Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
Dong He, Wen Zeng, Yi Wang, Yifan Xing, Kang Xiong, Nan Su,Chong Zhang,c, Yuan Lu, Xinhui Xing,c,d,e,*
a School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, China
b Key Laboratory for Industrial Biocatalysis, Ministry of Education, Institute of Biochemical Engineering, Department of Chemical Engineering,China Center for Synthetic and Systems Biology, Tsinghua University, Beijing 100084, China
c Center for Synthetic and Systems Biology, Tsinghua University, Beijing 100084, China
d Institute of Biopharmaceutical and Health Engineering, Tsinghua Shenzhen International Graduate School, Shenzhen 518055, China
e Institute of Biomedical Health Technology and Engineering, Shenzhen Bay Laboratory, Shenzhen 440300, China
ABSTRACT
Ulcerative colitis (UC) is an incurable and highly complex digestive disease affecting millions of people worldwide. Compared to the current therapeutic drugs, bioactive peptides are more promising and safe substances as functional foods or drugs for the prevention and treatment of UC. The alcohol-soluble components from fermentation broth by fresh wheat germ and apple (AC-WGAF) were found to be effective in UC prevention in dextran sulfate sodium-induced mice in vivo. Herein, 4 novel peptides are identified from AC-WGAF by membrane ultrafiltration, recycling preparative high-performance liquid chromatography, and matrix-assisted laser desorption–ionization time-of- flight/time-of- flight mass spectrometry, possessing anticolitis activity via using an in vitro model. One of those peptides named T24 (PVLGPVRGPFPLL) exhibited the most remarkable anti-colitis activity by preventing tight junction protein loss, maintaining epithelial barrier integrity, and promoting cell proliferation during in vitro and in vivo studies by regulating mitogen-activated protein kinase signaling pathways. Thus, T24 is a promising peptide as a functional food or novel drug for UC prevention and treatment.
Keywords:
Ulcerative colitis
Peptide
Fermentation product
Intestinal epithelial barrier
Cell proliferation
Mitogen-activated protein kinase signaling pathways
1. Introduction
Inflammatory bowel diseases (IBDs), such as ulcerative colitis(UC) and Crohn’s disease (CD), are idiopathic intestinal in flammatory diseases involving the ileum, rectum, and colon [1]. Patients with UC usually suffer from persistent diarrhea, abdominal pain, cramps, rectal bleeding, and fatigue, leading to a dramatic decrease in the quality of life. Moreover, UC also entails significantly increased risks of colorectal cancer, thrombosis, and primary sclerosing cholangitis in patients [2]. The global incidence of UC has increased rapidly over the past few years, and millions of people worldwide suffer from UC.However, there is still no clear conclusion regarding the pathological mechanisms of UC. A consensus is that UC is caused by the complex interaction of in fluencing factors, such as the environment(including intestinal microorganisms and diet), genes, immune system, and in flammatory mediators [3]. Although the application of immunosuppressants, antibiotics, and corticosteroids can effectively suppress inflammation at the onset of UC [4], the remission rate of these drugs in UC treatment is still limited and unsatisfactory.Furthermore, all the current therapeutic drugs have different degrees of side effects, some of which are profoundly serious [5]. Therefore,there is an urgent need to develop new therapeutic treatments with fewer side effects and safer [6].
In addition to the current UC-treating drugs, food-derived bioactive peptides may become a promising alternative as a preventative and safer treatment for UC patients with better convenience and compliance [7]. Bioactive peptides can be present in foods or obtained from protein digestion and microbial fermentation of protein-rich feed stalks. Some of food-derived bioactive peptides had been reported to possess the ability to resist digestion from gastric juice and intestinal juice, and they successfully reached the intestine and passed through the intestinal barrier, thereby exerting biological effects at both the intestinal and systemic levels [8]. Therefore, foodderived bioactive peptides have been investigated as therapeutic agents for relieving intestinal inflammation and intestinal mucosa damage because of their potential nutraceutical properties [9]. For example,a peptide derived from the antrum mucosal protein (AMP)-18(gastrokine-1) reduced the extent of mucosal erosion and clinical severity in mice with dextran sulfate sodium (DSS)-induced colonic damage [10]. A 20-amino acid antimicrobial peptide named LFP-20 increased the expression of zonula occludens-1 (ZO-1), occludin,and claudin-1 and reduced the permeability and apoptosis of the colonic epithelia in lipopolysaccharide-treated mice [11]. The antiinflammatory soy peptide Val-Pro-Tyr reduced IL-8 secretion in TNF-α-induced Caco-2 cells and reduced the severity of colitis in a DSS-induced mouse model [12]. Casein peptides can act on intestinal tight junctions by stimulating occludin expression in Caco-2 cells. Another study demonstrated that the casein peptide Asn-Pro-Trp-Asp-Gln enhanced epithelial barrier function owing to its ability to upregulate occludin expression [13]. These active peptides have(or partially have) the biological capability to regulate immune response, inhibit intestinal inflammation, and alleviate intestinal mucosal damage, thereby playing a positive role in the alleviation of intestinal inflammation [14]. Murine colitis models and Caco-2 cell lines are widely adopted in the studies mentioned above.However, conducting murine colitis studies requires conformance of animal ethical guidelines and is relatively laborious, which is not suitable for screening numerous bioactive peptides. The Caco-2 cell line is a human cancerous cell line with a physiological state that is significantly different from that of natural colonic epithelial cells.Hence, there is still a lack of rapid, efficient, and effective models for screening bioactive peptides against UC.
