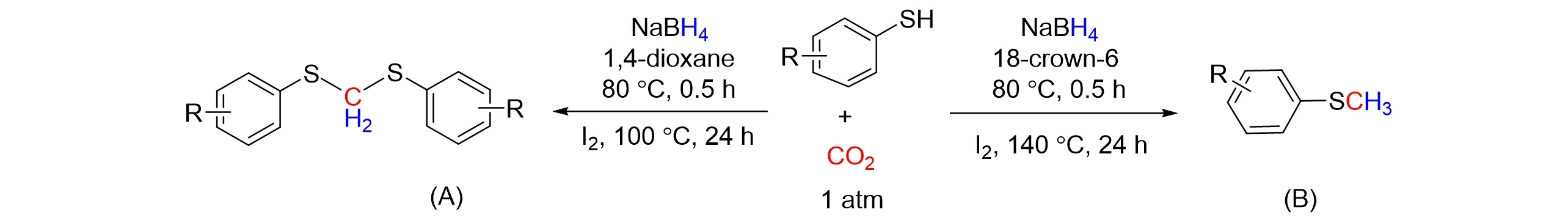
Fig.5 The selective reductive functionalization of CO2 using thiophenol with NaBH4
2.2 Reduction of CO2 with KBH4
In 2015,Amalet al.[36]developed an efficient route for the reduction of CO2with KBH4(potassium borohydride)to produce formic acid in 24% yield at mild conditions. The effects of reaction temperature,reaction time,CO2pressure and borohydride concentration on the reaction yields were investigated. It was found that 0.15 mol/L formic acid could be produced in a borohydride solution of 0.5 mol/L at room temperature and atmospheric pressure,the yield increased with the increase of pressure. This work offers the possibi-lity of sustainably reducing CO2to formic acid,accompanied by hydrogen production,as shown in Fig.6.

Fig.6 Reduction of CO2 with KBH4
Next,Filinchuk and cooperator[37]studied the solid-gas catalyst solvent-free reaction of KBH4with CO2to produce the main product of formyl potassium borohydride K[HxB(OCHO)4‒x](x=1—3)under mechanochemi⁃cal and thermal induction conditions in 2016,as shown in Fig.7. The first crystal of the main product,potassi⁃um triformylborate K[HB(OCHO)3]was obtained by the mechanochemical reaction. Notably,one possible continuation of the project is the recycling of CO2with complex hydrides that can create optimum conditions for the selective and sustainable production of organic fuels or useful organic matter.
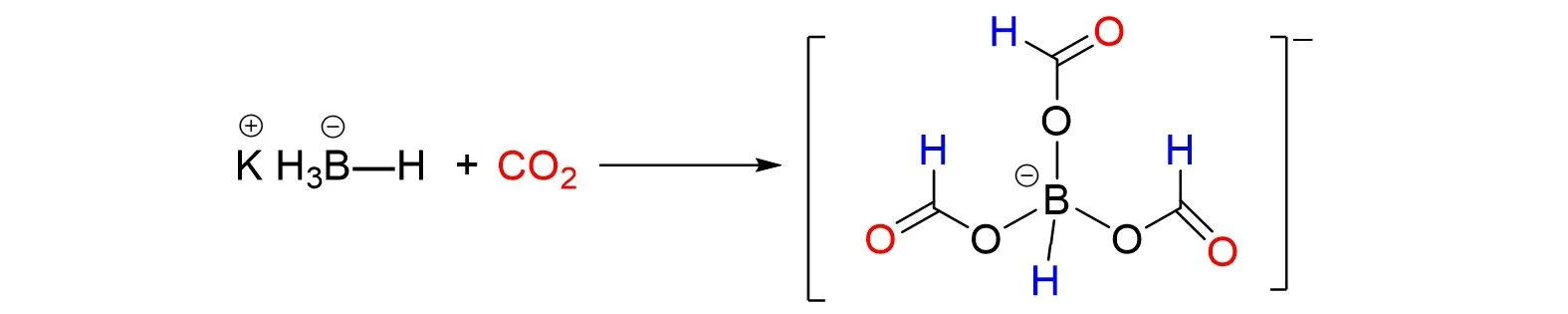
Fig.7 Catalyst⁃free reaction of KBH4with CO2 to K[HB(OCHO)3]
2.3 Reduction of CO2 with NaBH(OAc)3
Yuand co-workers[38]came up with an approach forN-formylation of amines with NaBH(OAc)3as a reductant under atmospheric pressure of CO2at 50 °C in 2019. The corresponding formylated products of various amines,including aliphatic,aromatic amines,and the amines with reductive-sensitive nitro groups and alkynyl groups and benzamides were obtained in good to excellent yields,as shown in Fig.8.
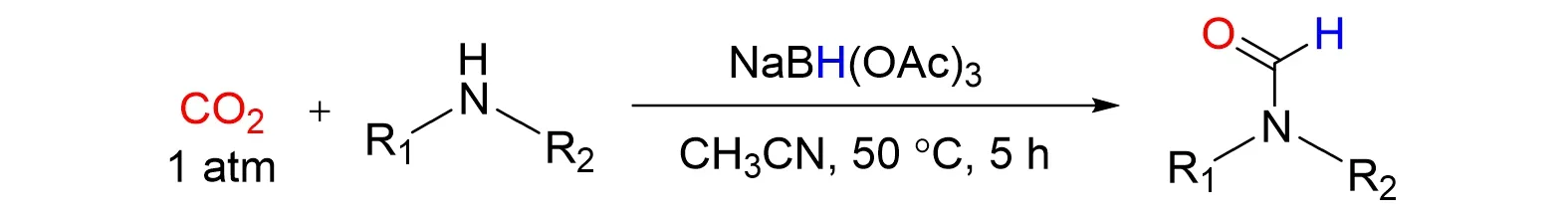
Fig.8 N⁃formylation of amines with NaBH(OAc)3 as reducing agent
3 Reduction of CO2 with Ammonia Borane
3.1 Reduction of CO2 with NH3‧BH3
In 2010,Stephan’s group[39]found that a combination of AlX3(X=Cl or Br)and PMes3(Mes3=2,4,6-C6H2Me3)could irreversibly capture CO2molecules after continuous exploration,as shown in Fig.9. This kind of Lewis acid-base combination shows the characteristic of blocked Lewis acid-base pair,and the reaction is irreversible when the ratio between this combination and CO2is 2∶1. At the same time,they provide a new method for rapid conversion of frustrated Lewis acid-base pair(FLP)-activated CO2to CH3OH at room tempera⁃ture with NH3‧BH3as hydrogen source.
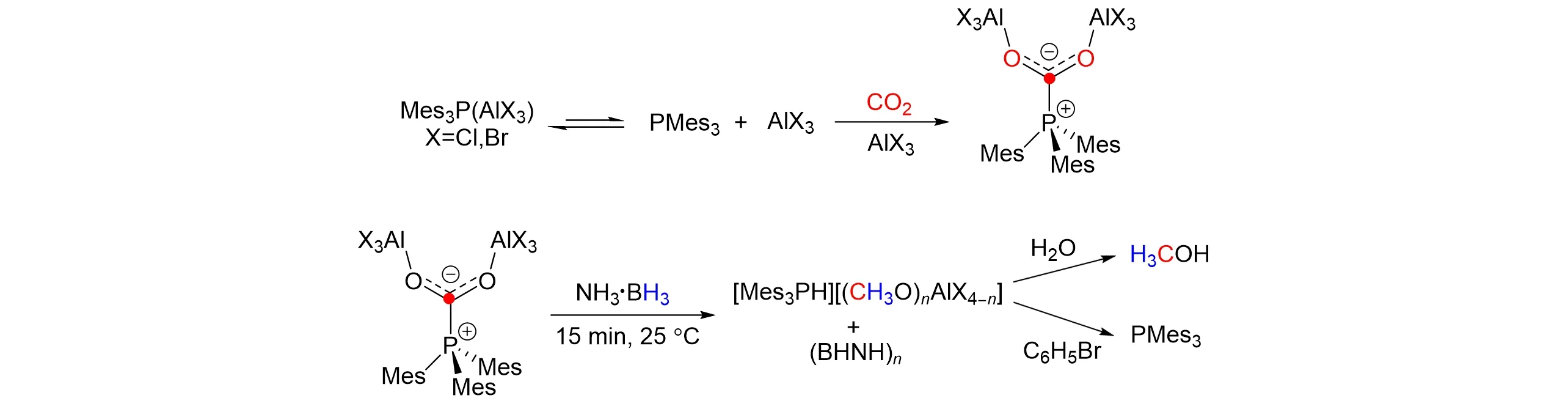
Fig.9 Reduction of CO2 to CH3OH with Al⁃based frustrated Lewis pairs and BH3‧NH3
Wuet. al.[40]developed a green,catalyst-free and effective procedure forN-formylation using CO2as the C1 source and NH3‧BH3as the reductant under mild conditions in 2017. TheN-formylation works quite well in the absence of catalyst whereas theN-methylation is highly catalyst dependent,which makes the reactions afford the formamides in good to excellent yields. The reactions do not require an inert atmosphere and are applicable to a wide range of substrates. At the same time,Xiet al.[41]reported Lewis base promoted selective reduction of CO2into boryl formates using NH3‧BH3as a reductant under mild conditions. The boryl formats generatedin situwere shown to be reactive and versatile sources of formyl compounds for creating new C—N,C—O,and C—C bonds. The reactivities of the boryl formates to yield formic acid,formamides,formates,secondary alcohols,and benzoheterocyclic rings were investigated,as shown in Fig.10.
