A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas
Shuzhen Cheng, Di Wu, Hnxiong Liu, Xinbing Xu, Beiwei Zhu,*, Ming Du,*
a Department of Food Science and Engineering, National Engineering Research Center of Seafood, Dalian Polytechnic University, Dalian 116034, China
b Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food Science and Nutritional Engineering,China Agricultural University, Beijing 100083, China
ABSTRACT
A comprehensive method was applied to evaluate the anticoagulant activity of a novel anticoagulant peptide(NAESLRK) derived from oyster (Crassostrea gigas). The anticoagulant peptide drastically reduced the extrinsic clotting activity and also impaired the intrinsic clotting activity slightly. Consistent with clotting data, the thrombin peak height was reduced to 84.7 nmol/L from 123.4 nmol/L, and thrombin generation time was delayed to 4.67 min from 4.42 min when the extrinsic trigger was applied. The inhibitory kinetics of FXIa, FIXa, FXa, FIIa, and APC in a purified component system rationally explained the reduction of extrinsic clotting activity and impairment of thrombin generation. Besides the inhibition of FXa and FIIa activity, the activation processes of FX and FII by intrinsic/extrinsic tenase complex and prothrombinase were also damaged. The anticoagulant activity in the plasma system was the result of comprehensive inhibition of various factors. The research provided a novel method for anticoagulant evaluation and inhibitory mechanism of bioactive peptides from food products.
Keywords:
Oyster
Anticoagulant peptide
Inhibitory kinetics
Coagulation factor
Non-specific inhibitor
1. Introduction
Cardiovascular diseases (CVDs), which are a group of disorders of the heart and blood vessels, are the number 1 cause of death globally, and more people die annually from CVDs than from any other causes (https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)). According to the World Health Organization (WHO) statistics in June 2021, ischaemic heart diseases and stroke, two main components of CVDs, remained the world’s biggest killers, causing 17.9 million deaths, which accounted for 32% of all deaths worldwide in 2019 (Fig. S1). Pathologic and angiographic reports had documented intraluminal thrombi in unstable angina and acute myocardial infarction, which were two typical myocardial ischemia diseases [1-3]. Thrombosis was associated with three of the four causes of ischemic stroke in which blood supply to part of the brain was decreased, leading to dysfunction of the brain tissue in that area [4,5]. Besides ischaemic heart diseases and stroke, thrombosis was also involved in other CVDs, such as deep vein thrombosis and pulmonary embolism [6,7]. Currently, abundant evidence proved that thrombosis was involved in a group of heart and blood vessels disorders by preventing blood from flowing to the heart or brain. Thrombi, the primary blockage, was one of the chief culprits of a series of CVDs.
There were many risk factors for thrombosis, such as tobacco,high cholesterol, obesity, cancer, diabetes, inactive lifestyle, etc.Virchow’s triad gave the leading causes of thrombosis, which listed thrombophilia, endothelial cell injury, and disturbed blood flow [8].The factors which could induce the three broad categories of causes all might be involved in thrombosis. At least three-quarters of the world’s deaths from CVDs occurred in low- and middle-income countries. People in low- and middle-income countries who suffered from CVDs and other noncommunicable diseases had less access to adequate and equitable health care services which responded to their needs. As a result, many people in low- and middle-income countries were detected late in the disease course.
Thrombosis in pathological and hemostasis in physiological were two extraordinarily similar and intricate processes involved in the blood vessels, platelets and coagulation factors. On various occasions (i.e. vascular trauma, inflammation, sepsis, etc.),hemostasis and thrombosis would be initiated/induced. Coagulation cascade is a complex reaction that involves amounts of coagulation factors. It contains intrinsic and extrinsic pathways. The common pathway is followed (Fig. 1). Based on the thrombotic pathobiology,antithrombotic agents could mainly be classified into three major categories, i.e., anticoagulants, antiplatelets and thrombolytics. Table S1 and Fig. S2 summarized the action mechanism, administration route and side effects of main antithrombotic agents and their part structure in clinical in recent years. Although the drugs had an excellent therapeutic effect on thrombus, the absence of an adequate reversal strategy to prevent and stop potential life-threatening bleeding complications was a major drawback to the clinical use of these anticoagulants [9].