The NCM460 cell line is an epithelial cell line derived from normal human colon tissues [15]. It has been widely applied in the in vitro study of colonic functions because it is non-cancerous and is not infected or transfected with any genetic information [16,17].In UC animal models, the oral administration of DSS induces colitis symptoms that are comparable to the clinical and histological features of IBD. It has also been found that DSS treatment induces cytotoxicity in intestinal epithelial cell lines and results in intestinal barrier injury, a major pathological change observed in UC [18]. Therefore,constructing an in vitro model of the NCM460 cell line with DSS treatment will promote the target peptide screening, thus improving human health, and could become an effective way to screen and characterize anti-colitis peptides from natural protein resources.
Damage to the intestinal epithelial barrier is an important factor in the occurrence and exacerbation of UC, and restoring the damaged intestinal barrier function is a key step in the prevention and treatment of UC [19,20]. Therefore, screening by repairing the intestinal barrier function will help to obtain peptides with the ability to prevent and treat UC. Wheat germ, which has a relatively high protein content(~35%) and contains essential amino acids, is an ideal source of abundant bioactive peptides, including antioxidant peptides, calciumbinding peptides, and antimicrobial peptides after protease hydrolysis or fermentation [21-23]. To thoroughly investigate and excavate the anti-colitis peptides originating from wheat germ, in our previous study, we obtained the alcohol-soluble components of fresh wheat germ-apple fermentation (AC-WGAF) using a mixed culture of four Lactobacillus spp. [24]. They showed pronounced preventive effects on DSS-induced experimental colitis in mice, as well as possessed abundant peptides. Peptide-enriched AC-WGAF significantly alleviated the loss of ZO-1 expression by DSS-induced colitis and exerted protective effects against DSS-induced murine colitis [24].Based on these investigations, we speculate that AC-WGAF may contain certain protective peptides against intestinal mucosal injury and colitis after fermentation via Lactobacillus spp. However, a detailed study of the peptides in AC-WGAF is ongoing. Thus, the present study focused on the identification and characterization of anti-colitis peptides derived from AC-WGAF by adopting membrane ultrafiltration, recycling preparative high-performance liquid chromatography (HPLC), and matrix-assisted laser desorptionionization time-of-flight/time-of-flight mass spectrometry (MALDITOF/TOF-MS). Moreover, the bioactive effect and supposed mechanism of the identified anti-colitis peptides were investigated using DSS-induced NCM460 cells and experimental UC mice. Thesefindings would be of significance in utilizing fermented peptides as anti-colitis ingredients in functional foods.
2. Materials and methods
2.1 Reagents
All reagents were obtained from Sigma–Aldrich (St. Louis, MO,USA) unless otherwise indicated. The DSS (Mw 36 000–50 000) was purchased from MP Biomedicals Inc. (California, United States).The Roswell Park Memorial Institute (RPMI) 1640 medium, Opti-MEMTMreduced serum medium, fetal bovine serum, and penicillinstreptomycin solution were obtained from Thermo Fisher Scientific(Waltham, MA, USA). The AC-RWGA (alcohol-soluble components of raw wheat germ-apple juice) and AC-WGAF were prepared using the procedure developed by us in a previous study [24].
2.2 Purification, identification and synthesis of anti-colitis peptides
Scheme of anti-colitis peptides purification, identification and synthesis of from AC-WGAF was shown in Fig. 1. AC-RWGA and AC-WGAF were collected and filtered sequentially using 30, 10,and 3 kDafilter membranes at centrifuging rates of 5 095, 6 654, and 8 422 g, respectively. The retentate of the filtrate was collected as Mf-NOM (> 30 kDa, 10–30 kDa, 3–10 kDa, and < 3 kDa, respectively).All recovered peptide fractions were lyophilized in a freeze dryer and stored at 20 °C. The < 3 kDa component of AC-WGAF was further analyzed via liquid chromatography (Japan Analytical Industry Company) containing a JAIGEL-GPC column. The parameter settings were as follows: the wavelength of the UV detector was set to 214 nm, the samples were prepared in an aqueous solution (10 mg/mL),the flow rate was 2.0 mL/min and the injection volume was 10 mL.The system was operated at room temperature (25 °C). The molecular weight of the peptides was analyzed on a 4800 Plus MALDI TOF/TOFTM(Applied Biosystems, Foster City, USA) according to the reference [25], while the peptide sequence was analyzed using an OrbiTrap Fusion Lumos (Thermo Fisher Company, MA, USA)at the Protein Research Technology Center at Tsinghua University according to the reference [26]. The synthesis of all anti-colitis peptides with the identified sequence were accomplished by Nanjing yuanpeptide Biotechnology Co., Ltd. with a solid-phase synthesis method. The purity analysis and characterization were performed by high performance liquid chromatography as shown in Fig. S4. The purity of the peptides was above 95%.