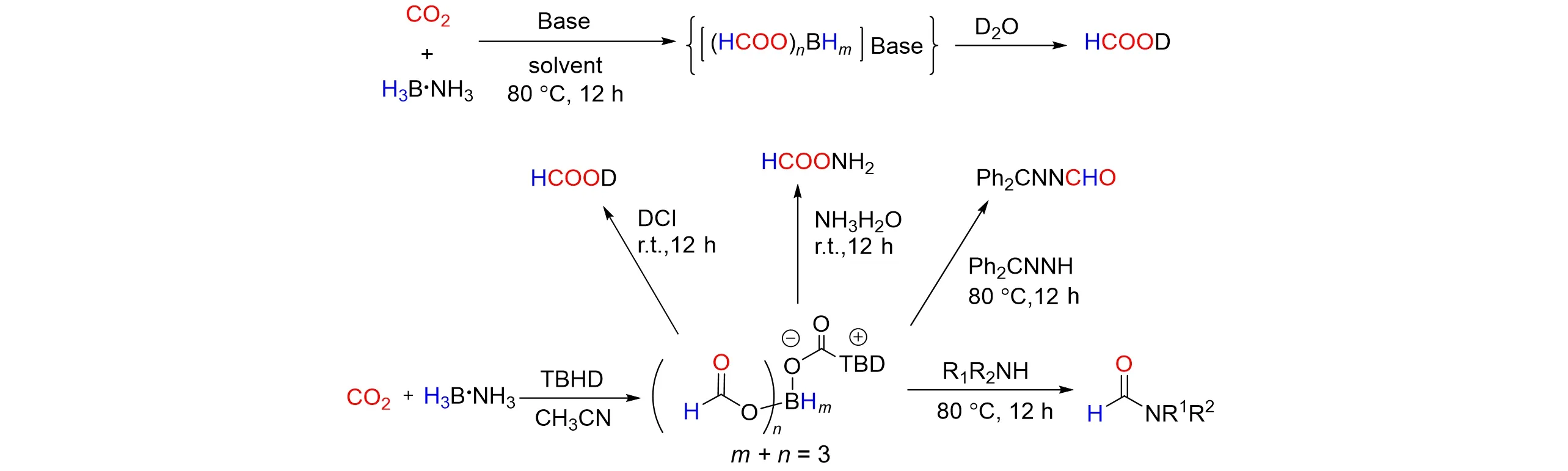
Fig.10 Selective reduction of CO2 to boryl formates by using BH3‧NH3
In 2021,Zhu’s group[42]selectedo-phenylenediamine with two amino groups and CO2to form a series of 1H-benzimidazole derivatives with NH3‧BH3as the reducing agent and catalyst under mild conditions,as shown in Fig.11. The mechanism study showed that the coordination ofo-phenylenediamine with boron atoms of NH3‧BH3was favorable for formyl group transfer to form stable intermediates,so that the intermolecular nucleophilic addition-elimination reaction would be transformed into the intramolecular nucleophilic additionelimination reaction to form target products. Therefore,NH3‧BH3plays an important role as a reducing agent and formylation catalyst in the reaction. In addition,Huet al.[43]reported the base-assisted reduction of CO2to formate with water as a green solvent without any catalysts under mild conditions. In this process,CO2reacted with water and base to give bicarbonate intermediatein situwhich can be further reduced to formate.Choudhuryet al.[44]descripted a catalytic CO2transfer hydrogenation process for generating formate with BH3‧NH3using Ir-NHC catalyst,this protocol was free of an external base,and exhibited a turnover frequency of 686 h−1and a high turnover number under 1 atm CO2stream at 30°C.
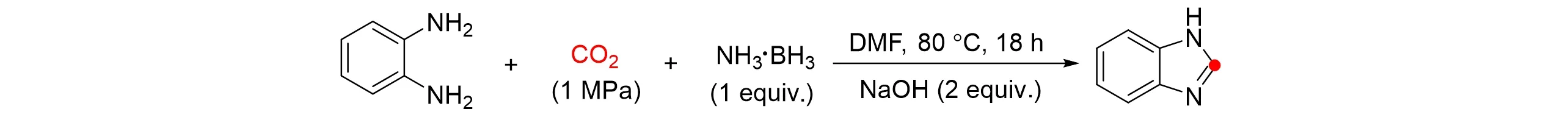
Fig.11 Reductive cyclization of o⁃phenylenediamine with CO2 and BH3‧NH3
3.2 Reduction of CO2 with BH3‧NHMe2
Bhanageet al.[45]prepared the mesoporous nanocomposites(amine modified meso Al2O3@MCM-41)in 2016,consisting of mesoporous silica and alumina. As an easily recovered and reused catalyst,it was used for the formylation of amines with CO2and using dimethylamine-borane(DMAB)as a green reducing source,as shown in Fig.12(A). This catalytic system represents a heterogeneous and environmentally benign protocol.Besides,the catalyst could be reused for five consecutive cycles without any significant loss in its catalytic activity towards the synthesis of formamides.

Fig.12 Synthesis of formamide derivatives with CO2 and BH3‧NHMe2
They also reported the B(C6F5)3as an organocatalyst for the transition-metal-freeN-formylation of amines using CO2as a C1 source in 2018[46],as shown in Fig.12(B),and dimethylamine borane(BH3‧NHMe2,DMAB)as a green hydrogen transfer source at 80 ℃. The bulky boron[B(C6F5)3]catalyst reacts with amines to form a frustrated Lewis acid-base pair(FLP),which activates CO2and BH3‧NHMe2molecules. Additionally,this boron catalyst shows a high catalytic activity for the cyclization ofo-phenylenediamines using CO2and BH3‧NHMe2to synthesize benzimidazoles. In the same year,they also reported the synthesis and characteriza⁃tion of ruthenium nanoparticles(Ru/NPs)supported on polymeric ionic liquids(PILs)[47]. This catalyst shows a high catalytic activity towards theN-formylation of amines and synthesis of benzimidazoles from 1,2-diamines and CO2by reductive dehydrogenation of dimethylamine borane. This methodology shows excellent functional group tolerance with broad substrate scope towards the synthesis ofN-formamides and benzimid⁃azoles. In addition,this protocol also provides the tandem reduction of 2-nitroamines and CO2to synthesize benzimidazoles. Moreover,the catalyst was found to be recyclable in nature and did not show significant loss in its activity during five consecutive recycling runs,as shown in Fig.12(C).
Furthermore,they proposed a green and sustainable approach by using CO2[48]and Cu@U-g-C3N4as multiphase cyclic catalysts for the synthesis of benzimidazole through the formation of carbon-nitrogen bonds,as shown in Fig.12(D). In the experiment,Cu@U-g-C3N4catalyst was synthesized by urea precipitation method and easy precipitation method,and used for the first time in the CO2cyclization reaction ofo-phenylenediamine(OPD)with DMAB as the reductant. In addition,the catalyst has high stability and excellent catalytic activity,and can be reused for many times. The use of green solvent makes the synthesis of benzimidazole more diversified. Because propylene carbonate/water is a suitable,biodegradable,economical,and environmentally friendly solvent system,the proposed solution becomes efficient and sustainable. The catalytic system has a wide range of substrates for the synthesis ofp-benzimidazole with high yield.