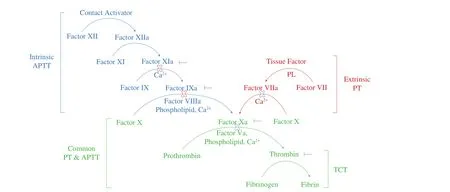
Fig. 1 The coagulation cascade. This depicts the intrinsic, extrinsic and common pathway of the revised model of the coagulation cascade. It provides a framework for interpreting basic coagulation tests - the activated partial thromboplastin time (aPTT), prothrombin time (PT) and thrombin time (TT). aPTT is a re flection of the intrinsic pathway. Isolated prolongations of aPTT should prompt considerations of factor VIII, IX, XI or XII deficiency. PT is a re flection of the extrinsic pathway. Isolated prolongations of the PT are most often due to factor VII deficiency. Deficiencies in factors V, X, thrombin, andfibrinogen prolong both aPTT and PT, as they are in the common pathway. TT is a measure of thefinal step in the coagulation pathway, the conversion offibrinogen tofibrin via the action of thrombin. It is hence sensitive to deficiencies infibrinogen and drugs such as direct and indirect thrombin inhibitors. The activities and activation processes of FX(a) and prothrombin were inhibited by P-3-CG were indicated.
With the improvement of human health consciousness, the relationship between food and many prevalent diseases (such as thrombus) is under extensive research [10-13]. Functional foods,defined as “those that contain a component that benefits a limited number of functions in the body, providing welfare and health,reducing the risk of disease”, have been in a rapidly developing phase [14-16]. As the main component of functional foods, bioactive peptides derived oyster showed satisfactory health effects on antioxidant and anticancer activity [17], antimicrobial properties [18],antihypertensive activity [19,20], sexual reproduction promoting activity [21,22], anti-inflammatory activity [23] and antithrombotic activity [24,25]. 4 325 peptides involving approximately 50 types of bioactivities were included in the BIOPEP database(accessed 07-07-2021) [26,27]. The peptides with the antithrombotic activity were summarized into Table S2 based on their source, amino acids sequence, action site, etc.
Although many researchers had studied various anticoagulant substances derived from food products, they only focused on the anticoagulant activity of bioactive substances by determining the plasma clotting activity [28-30]. The anticoagulant mechanism of bioactive substances was not fully clear, which was not conducive to developing anticoagulant substances, even other bioactive products from food. Some researchers tried to explain the anticoagulant mechanism of bioactive products from food by determining the association of bioactive substances and targeted enzymes [24,25,31,32].Besides examining the binding capacity between inhibitors and targeted enzymes, the method to research the inhibitory activity thoroughly in coagulation cascade needed to be established to study the anticoagulant mechanism of bioactive substances from food products and promote food-derived food functional anticoagulants.
By the classical approach (oyster protein isolation, protein hydrolysis, hydrolysate isolation, peptides identification), a novel anticoagulant peptide (NAESLRK, named by P-3-CG) from oyster pepsin hydrolysate was obtained [25]. We aimed to examine the coagulation factors activity under our anticoagulant peptide condition similar to other research and study the inhibition of the coagulation factor activation dynamics process. We explained the clotting activity reduction and thrombin generation decreasing in the plasma system that P-3-CG, a non-specific inhibitor, presented anticoagulant activity by inhibiting FX(a), FIX(a), FII(a) and so on. As far as we know, this was thefirst time to provide such a frame to explore the anticoagulant mechanism of anticoagulant bioactive substances from food products.
2. Material and methods
2.1 Material and reagents
Normal pooled plasma (NPP) was from Sanquin (Amsterdam,the Netherlands). FII-depleted, FV-depleted, FVIII-depleted, FIX-depleted, FX-depleted and FXI-depleted human plasma, Neoplastine CI Plus 10 PT reagent and TriniCLOT automated aPTT reagent were from Diagnostica Stago (Paris, France). The peptidyl substrate N-α-benzyloxycarbonyl-D-Arg-Gly-Arg-pNA (S2765), H-D-Phe-Pip-Arg-pNA (S2238) and pyroGlu-Pro-Arg-pNA·HCl (S2366)were from Instrumentation Laboratories (Bedford, MA, USA), and spectrozyme FXa was from Sekisui Diagnostics (Stamford, CT,USA). The Pefachrome FIXa was from Pentapharm (Dornacherstrasse,Switzerland). Dansylarginine-N-(3-ethyl-1,5-pentanediyl) amide(DAPA) was from Haematologic Technologies (Essex Junction, VT).Phospholipid vesicles (0.5 mmol/L, PCPS) composed of 42% (m/m)phosphatidylcholine (PC), 28% (m/m) phosphatidylserine (PS)and 30% (m/m) sphingomyelin was from Rossix (Taljegårdsgatan,Mölndal, Sweden). Calibrator and fluorescent substrate (FluCa) were from Thrombinoscope (Maastricht, the Netherlands). The SDS-PAGE loading buffer, sample reducing agent (DTT) and Invitrogen NuPAGE Bis-Tris protein gels were from ThermoFisher (American, Waltham,Massachusetts). All functional assays were performed in HEPES-buffered saline (HBS: 20 mmol/L HEPES, 150 mmol/L NaCl, pH 7.5)supplemented with 5 mmol/L CaCl2, 0.1% PEG8000, and filtered over an 0.2 μmfilter (assay buffer, AB).