In 2019,they developed a more efficient method forN-formylation using CO2. They first prepared UiO-66 and UiO-66-NH2MOFs containing Zr metal-organic frameworks and used them as the catalysts[49],as shown in Fig.12(E). Formamide was prepared by using DMAB as a reducing agent. It was found that both catalysts have good catalytic activity and can obtain ideal products. The catalytic system is very effective for many amines including primary amines,secondary amines,cyclic amines and aromatic amines. In addition,when UIO-66-NH2MOFs were used as the catalysts,they could be recycled up to four times without a signifi⁃cant decrease in the catalytic activity.
3.3 Reduction of CO2 with BH3‧NMe3/BH3‧NEt3
In 2019,Xi’s group[50]first proposed to use CO2and amborane to methylate the C(sp3)-H bond of 2-aryl acetonitrile. Aminoboranes were chosen because the amines and boranes compete with each other in CO2reduction and capture two-electron intermediates. Trimethylamine-borane(BH3‧NMe3)could effectively avoid side reactions and promote the reduction of CO2by six electrons. Various 2-aryl propionitrile can be obtained with high yield,as shown in Fig.13.

Fig.13 Methylation of 2⁃arylacetonitrile with CO2 and BH3‧NMe3
In 2021,Gaoet al.[51]proposed a cheap and stable intramolecular FLP catalytic system based on 6-amino-2-pyridine(I)and borane. In the absence of metals,the system successfully used boranetrimethylamine(BH3‧NMe3)as reducing agent and 1 atm CO2as the carbon source to achieve methylate amine,as shown in Fig.14. Studies have shown that the use of 6-amino-2-pyridine can significantly improve the methylation yield of various amines. If the system did not use 6-amino-2-pyridine,the boranes contained in the system also had high chemical selectivity to various primary and secondary amines,and would carry out formylation reaction. In addition,the mechanism studies showed that boranes are Lewis acids and reductants for forming intramolecular FLP catalytic system. At the same time,Huet al.[52]reported the catalyst-free selective hierarchical reduction of CO2with BH3‧NEt3to formamide andN-methylanilines,the mechanistic research indicated the formamide could be regarded as intermediate species during theN-methyla⁃tion process.
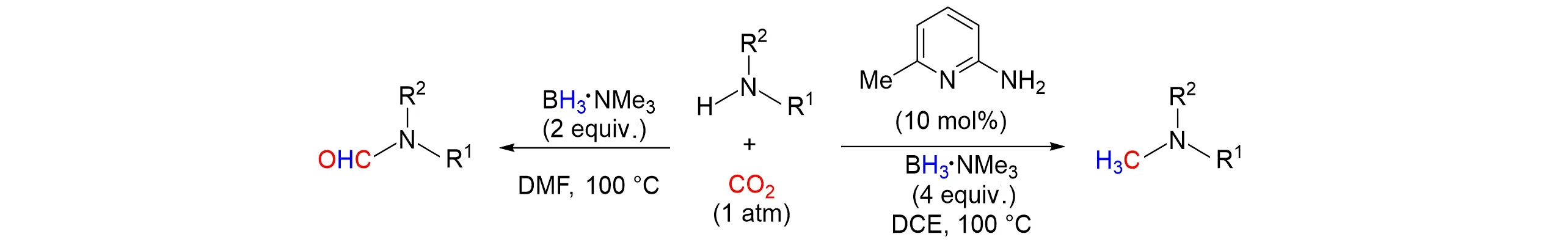
Fig.14 Selective methylation and formylation of amines with CO2 and BH3‧NMe3
3.4 Reduction of CO2 with Borohydride Ionic Liquid
In 2020,Zuttelet al.[53]described the synthesis of[EMPY][BH4](EMPY=1-ethyl-1-methyl-pyrrolidini⁃um cation)by combining pyrrolidone based ionic liquid with borohydride anion),and achieved metal-free and solvent-free capture and reduction of a large amount of CO2to formate at room temperature and ambient pressure. Even the air was used as CO2source,more than 1 molCO2/mol[EMPY][BH4]could be captured and reduced to formate. The product can be treated with HCl to give formic acid and the corresponding chloride ionic liquid,which can be recycled. It was clear that[EMPY][BH4]not only is very good at capturing CO2in the environment,but also can provide valuable chemicals by reducing atmospheric CO2,as shown in Fig.15.
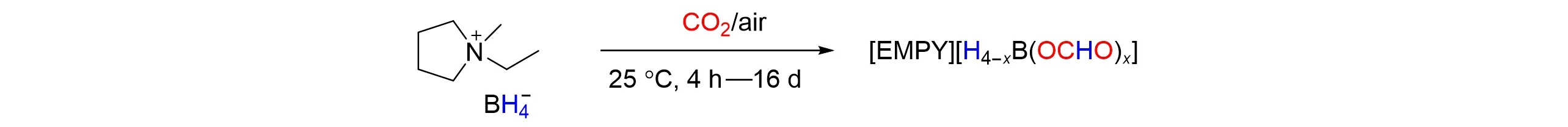
Fig.15 Reduction of CO2 to to formatoborohydride with[EMPY][BH4]
4 Catalytic Reduction of CO2 with Borohydrides
4.1 Transition Metal-catalyzed Reduction of CO2 with Borohydrides
Although Lewis acid-base pair chemistry has led to alternative strategies for the reduction of CO2to the methoxide level,the cleavage of these formed robust MeO—B and MeO—Al chemical bonds in this FLP systems have proven to be difficult,and stoichiometric amounts of FLPs are often required. In 2010,Guan’s group[54]reported a highly efficient nickel system for the catalytic hydroboration of CO2to methoxyboryl species with the highest turnover frequencies(TOF)(495 h‒1based on B—H),as shown in Fig.16(A). In 2012,they utilized the similar nickel hydride complex with different sizes of alkyl groups on the phosphorus donor atoms to reduce CO2rapidly at room temperature to give the corresponding nickel formate complex[55]. The catalytic reduction of CO2with catecholborane suggested the steric environment around the nickel center is critical to the efficiency of the catalysis. The reaction was favored by a bulky Guan’s group,as shown in Fig.16(B). They further synthesized or spectroscopically observed nickel dihydridoborate complexes from the reactions between POCOP-pincer-ligated nickel hydrides and various boranes[56]. The results showed that the efficiency of the nickel hydrides in catalyzing the reduction of CO2depends on not only the substituents on the phosphorus donor atoms,but also the type of boranes. The best catalyst involved bulky substituents on the phosphorus donor atoms. Catalytic reactions were less efficient because of the formation of dihydridoborate complexes as the dormant species as well as partial decomposition of the catalysts by the boranes. In 2017,Chenet al.[57]reported the hydroboration of CO2with catecholborane catalyzed by a series of bis(phosphinite)pincer ligated nickel thiolate complexes. The TOFs up to 2400 h−1were achieved at room temperature under 1 atm pressure of CO2. This represents the most efficient homogeneous catalytic system known to date for the reduction of CO2to the methoxide level under mild conditions. The related results indicated the present system is significantly different from the nickel hydride system in which POCOP-pincer ligated nickel hydrides are used to catalyze the reduction of CO2with HBcat under mild conditions,and the nickel hydride complexes were not the only species in this catalyzed process.
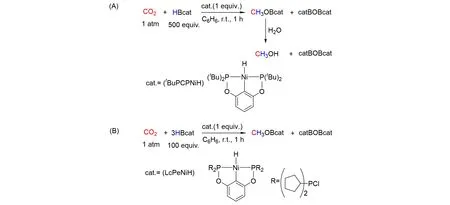
Fig.16 Reduction of CO2 using nickel catalyst and borane as reductant
In addition,Turculetet al.[58]reported the synthesis and characterization of Ni,Pd,and Pt hydride com⁃plexes supported by bis(indolylphosphino)silyl ligand. These hydride species exhibited divergent selectivity in the catalytic hydroboration of CO2with pinacolborane(HBPin). The Ni species exhibited a higher selectivity(97%)for the hydroboration of CO2to the formaldehyde level to give the bis(boryl)acetal PinBOCH2OBP with high yield,while the Pd species exhibited moderate activity for CO2hydroboration to the formate level under mild conditions. The HBPin-derived bis(boryl)acetal can be successfully isolated and utilized as an effective methylene transfer reagent for the formation of C—N and C—P bonds,as shown in Fig.17.