2.2 Proteins
The human plasma-derived coagulation proteins FXI(a), FX(a),FIX(a), FVIIa, prothrombin, α-thrombin, protein C and activated protein C were from Haematologic Technologies (Essex Junction,VT, USA). NovoEight was from Novo Nordisk (Plainsboro, NJ,USA). The human tissue factor (TF, Innovin) was from Siemens(Newark, NY, USA), and recombinant hirudin was from Hyphen Biomed (Neuville-sur-Oise, France).
The oyster-derived peptide NAESLRK (designated peptide P-3-CG) was synthesized and purchased from Genscript (Piscataway,NJ, USA) and dissolved in dilution buffer (DB, 20 mmol/L HEPES,150 mmol/L NaCl, 0.1% PEG-8000, pH 7.5) at 5.0 mmol/L.
2.3 The inhibitory ability of P-3-CG towards normal or specific clotting activity
The normal extrinsic clotting activity was determined using a normal PT-based assay [9]. The anticoagulant peptide (25 μL) was serially diluted and incubated with normal pool plasma (25 μL) for 60 s at 37 °C. Coagulation was initiated by adding 50 μL PT reagent,and the time to fibrin clot formation was monitored using a Start4 coagulation instrument (Diagnostica Stago). The normal intrinsic clotting activity was determined using a normal aPTT-based assay [9].Normal pool plasma (25 μL) was mixed with serially diluted anticoagulant peptide (25 μL) and aPTT reagent (50 μL), followed by a 180 s incubation period at 37 °C. Coagulation was initiated by adding 25 μL of 25 mmol/L CaCl2, upon which the time tofibrin clot formation was monitored.
The specific extrinsic clotting activity was determined using a modified coagulation factors-specific PT-based assay. Plasma-derived factors (FII, FV and FX) were diluted to normal plasma concentration based on Table S3 in assay buffer with 0.1% BSA that contained serially diluted anticoagulant peptide. The specific intrinsic clotting activity was determined using a modified coagulation factors-specific aPTT-based assay. Plasma-derived factors (FII, FV, FVIII, FIX, FX and FXI) were diluted to normal plasma concentration based on Table S3 in Owren-Koller buffer that contains serially diluted anticoagulant peptide. Other procedures were the same with normal PT/aPTT-based assay. Reference curves consisted of serial dilutions of NPP.
2.4 The inhibitory ability of P-3-CG towards coagulation factors activity
The inhibitory ability of P-3-CG towards key coagulation factors(Factor IIa, FIXa, Factor Xa, Factor XIa, and activated protein C)were assessed with their corresponding chromogenic substrates.Briefly, the coagulation factors (10 nmol/L) were incubated with serially diluted anticoagulant peptide (0–1 mmol/L) for 2 min at room temperature. The chromogenic substrates were added into the pretreated coagulation factors-peptide mixes and monitored kinetics for 30 min at 405 nm immediately. The chromogenic substrate for factor IIa (thrombin) was S2238 (400 μmol/L), for factor IXa was Pefachrome FIXa (2 mmol/L), for FXa were SpecXa (250 μmol/L)and S2765 (250 μmol/L), For factor XIa and APC was S2366(100 μmol/L). The raw data was normalized with blank control. The inhibitory constant (IC50) was determined byfitting the data sets to a one-phase decay function with GraphPad Prism 8.0.1 software.
2.5 The inhibitory ability of P-3-CG towards coagulation factors dynamics activation process
The prothrombin was converted into thrombin by prothrombinase.Steady-state initial velocities of macromolecular substrate cleavage were determined discontinuously at 37 °C in assay buffer [33].Progress curves of prothrombin activation by FXa that was incubated previously with P-3-CG (1 mmol/L) for 2 min at 37 °C were obtained by incubating PCPS (50 μmol/L) and hFVa (20 nmol/L) for 5 min, and the reaction was initiated by adding of plasma-derived prothrombin (400 nmol/L). At selected time points, aliquots were quenched in quench buffer (dilution buffer with 50 mmol/L EDTA)and determined the formed thrombin with chromogenic substrate S2238. Aliquots of the reaction mixtures were also withdrawn at the indicated time intervals and quenched in loading buffer-DTT mixes immediately. The samples for SDS-PAGE were cooked at 100 °C for 10 min and loaded on high-performance precast polyacrylamide gels.