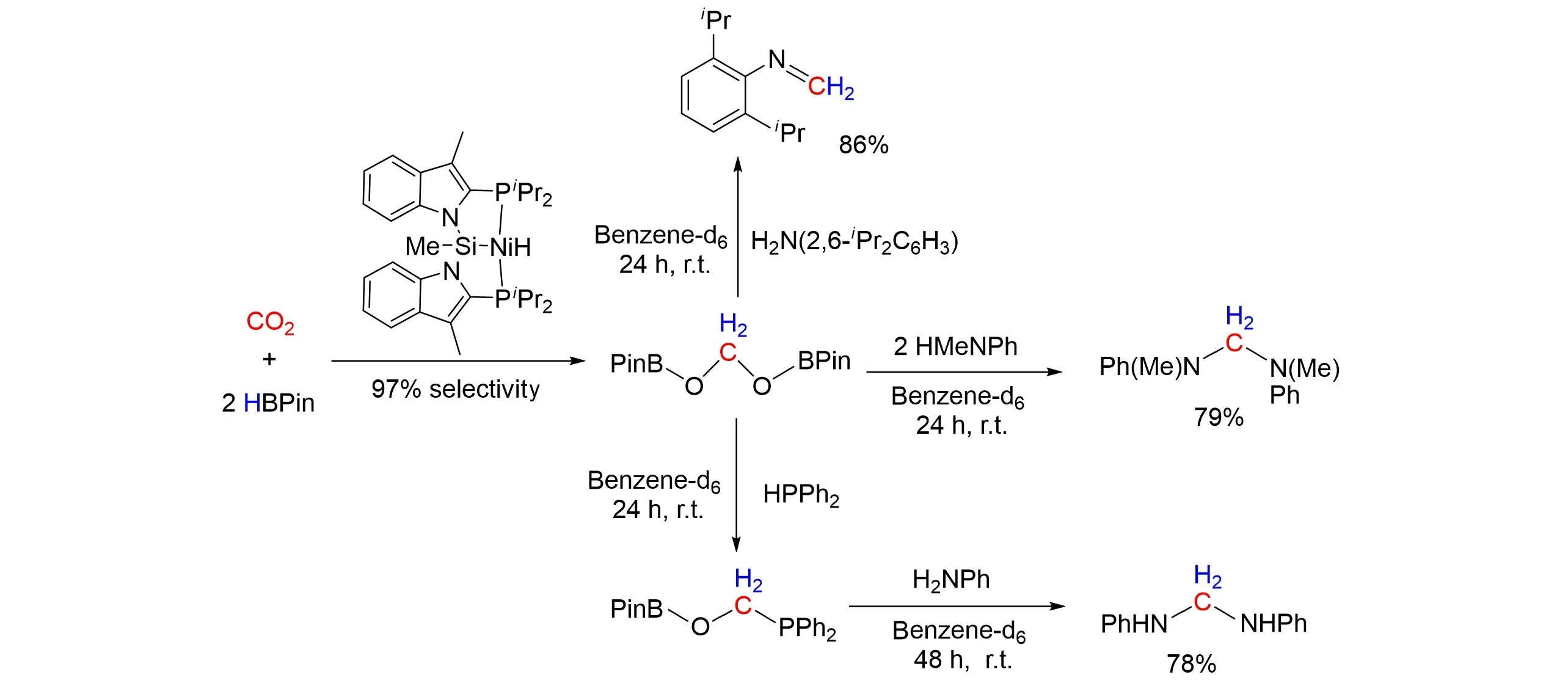
Fig.17 Selective hydroboration of CO2 to the formaldehyde level by Ni⁃catalyst
Ruthenium complexes are known to be efficient catalysts for the reductive functionalization of CO2.In 2012,Sabo-Etienne’s group[59]reported a catalyzed reduction of CO2by bis(dihydrogen)complex[RuH2(H2)2(PCy3)2](Cy=cyclohexyl)with pinacolborane(HBpin),as shown in Fig.18. After the detailed NMR spectroscopy study using labeled13CO2,multinuclear NMR spectroscopy analyses demonstrated the formation of(pinB)2O,pinBO13CH3,pinBO13CHO,pinBO13CH2OBpin,and pinBO13CH2O13CHO. It disclosed an unprecedented reductive coupling of two CO2molecules,the species pinBO13CH2OBpin has not been reported with boron so far,and the species pinBO13CH2O13CHO represents the first direct reductive coupling of two CO2units. In 2014,they observed directly the free formaldehyde from the borane reduction of CO2catalyzed by analogous bis(dihydrogen)complex[60]. Guided by mechanistic studies,they disclosed the selective trapping of formaldehyde byin situcondensation with a primary amine affording the corresponding imine under mild conditions. Subsequent hydrolysis regenerated amine and afforded a formalin solution. It was confirmed that CO2can be used as a C1 feedstock to produce formaldehyde by experiments for the first time.
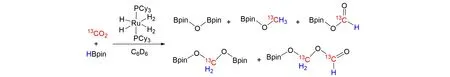
Fig.18 Reduction of CO2catalyzed by bis(dihydrogen)complex[RuH2(H2)2(PCy3)2]
At the same time,Stephanet al.[61]also developed an acidic ruthenium species with a pendant phosphine donor in 2012. They utilized a hybrid approach by exploiting both transition-metal systems and the concept of FLPs for activa⁃tion to CO2reduction. This FLP-like system was shown to capture and activate CO2for the catalytic reduction by HBpin to yield MeOBpin and O(Bpin)2. Analogous products were also obtained with catecholborane and 9-BBN,as shown in Fig.19.