2.5.1 Factor X was activated by the human intrinsic and extrinsic tenase complex
Coagulation factor X can be activated by the extrinsic tenase complex (TF-FVIIa-PCPS) and intrinsic tenase complex (FVIIIa-FIXa-PCPS). For the activation with extrinsic tenase complex, tissue factor (100 pmol/L) and PCPS (50 μmol/L) were pre-incubated for 15 min at 37 °C, and factor VIIa and anticoagulant peptides (1 mmol/L)were pre-incubated for 2 min at 37 °C. The pre-warmed factor VIIapeptides complex was added into the pre-treated TF-PCPS complex for another incubation for 5 min. The reaction was initiated by the addition of factor X (250 nmol/L). At selected time points, aliquots were quenched in the quench buffer. The amidolytic activity of each timepoints sample was determined by SpecXa conversion(250 μmol/L), measuring the absorbance at 405 nm. The initial rates of chromogenic substrate hydrolysis were converted to nanomolar of the product by reference to an FXa standard curve.
For the activation with intrinsic tenase complex, a similar approach with extrinsic activation was used. FVIII (NovoEight,40 nmol/L) was activated by thrombin (100 nmol/L) for 30 s at 25 °C, and the reaction was stopped by adding a 10-fold molar excess of hirudin. The activated FVIIIa-PCPS complex was added into the pre-incubated FIXa (0.5 nmol/L)-P-3-CG (1 mmol/L) system immediately. The reaction was initiated by the addition of factor X(250 nmol/L). At selected time points, aliquots were drawn as chromogenic and SDS-PAGE samples.
2.5.2 Factor IX was activated by the human extrinsic tenase complex and factor XIa
The processes of factor IX activation by human extrinsic tenase complex and factor XIa were assessed via the SDS-PAGE approach.The protocol of FIX activation by extrinsic tenase complex was similar to that of FX, and the only difference was that the substrate was changed into factor IX (1.8 μmol/L). Factor XIa (50 nmol/L) was pre-treated with P-3-CG (1 mmol/L) for 2 min at 37 °C, and FIX was added into the mix to initiate the reaction. Aliquots were taken and quenched in loading buffer at selected time points and analyzed by SDS-PAGE.
2.6 The inhibitory ability of P-3-CG towards thrombin generation in plasma
Thrombin generation (Calibrated Automated Thrombogram,CAT) was adapted from the protocol earlier described [34]. The diluted normal pool plasma (5-fold) was incubated with trigger and serially diluted anticoagulant peptide at 37 °C for 10 min in the Thrombinoscope Instrument. The reaction system was initiated by adding chromogenic substrate buffer (FluCa) to the plasma. Thefinal reaction volume was 120 μL, of which 80 μL was plasma (included anticoagulant peptide), 20 μL was the trigger, and 20 μL was FluCa.Thrombin formation was determined every 15 s for 60 min and corrected for the calibrator using Thrombinoscope software.
The specific thrombin generation in coagulation factor (FII,FIX and FX) deficient plasma was conducted by supplementing the corresponding coagulation factor, which was the same concentration as that in normal plasma (Table S3). The coagulation factor was incubated previously with anticoagulant peptides. Other procedures for coagulation factor deficient plasma were the same as that of normal pool plasma. The extrinsic pathway trigger (TF, 1 pmol/L) and intrinsic pathway triggers (Factor XIa, 0.25 nmol/L, silica, 1 nmol/L)were used in thrombin generation in NPP or coagulation factor deficient plasma.
2.7 Data analysis
All data are presented as mean ± standard deviation. Statistical analyses were performed using the one-way ANOVA, with the software of GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, USA).
3. Results and discussion
3.1 The inhibitory ability of P-3-CG towards normal/specific clotting activity
Firstly, the inhibitory ability of P-3-CG to normal intrinsic and extrinsic clotting activity was examined in normal pool plasma(Fig. 2A). The intrinsic and extrinsic clotting activity of normal pool plasma was defined as 1 U/mL. The intrinsic/extrinsic clotting activity of diluted NPP and aPTT/PT were respectively transformed by logarithmic and plot the standard curve based on linear regression(Fig. S3). According to the standard curve, the clotting time of normal pool plasma treated with different concentrations of P-3-CG was processed into intrinsic/extrinsic clotting activity. The inhibitory ability of P-3-CG was normalized based on blank control plasma without anticoagulant peptides. The data processing method of coagulation factor deficient plasma was the same as that of normal pool plasma. Liu et al. [35] isolated and identified an anticoagulant peptide from beta-casein which prolonged aPTT to 65 s from 54 s at 13 mmol/L. Xu et al. [36] isolated and identified an anticoagulant peptide from lactoferrin which prolonged PT to 22 s from 15 s at 20 mmol/L. Although these peptides have shown anticoagulant activity, their concentration were all much higher than P-3-CG.Furthermore, the characterization of anticoagulant peptides activity using the prolongation of aPTT/PT was not reliable to compare different research as different experiment conditions. The normalization of different anticoagulant peptides inhibitory clotting activity with normal pool plasma would be a good method to solve the problem. The addition of P-3-CG into plasma reduced its clotting activity (Fig. 2A). P-3-CG can inhibit the intrinsic and extrinsic clotting activity in a dose-dependent manner, while the inhibitory ability to intrinsic clotting activity was lower than extrinsic clotting activity. It demonstrated that P-3-CG might not only inhibit intrinsic clotting factor (VIII, IX, XI and XII) but also inhibit intrinsic clotting factor (tissue factor) [37]. Consistent with normal pool plasma, the specific intrinsic and extrinsic clotting activity in coagulation factors deficient plasma could also be inhibited significantly by P-3-CG.
P-3-CG gave stronger anticoagulant activity (intrinsic specific clotting activity, aPTT activity) in intrinsic coagulation factor (factor XI, IX and VIII) deficient plasma (Figs. 2E and 2F), comparing with that of common coagulation factor (factor II, V and X) deficient plasma (Figs. 2B-2D). Here, we speculated that P-3-CG had a higher inhibitory ability to coagulation factors in the intrinsic pathway (FXI,FIX, etc.). P-3-CG showed higher extrinsic anticoagulant activity in FX, FV and FII deficient plasma (Figs. 2B-2D). Factor Va participated as a cofactor in prothrombin activation by increasing the Vmax of the reaction about 1 000-fold [38]. Thus, as a whole demonstrated that P-3-CG presented higher extrinsic anticoagulant activity in normal pool plasma (Fig. 2A). We concluded that the anticoagulant peptide was not a specific inhibitor for coagulation factors based on the clotting data.

Fig. 2 The normalized inhibitory ability of P-3-CG to intrinsic and extrinsic clotting activity in normal pool plasma (A), FX deficient plasma (B), FII deficient plasma (C), FV deficient plasma (D). The normalized inhibitory ability of P-3-CG towards intrinsic specific clotting activity in FIX and FXI deficient plasma (E),and FVIII deficient plasma (F). The clotting activity (U) and clotting time (s) were all transformed by logarithmic. Graphs represent the mean ± SD. One-way ANOVA followed by Tukey’s multiple comparisons test was performed; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
3.2 The inhibitory ability of P-3-CG to coagulation factors activity
The activated coagulation factors can activate its physiological substrate factor to be a cascade reaction. Then the final product,thrombin cleavages fibrinogen into fibrin. To test the activity of activated coagulation factors, chromogenic substrates were used.The reaction velocity of activated factors toward their specific chromogenic substrates was recorded and normalized by the blank control group (minus anticoagulant peptides). The IC50was determined byfitting the data sets to a one-phase decay function with GraphPad Prism 8.0.1 software.