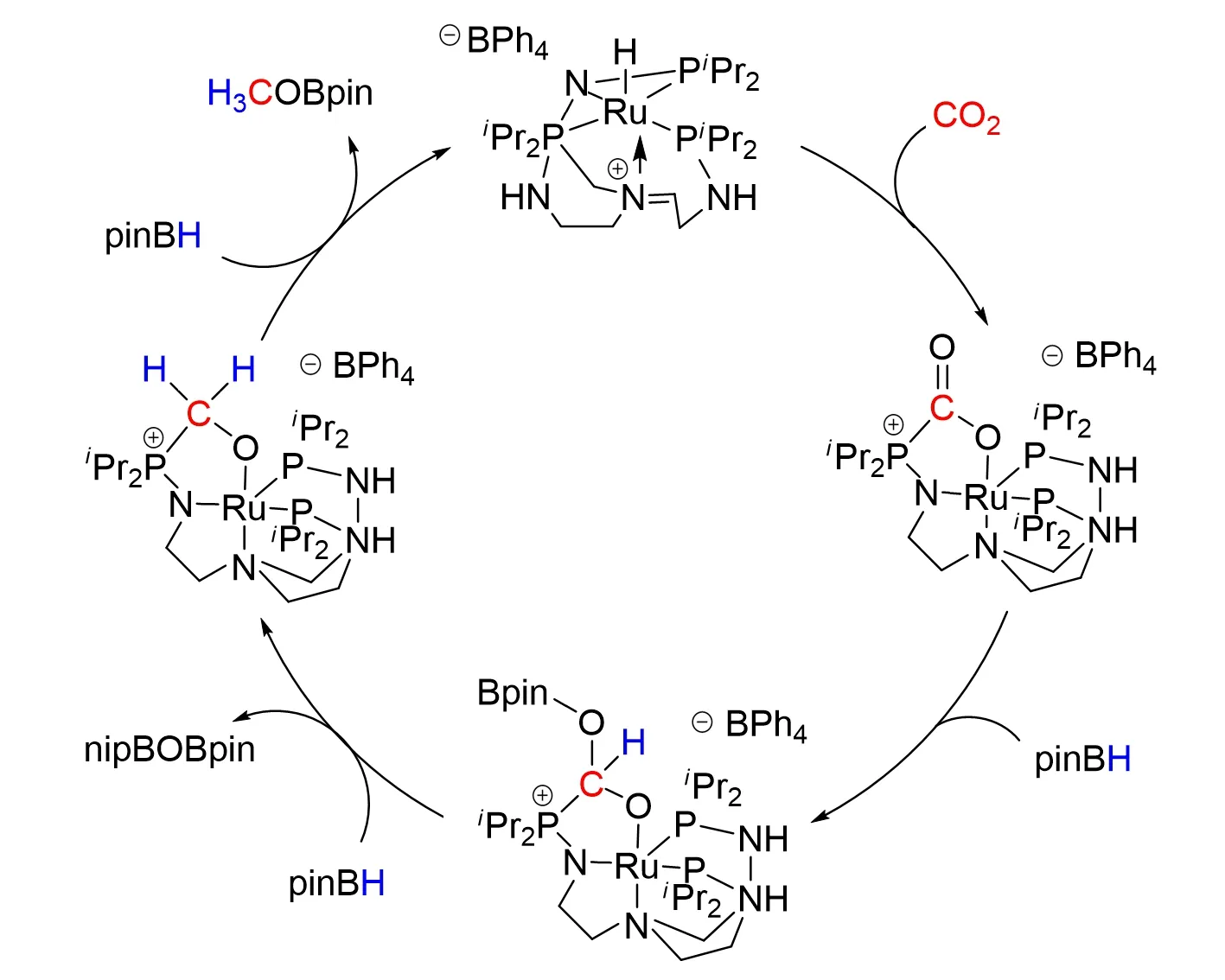
Fig.19 Frustrated Lewis pair inspired CO2 reduction by a ruthenium tris(aminophosphine)complex
Based on thatN-heterocyclic carbene copper(I)complexes could serve as efficient catalysts for the carboxylation of various nucleophiles,Hou’s group[62]reported the first catalytic boracarboxylation reaction of alkynes with diborane and CO2usingN-heterocyclic carbene copper complex as a catalyst,which gave a novel family ofα,β-unsaturatedβ-boralac⁃tone derivatives,as shown in Fig.20(A). In this catalytic system,the NHC-copper complex[(SIMes)CuCl]reacted with LiOtBu,affording[(SIMes)Cu-(OtBu)]firstly,which upon reaction with B2(pin)2generated the borylcopper complex[(SIMes)CuB(pin)]. Then,an alkyne was inserted into the Cu—B bond,givingβ-boryl alkenylcopper complex. Subsequently,nucleophilic addition of the alkenylcopper species to CO2followed by replacement of the Cu moiety in the resulting carboxylate with the B atom gaveβ-boralactone derivative. Final⁃ly,β-boralactone derivative regenerated[(SIMes)Cu(OtBu)]and released the final product by the transmeta⁃lation reaction. In 2016,they described the selective multi-component coupling of CO2,bis(pinacolato)dibo⁃ron,and various aldehydes using NHC-copper catalyst[63],as shown in Fig.20(B). The catalytic reaction mechanism was similar to that of the catalytic boracarboxylation reaction of alkynes with diborane and CO2usingN-heterocyclic carbene copper complex. This protocol could afford a series of novel lithium cyclic boracarbonate ion pair compounds with available starting materials. It can not only provide a practical catalytic process for the utilization of CO2,but also offer a novel route for the synthesis of lithium borate compounds that might be of interest as potential electrolyte candidates for lithium ion batteries.
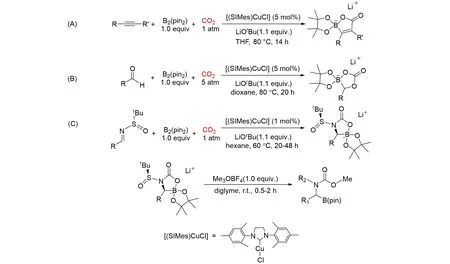
Fig.20 Boracarboxylation of nucleophiles with B2(pin)2 and CO2 by using NHC⁃copper complex
In 2020,Houet al.[64]developed an efficient and highly selective difunctionalization of iminesviaCO2activation by intramolecular N/B Lewis pairs into a copper catalytic cycle,as shown in Fig.20(C). It is important that a cyclic boracarbamate copper reactive intermediate was successfully isolated and full characterized. The computational investigations confirmed an unusual activation mode of CO2byα-borylalkylamidobased Lewis pair,which is different from traditional CO2insertion into transition-metal-element bonds. Using the reactivity of this unique lithium cyclic boracarbamate product,a series of attractiveα-amino boronic esters was explored. During the same period,Nozakiet al.[65]also reported the hydroboration of CO2to give a formic acid derivative using a copper/N-heterocyclic carbene as catalyst. And it was found that the HCO2B(pin)pro⁃duced in the present catalytic system could be directly used as a formylation reagent for various amines.
The carboxylation of allenes with CO2is a potentially significant protocol for the formation of unsaturated carboxylic acids. In 2014,Hazari’s group[66]studied the mechanism of the catalytic carboxylation of allenes usingCyPSiP[CyPSiP=Si(Me)(2-PCy2-C6H4)2]supported Pd complexes. And they have isolated and characterized all of the proposed intermediates in the catalytic cycle. The mechanistic study allowed them to exploit a kind of new catalyst for the hydroboration of CO2,which gave a maximum turnover number(TON≥60000)to date,as shown in Fig.21.
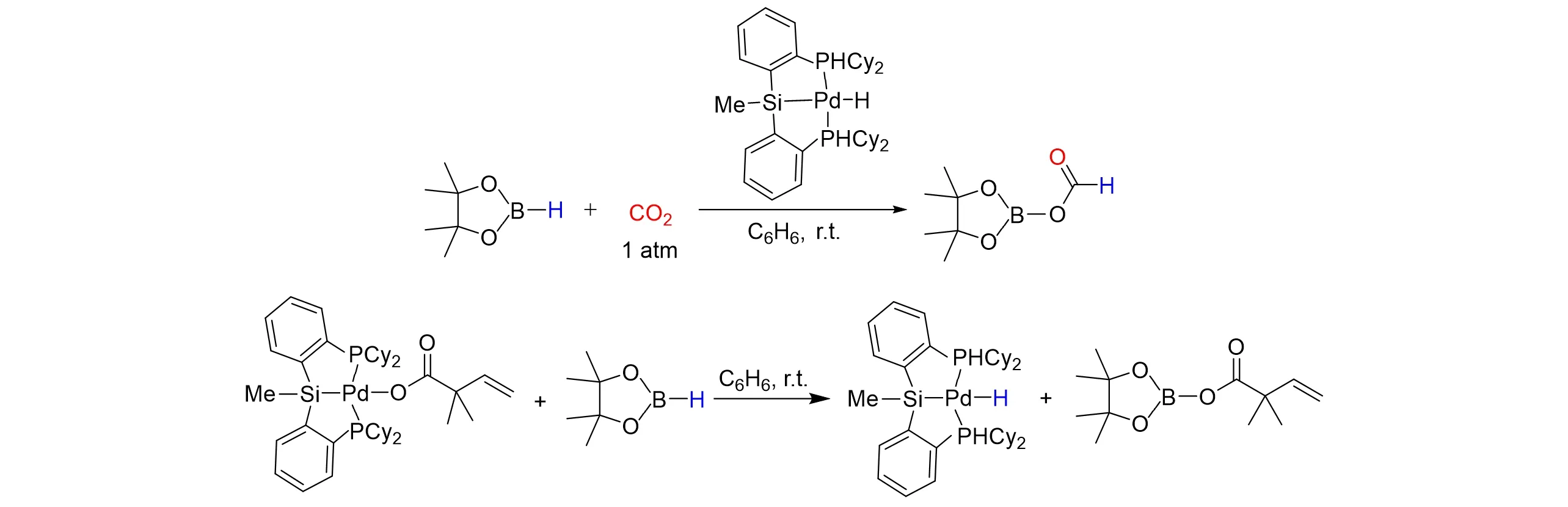
Fig.21 Catalytic carboxylation of allenes using CyPSiP supported Pd complexes
Bontempset al.[67]choosed the dihydride iron complex[Fe(H)2(dmpe)2]as catalyst precursor for the selective reduction of CO2into either bis(boryl)acetal or methoxyborane using hydroborane as reductant. As shown in Fig.22,thein situgenerated bis(boryl)acetal was shown to be a versatile and reactive methylene agent to construct C—N,C—O,C—C,C=N,and C=C bonds in a one-pot two-steps strategy. These results further highlight the importance of iron hydride complexes in the controlled reductive functionalization of CO2. In 2021,they achieved the selective reductive dimerization of CO2into glycolaldehyde in a one-pot two-step process[68].