Fig. 3 (Continued)
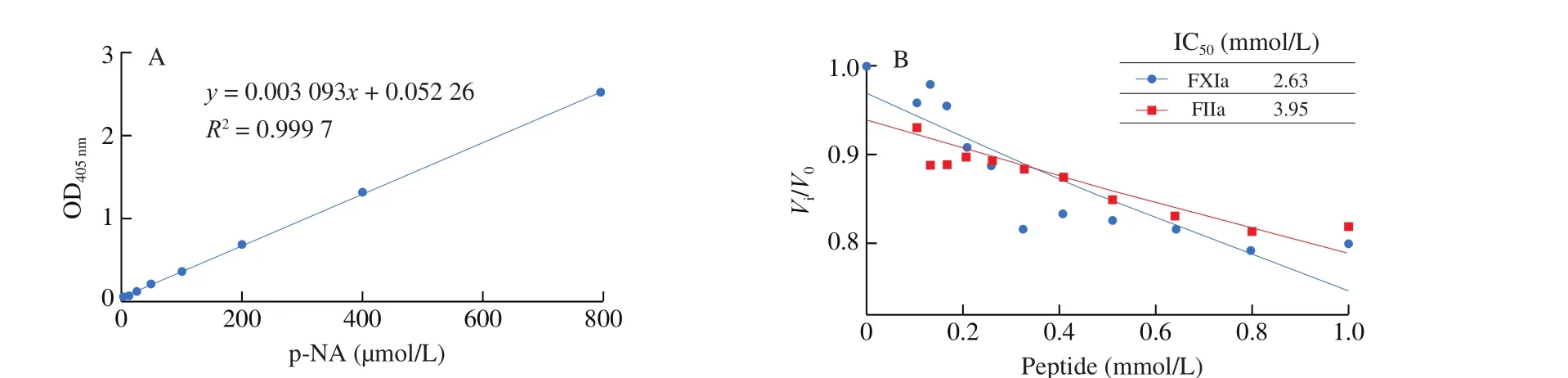
Fig. 3 The normalized inhibitory ability of P-3-CG to activated factors (thrombin, FXa, FIXa, FXIa and APC) in coagulation cascade system was assessed, upon which the IC50 was determined byfitting the data sets to a one-phase decay function with GraphPad Prism 8.0.1 software. The activity of FXa was assessed with two substrates (SpecXa and S2765). (A) p-NA standard curve line; the inhibitory rate towards (B) FIIa and FXIa, (C) FXa, (D) FIXa and APC.
3.3 The inhibitory ability of P-3-CG to coagulation factors activation
3.3.1 The activation of FIX by factor XIa and extrinsic tenase complex (TF-FVIIa-PCPS)
As a contactor trigger, the aPTT agent (silica) could activate factor XII and trigger intrinsic clotting (Fig. 1). Then factor XIIa cleaved factor XI into XIa [40]. We examined that P-3-CG could inhibit factor XIa action to its chromogenic substrate (IC502.63 mmol/L).Factor IX, the physiological substrate of factor XIa, was also used to examine the anticoagulant capacity of P-3-CG. It was clear that factor IX was activated into FIXa in a time-dependent manner (30–300 s)(Fig. S4A). Preincubation with P-3-CG (1 mmol/L) inhibits the factor XIa function to factor IX. Less factor IXa formed in plus anticoagulant peptides group, comparing with that of minus peptide groups (Figs. S4A and S4B). However, the inhibitory rate drastically reduced with time (38% at 30 s, 27% at 300 s).
Besides intrinsic factor XIa, factor IX could also be activated by extrinsic tenase complex (TF-FVIIa-PCPS) [41]. Surprisingly,the difference of FIX activation by extrinsic tenase complex and intrinsic coagulation FXIa was that the inhibitory ability of P-3-CG was not decreased with time (Figs. S4C and S4D). Conversely, the inhibitory rate was improved from 26% (15 min) to 33% (60 min).This supplied another explanation certification for higher extrinsic anticoagulant activity.
3.3.2 The activation of FX by intrinsic tenase complex (FIXa-FVIIIa-PCPS)
Activated factor IXa assemble with its cofactor FVIIIa on the negatively charged phospholipid into intrinsic tenase complex, which covers factor X into Xa [40]. We had examined the inhibitory rate to factor IXa by its chromogenic substrate (Pefachrome FIXa). P-3-CG had the potent inhibitory ability (IC500.71 mmol/L). Firstly, we examined the activation of factor X by intrinsic tenase complex by determining its chromogenic substrate (SpecXa). As we can see,the velocity of FXa generation (kcat) was inhibited by P-3-CG(Fig. 4A). Because the anticoagulant peptide could inhibit FXa cleavage its chromogenic substrate (Fig. 3C), the presence of anticoagulant peptides in the reaction system affected determining the formed FXa by the chromogenic method. To verify the inhibition for factor X activation by intrinsic tenase complex by P-3-CG furtherly,formed FXa was presented via SDS-PAGE (Fig. 4B). It was similar to FIX activation by intrinsic factor XIa (Fig. S2A). The inhibitory rate reduced with time. But the inhibitory rate of P-3-CG could go as high as 28% in 30 min (Fig. 4C).
3.3.3 The activation of FX by extrinsic tenase complex (TFFVIIa-PCPS)
The intrinsic and extrinsic pathways converge into a common pathway by factor X activation (Fig. 1) [40]. Thus, the factor X activation by extrinsic tenase complex was also examined with the presence of P-3-CG. It was similar to FX activation by intrinsic tenase complex. Formed FXa was also determined by chromogenic method and SDS-PAGE (Figs. 4D-4F). Although the FX activation velocity was not different significantly when the chromogenic method was applied (Fig. 4D), the grey-scale value of FXa heavy chain in SDS-PAGE was significantly lower under the presence of P-3-CG condition, especially at the initial stage (30 min) (Fig. 4E).Furthermore, the inhibitory efficiency was independent of time. The inhibitory rate was stable (~30%) with time.
采用RTK进行人工实测,利用测量仪器在潮位线附近每隔一定距离采集特征点,标记在数字地形图上并连接成高潮线、低潮线。外业核查比例不小于总岸线的10%。
3.3.4 The activation of prothrombin by prothrombinase(FXa-FVa-PCPS).
The activated FX assembled with its cofactor FVa at the negatively charged phospholipid into prothrombinase, which converted prothrombin into thrombin (Fig. 1) [40]. Although thrombin generation velocity was not different significantly when the chromogenic method was applied (Fig. 4G), we found the inhibitory effect from the SDS-PAGE profile that prothrombin was activated to thrombin in a time-dependent manner (Figs. 4G and 4H). It was strange that the grey-scale value of the thrombin lane at 180 s decreased in control groups. Maybe the reason for that was not homogeneity in the reaction system after 120 s.
Furthermore, the inhibitory activity also decreased with time. The anticoagulant peptides could inhibit thrombin cleave its chromogenic substrate (S2238) (Fig. 3B). The cofactor V could also be activated by thrombin in negative feedback [42]. To examine whether the anticoagulant peptide can inhibit thrombin activating cofactor V,the SDS-PAGE was used to show the generation of FVa. As we can see, there was no difference for the FVa lane between control and anticoagulant peptide treated groups (Figs. S4E and S4F). One apparent reason for the phenomenon is that the activation reaction time (1 min) was too long. Because thrombin can also activate another cofactor VIII in the same manner, and 30 s was enough to do it [33].
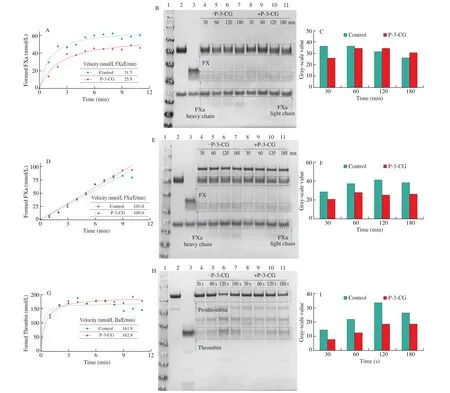
Fig. 4 The inhibitory ability of P-3-CG to FXa and thrombin generation in solution. Factor X was activated by intrinsic tenase complex (FIXa-FVIIIa-PCPS,A-C) and extrinsic tenase complex (TF-FVIIa-PCPS, D-F). Prothrombin was activated by prothrombinase complex (FXa-FVa-PCS, G-I). The grey-scale value of formed FXa and thrombin lanes were assessed with ImageJ software (C, F and I). Reduced protein (30 μL, 2 μg) was loaded to every lane.
3.4 The inhibitory ability of P-3-CG to thrombin generation in plasma
Thrombin generation in the purified-component system had been examined, P-3-CG had inhibitory potency. To link the earlyfindings obtained in the purified system to the clotting data, we studied thrombin generation in normal pool plasma and coagulation factor(FIX, FX and FII) deficient plasma supplemented with the plasma concentration FIX, FX and FII. The intrinsic triggers (FXIa and silica)and extrinsic triggers (TF) were used to initiate the reaction system(Fig. 5). Consistent with the results in the purified-component system,thrombin generation in normal pool plasma could also be impaired by P-3-CG. Thrombin generation impairment was independent of anticoagulant peptides concentration. Thrombin peak was drastically decreased, and LagTime was prolonged significantly when extrinsic pathway trigger TF was used (Fig. 5A). The phenomenon that the inhibitory efficiency to TF-FVIIa complex cleaving FIX was independent on time could also explain P-3-CG had a more potent effect on the extrinsic pathway (Fig. S4D). Furthermore, thrombin generation impairment was relatively slight when intrinsic pathway triggers were used, especially silica (Figs. 5B and 5C). It was consistent with the intrinsic anticoagulant activity of P-3-CG. The findings were consistent with clotting activity assays. P-3-CG had higher inhibitory potency to the extrinsic pathway.