Fig.22 Iron⁃catalyzed selective reduction of CO2 into bisborylacetal and subsequent functionalization
In 2018,Findlateret al.[69]reported an effective and higher selective reduction of CO2to methanol using a simple cobalt precatalyst under mild reaction conditions. The“Co-H”species generated by the addition of NaHBEt3to Co(acac)3was proved to be essential for the formation of catalyst. After scanning several different borylating reagents,the BH3·SMe2afforded exclusive conversion to methanol(98%yield)upon hydrolysis,as shown in Fig.23.
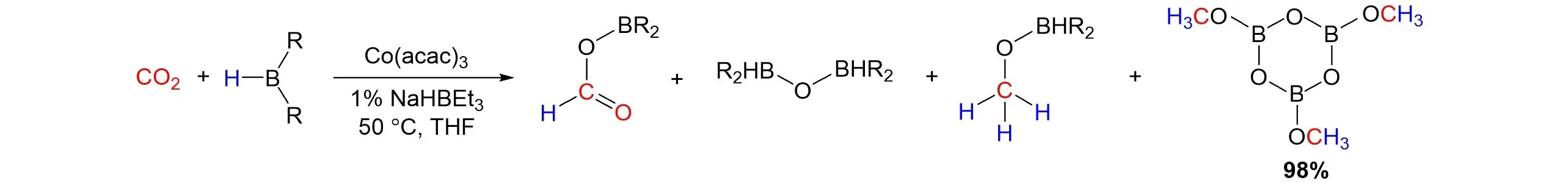
Fig.23 Cobalt⁃catalyzed hydroboration of CO2 with borane
4.2 Main Group Metal-catalyzed Reduction of CO2 with Borohydrides
Due to the ambiphilic molecules possesses both an electrophilic carbon atom and nucleophilic oxygen atoms,it maybe has a potential significant impact on the activation and functionalization of CO2. In 2013,a novel ambiphilic species Al[C6H4(o-PPh2)]3was synthesized and fully characterized through single crystal X-ray structural analysis by Fontaine’s group[70]. Its structure exhibits pseudo-bipyramidal-trigonal geometry caused by the two Al—P interactions. Remarkably,it reacted with CO2in a reversible way and generated a new CO2coordination complex. This ambiphilic species served as a precatalyst for the reduction of CO2using catecholborane(HBcat)to produce CH3OBcat,which can be readily hydrolyzed to methanol,as shown in Fig.24. In 2019,Inoueet al.[71]reported the applicability of various aluminum and boron hydride complexes as well as the organic superbase for the catalytic reduction of CO2with different hydride sources.The results indicated the“free”bis(NHI)ligand was the most active precatalyst,and the bulkier aluminum hydride exhi-bited comparable conversion rates,while the less congested aluminum hydride was significantly less active.

Fig.24 Reduction of CO2 with catecholborane using tris(triphenylphosphine)aluminum ambiphilic precatalyst
In 2021,Soet al.[72]reported bis(phosphoranyl)-methanido aluminum hydride([ClC-(PPh2NMes)2AlH2])catalyzed hydroboration of CO2. Using BH3·SMe2instead of HBpin,the catalytic reaction was higher selective,resulting in the formation of trimethyl borate B(OMe)3. This aluminum hydride species not only efficiently reduces CO2with HBpin to form methoxyborane,but also shows a good catalytic activity toward the hydroboration of carbonyl,nitrile,and alkyne derivatives into borate esters,diboryl amines,andtrans-boryl alkenes,respectively.
In addition,Aldridgeet al.[73]reported the synthesis of a single-component ambiphilic system capable of cooperative activation of protic,hydridic,and a polar H—X bond across a gallium/ activatedβ-diketiminato ligand framework,as shown in Fig.25. The hydride complex derived from the activation of H2is a competent catalyst for the selectivity to a methoxy derivativity using HBpin. It represents the first example of selective reduction of CO2catalyzed by a molecular gallium species.
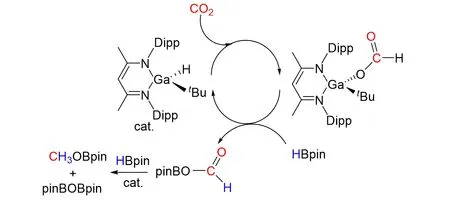
Fig.25 Reduction of CO2 to form MeOBpin by HBpin catalyzed by gallium hydride
Xuet al.[74]synthesized neutral molecular zinc(II)dihydrides supported byN-heterocyclic carbene ligands bearing a tethered phosphine arm. The obtained zinc dihydrides were found to be active catalysts for hydroboration of CO2under mild conditions,as shown in Fig.26. The selectivity of the hydroboration reactions into boryl formate,bis(boryl)acetal,or methoxy borane compounds can be easily controlled by varying the borane reagent. This study represents the first example of zinc hydride as catalyst in the hydrobora⁃tion of CO2.
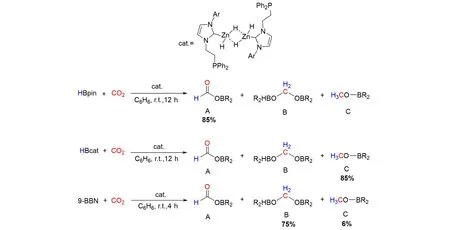
Fig.26 Zn⁃catalyzed reduction of CO2 with boranes
Okuda’s group[75]reported that a series of alkali metal hydridotriphenylborates[(L)M][HBPh3](M=Li,Na,K)was synthesized by treating M[HBPh3]with tris{2-(dimethylamino)ethyl}amine in THF. Their struc⁃tures were characterized by single crystal X-ray crystallography,the lithium and sodium compounds are sepa⁃rate ion pair,while the potassium compound is zwitterionic. These compounds could catalyze the hydrobora⁃tive reduction of CO2to give formoxyborane without any over-reduction,as shown in Fig.27. Especially,the lithium compound shows a remarkably high TOF of ≥17 s−1. In addition,they were used as efficient catalysts for the chemoselective hydroboration of a wide range of aldehydes and ketones with HBpin.
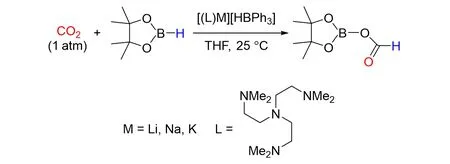
Fig.27 Hydridotriphenylborates catalyzed hydroboration of CO2 with HBpin
4.3 Organic Reagent-catalyzed Reduction of CO2 with Borohydrides
In 2013,Fontaineet al.[76]reported a metal-free system for the reduction of CO2in the presence of hydrob⁃oranes using 1-Bcat-2-PPh2-C6H4(cat=catechol)as organocatalyst. It generated exclusive formation of CH3OBR2with TOFs up to 853 h−1and TONs up to 2950 at 70 °C under 1 atm of CO2. The products can be readily hydrolyzed to methanol,as shown in Fig.28. The further results demonstrated that the 1-Bpin-2-PPh2-C6H4is also as an active catalyst for the CO2reduction using other hydroboranes including BH3·THF,BH3·SMe2,and 9-BBN. Then,they also described the hydroboration of CO2into methoxyboranes by boranedimethylsulfide using different catalysts in 2014[77]. Although limited activity was observed with monodentate species such as 1,8-diazabicycloundec-7-ene(DBU)and 2,2,6,6-tetramethyl-piperidine(TMP),bidentate amines such as proton sponge proved to be the active catalysts in this reaction. A non-nucleophilic proton sponge was found to be an active catalystviathe activation of BH3·SMe2into a boronium-borohydride ion pair,with TOF up to 64 h‒1at 80 ℃.