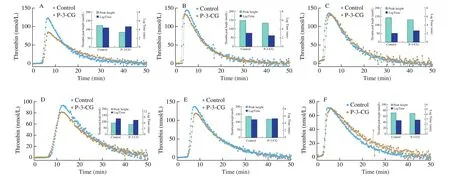
Fig. 5 The inhibitory ability of P-3-CG towards thrombin generation. Thrombin generation was measured for 60 min at 37 °C in NPP in the presence of 20 μm PCPS and 1 pmol/L tissue factor (A), 0.25 nmol/L FXIa (B), 1 nmol/L silica (C). Thrombin generation was measured for 60 min at 37 °C in factor X deficient plasma (D), factor IX deficient plasma (E) and factor II deficient plasma (F) in the presence of 20 μm PCPS and 1 pmol/L tissue factor. P-3-CG (1 mmol/L) was pre-incubated with NPP or factor deficient plasma for 10 min. We also indicated the thrombin peak height and thrombin generation time (lagtime) with a histogram(inset). Data sets are representatives of three independent experiments.
To link the clotting data in coagulation factor deficient plasma,thrombin generation in the factor deficient plasma was also examined by supplementing plasma concentration-specific factors. When extrinsic TF was used as a trigger, P-3-CG could decrease thrombin peak height (12%) and prolong the thrombin generation time (10%)in FX deficient plasma (Fig. 5D). But P-3-CG could only decrease thrombin peak height (13%) in FIX deficient plasma (Fig. 5E).However, P-3-CG could not impair thrombin generation in FII deficient plasma when extrinsic TF was used as a trigger (Fig. 5F).It was not consistent with clotting data that P-3-CG presented the highest extrinsic anticoagulant activity in FII deficient plasma.Here we could not give a rational explanation. We speculated that the reason for the different results in clotting assay and thrombin generation in FII deficient plasma was the difference of different triggers. The extrinsic trigger in clotting assay was rabbit brain and was purified TF in thrombin generation.
Interestingly, when intrinsic FXIa was used as a trigger, P-3-CG could decrease thrombin peak in FII deficient plasma (15%, Fig. S5C)and prolong the thrombin generation time (40%) in FIX deficient plasma(Fig. S5B). However, P-3-CG could not impair thrombin generation in FX deficient plasma when intrinsic FXIa was used as a trigger (Fig. S5A).
4. Conclusion
The anticoagulant activity of a novel anticoagulant peptide derived from oyster (Crassostrea gigas) was evaluated by a comprehensive method that involved many essential indexes that were involved in various coagulation factors (FXI, FIX, FX, FVIII, FV, FII and APC).As far as we know, there is no report to determine the anticoagulant activity for food-derived anticoagulants with the approaches that we used in the research.
P-3-CG drastically reduced the extrinsic clotting activity and also impaired the intrinsic clotting activity slightly. Consistent with clotting data, P-3-CG had more in fluence on thrombin generation in plasma when extrinsic TF was a trigger than intrinsic FXIa and silica.The inhibitory kinetics of P-3-CG to various coagulation factors(FXIa, FIXa, FXa, FIIa and APC) also explained the reduction of clotting activity and prolongation of thrombin generation time in plasma. P-3-CG could inhibit FXIa cleaving the chromogenic substrate.FIX activation via FXIa could also be inhibited by P-3-CG in a timedependent manner which the inhibitory potency decreased with time.However, P-3-CG could inhibit FIX activation mediated by extrinsic tenase complex (TF-FVIIa-PCPS) in a time-independent manner that the inhibitory potency was stable with time. Thus, we concluded that P-3-CG was a more potent extrinsic anticoagulant based on thesefindings.
Other than presenting high extrinsic anticoagulant potency, P-3-CG could also inhibit intrinsic coagulation factors (FXI and FIX) activity and activation. Furthermore, the activity and activation process of common coagulation factors (FX and FII) were also inhibited by the anticoagulant peptide seriously. Therefore, the anticoagulant property of P-3-CG in a non-purified component system (clotting assay and thrombin generation assay in plasma) resulted from comprehensive inhibition for various factors (FXIa, FIXa, FXa, FIIa) in the coagulation cascade.
Declaration of interest
All authors declare that they have no con flicts of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31771926) and the State Key Research and Development Plan “Modern Food Processing and Food Storage and Transportation Technology and Equipment” (No.2017YFD0400201). Thanks for the help from Dr Mettine Bos, Leiden University Medical Center.
Ethics approval and consent to participate
The blood coagulation assay was performed according to the Ethics Committee of the Dalian Polytechnic University and Leiden University Medical Center. It was performed following the tenets of the Declaration of Helsinki.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.06.006.
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- Protective effects of tilapia (Oreochromis niloticus) skin gelatin hydrolysates on osteoporosis rats induced by retinoic acid