Fig.28 Reduction of CO2 with HBcat and 1⁃Bcat⁃2⁃PPh2⁃C6H4
Cantatet al.[78]described that the containing-nitrogen bases are able to promote the catalytic hydrobora⁃tion of CO2. Guanidine and amidine derivatives,such as triazabicyclo[4.4.0]dec-5-ene(TBD),Me-TBD and DBU,were proved to be a kind of active catalysts for this transformation. Among them,Me-TBD catalyzed the reduction of CO2to methoxyborane with TONs up to 648 and TOFs up to 33 h‒1at room temperature. As shown in Fig.29. It was worth noting that experimental and computational results showed that TBD and Me-TBD follow different mechanisms. Me-TBD promotes the reduction of CO2by activating the hydroborane reagent to form the ion pairs,while the TBD is converted into an N/B frustrated Lewis pair to activate CO2and facilitate the hydride transfer.
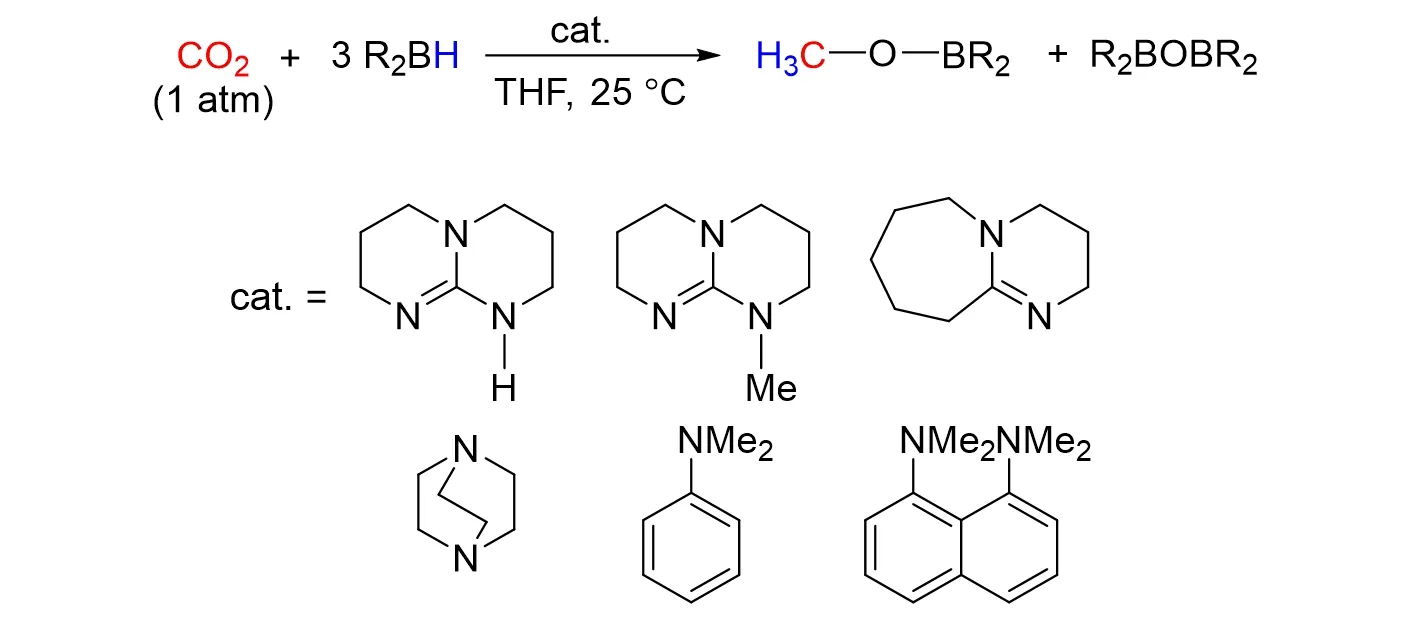
Fig.29 Reduction of CO2 to methanol with hydroboranes catalyzed by organobases
In the same year,they reported an unprecedented metal-free method for the methylation of N—H bonds with CO2in the presence of hydroboranes[79]. Proazaphosphatrane superbases prove to be highly active catalysts in this transformation,as shown in Fig.30. It is of interest that the efficiency of the metal-free methodology for the conversion of CO2to methylamines is comparable with the metal-catalyzed versions with H2or hydrosilanes as reductant. At the same time,Stephanet al.[80]reported the reactions of phosphine-derived carbenes with 9-BBN result in ring-expansion reactions to generate novel intramolecular frustrated Lewis pairs. These FLPs effected the catalytic reduction of CO2in the presence of boranes to give R2BOBR2[R=tBu,N(iPr)2]and methoxy-borate species.
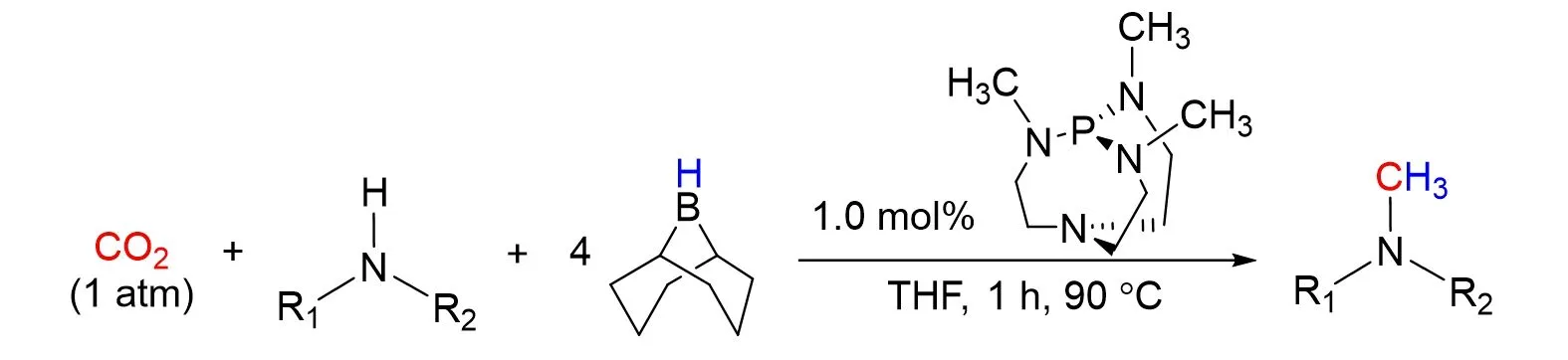
Fig.30 Methylation of amines with CO2 with hydroboranes
While Ong’s group[81]reported a series of carbodicarbene ligand frameworks that prepared by corporation of unsymmetrical pendant groups. And the carbodicarbenes used as organocatalysts for the methylation of amines with CO2in the presence of borane were investigated,as shown in Fig.31. The optimization study was performed with diphenylamine and 9-BBN under 1 atm of CO2using 10%(molar fraction)carbodicarbene catalyst. A unique B-H-activated species has been isolated and characterized by X-ray crystallographic studies. The key intermediate provided an important implication for the mechanistic understanding of the reaction as promoted not only by CDCs,but also by analogous NHCs.
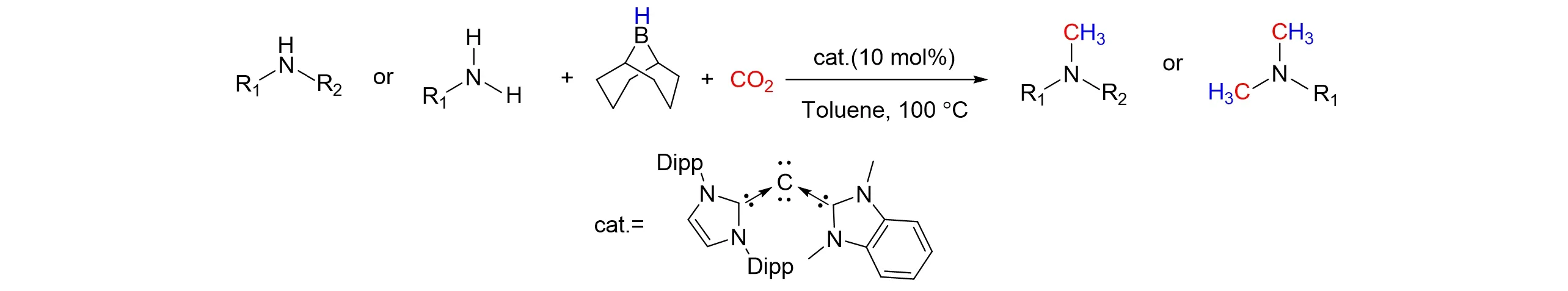
Fig.31 Methylation of amines with CO2 by using carbodicarbenes as organocatalysts
Cantatet al.[82]also reported a novel N/Si+FLP-CO2adduct prepared by the base-stabilized silylium species with CO2,these FLP-CO2adduct shows higher activity for the hydroboration of CO2to the methoxide level with 9-BBN,catBH,and pinBH,as shown in Fig.32. Based on experiment and DFT calculation results,two distinct mechanisms have been proposed,different from that the 9-BBN and catBH could reduce an intermediate FLP-CO2adduct,the hydroboration of CO2with pinBH followed a novel path where the B—H bond was activated by the silicon-based Lewis acid catalyst.
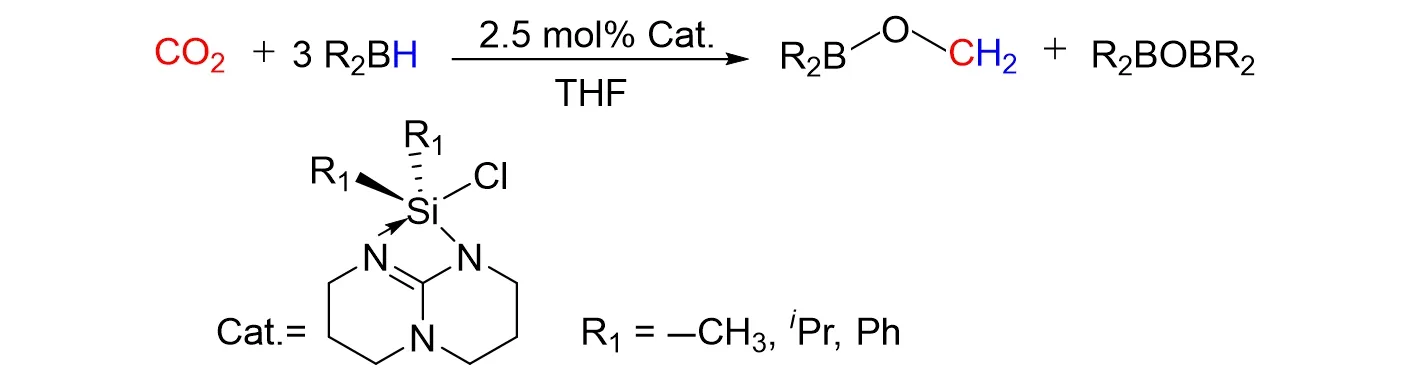
Fig.32 Hydroboration of CO2 to the methoxide level with 9⁃BBN,catBH and pinBH
As a special class of organic ligand with strong donor characteristics,N-heterocyclic olefins(NHOs)have received much attention recently. In 2016,Bhanageet al.[83]demonstrated their catalytic processes for theN-formylation of amines with 9-BBN as the reducing agent under mild conditions. The experimental results indicated that thein situgenerated zwitterionic NHO-CO2adducts play the important role for the activation of CO2,as shown in Fig.33. The syntheticN-heterocyclic olefins showed a high activity,and provide excellent turnover numbers and turnover frequencies.

Fig.33 NHOs⁃catalyzed formylation reaction with CO2
Dagorneet al.[84]used simple sodium,potassium,and tetrabutylammonium acetate salts to effectively catalyze the reduction of CO2by hydroboranes for the selective production of methanol-equivalent products,as shown in Fig.34. In addition to catalytic results,combined experimental and computational studies were also performed to provide insight into the hydroboration mechanism.
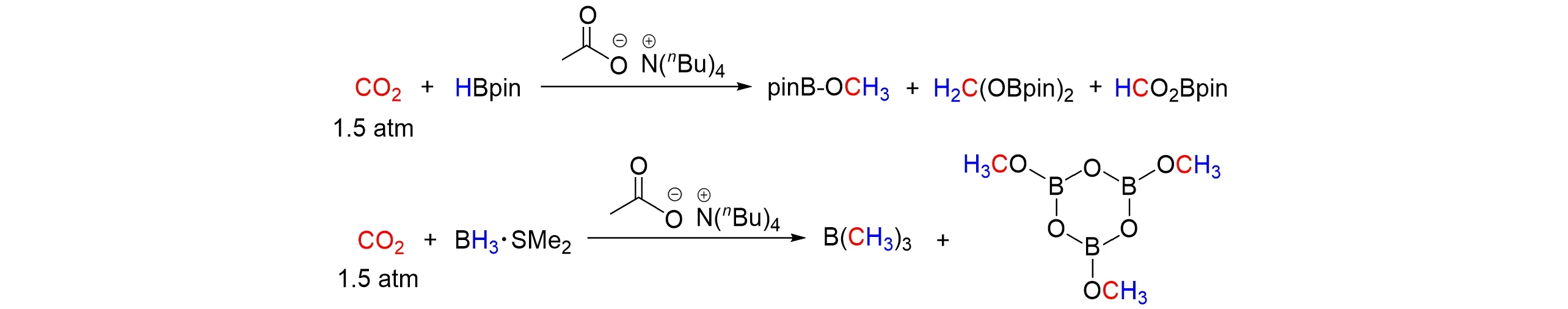
Fig.34 Acetate⁃catalyzed reduction of CO2 with HBpin/BH3·SMe2
Fernández-Galánet al.[85]detailedly studied the Ph2PCH2CH2B(C8H14)and its formaldehyde adduct in the catalytic reduction of CO2with four different hydroboranes and tried to identify mechanistically relevant inter⁃mediate species,as shown in Fig.35. The experiments proved the formaldehyde adduct of phosphinoborane and its formaldehyde-formate derivatives play an essential role in the catalytic hydroboration of CO2with four different hydroboranes. Reduction of CO2with H-BBN gave mixtures of CH2(OBBN)2and CH3OBBN using both catalysts. Reduction of CO2with HBcat led to MeOBcat exclusively. In contrast,using HBpin as the reductant usually led to mixtures of mono-,di-,and trihydroboration products of HCO2Bpin,CH2(OBpin)2,and CH3OBpin. In addition,only the formaldehyde adducts turned out to be active in the catalytic hydrobora⁃tion of CO2using BH3·SMe2,yielding a mixture of two methanol-level products[(OMe)BO]3and B(OMe)3.

Fig.35 Reduction of CO2 with different borohydrides using phosphinoborane as catalyst
6 Summary and Outlook
In this paper,we have summarized the current status,challenges and opportunities in the conversion of CO2into more valuable chemicals using borohydride reagents. Although there has been a lot of researches in this area,practical catalytic systems have proved elusive. The more active,stable,selective catalyst,and systems that do not require stoichiometric additives and harsh condition remains desired. The above method showed that boron reagents are important reducing agent for CO2reduction,which can selectively reduce CO2to value-added chemicals by 2e,4e,and 6e. It is not only suitable for the formation of organic sulfur and nitrogen compounds,but also for the synthesis of oxygen-containing organic compounds. In addition,catalysts such as ionic liquids used in organic synthesis can be recycled many times without significant decrease in activity.
As ideal one-carbon(C1)source,the application prospect of the reductive functionalization of CO2can not only reduce greenhouse gas in the atmosphere,but also help to convert CO2into high-value chemical products. For example,the selective formylation,methylenation,and methylation of mercaptan and thiophe⁃nol to organic sulfur compounds with CO2and borohydride reagents that can provide an adequate material basis for the synthesis of various drugs.
At present,although a larger number of catalytic systems and reduction methods have been reported for the reduction functionalization of CO2,it is still necessary to develop hydrogen as a reducing agent for the large-scale conversion of CO2when it is applied to industrial production. It is noted that the very efficient regeneration process of borohydride reagents has been reported recently. As one of the safest,inexpensive,most studied and exploited borohydrides sources,the commercial supply chain of sodium borohydride is already established. Importantly,a facile and cost-efficient,green method for regeneration of NaBH4from its aqueous hydrolytic product NaBO2by reacting it with CO2and Mg under the ambient conditions was developed. This made the borohydride reagent for CO2conversion an attractive reductant that can provide both cost as well as energy efficiency.