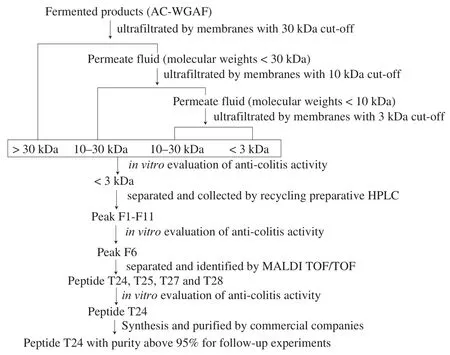
Fig. 1 Scheme of peptide purification, identification and synthesis of anticolitis peptides from AC-WGAF.
2.3 Cell culture and peptide treatment
For in vitro colitis bioactivity studies, human colonic epithelial cells, NCM460, were adopted and treated with peptides according to the referenced method [24] and cultured at 37 °C in humidified air containing 5% CO2in a cell incubator. The culture medium consisted of an RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. For peptide pre-treatment and DSS stimulation, cells were cultured in 6-well culture plates and grown to approximately 70% confluence, followed by pretreatment with purified peptides for 12 h Then, the cells were further challenged with 3% DSS in the reduced serum medium for another 48 h. After DSS treatment, the cells were washed with PBS (0.01 mol/L, pH 7.2)and harvested for subsequent analysis.
2.4 Cell viability assay
The viability of peptide-treated NCM460 cells was determined using the Cell Counting Kit-8 (CCK-8) assay kit (Solarbio Science & Technology Co., Ltd., Beijing, China). In brief, cells were seeded in 96-well plates at a density of 1 × 105cells/mL in a 250 μL culture medium overnight, followed by treatment with various concentrations of peptides and PBS alone (control)for 24 h. Then, each well was incubated with 10 μL CCK-8 solution for 2 h in the dark. Absorbance was measured at 450 nm using a microplate reader. Cell viability was calculated as:

where Asamplerepresents the absorbance of the peptide-treated cells at 450 nm, Ablankis the blank well absorbance, and Acontrolis the absorbance of the cells without peptide treatment at 450 nm.
2.5 Western blot analysis
Western blotting was performed as described in our previous study [24]. Briefly, cells were washed twice with cold PBS, then harvested by protein loading buffer (TransGen Biotech, Beijing,China). The samples were denaturized at 100 °C for 10 min before electrophoresis. Subsequently, the samples were separated through 10% SDS-PAGE at 120 V for 120 min, and electrotransferred onto 0.22 μm polyvinylidene fluoride membrane at 300 mA for 120 min.Then, the membranes were blocked in tris buffered saline containing 0.1% Tween-20 (TBST) with 5% defatted milk powder at room temperature for 60 min, and incubated with specific primary antibodies at 4 °C overnight. After being washed thrice with TBST,the membranes were incubated with corresponding second antibodies at room temperature for 30 min, followed by another thrice wash with TBST. Finally, protein bands were visualized using west pico plus chemiluminescent substrate kit (SageBrightness, Beijing, China).ImageJ software was applied to analyze the gray value of each band.
2.6 Characterization of barrier function in NCM460 cell monolayer
For permeability assays, NCM460 cells were cultured on a Transwell polycarbonate film, containing 500 μL of culture medium, for 5 days to obtain monolayers of NCM460 cells. The monolayers were then supplied with 3% DSS and peptides with different concentrations, with an equivalent PBS group as the control.After culturing for 24 h, fluorescein isothiocyanate (FITC)-dextran(4 000 Da) was added to each well at afinal concentration of 200 mg/mL.The monolayers were then incubated at 37 °C in 5% CO2for 12 h. After incubation, the monolayers were washed extensively, and the relative fluorescence intensity of FITC-dextran that had penetrated through the monolayers into the lower compartments was measured using a microplate reader. The mean fluorescence intensity of at least three independent experiments was obtained.
2.7 RNA-seq analysis of NCM460 cells treated by T24
The NCM460 cells were inoculated in a 96-well plate at a density of 105cells/well, followed by culturing for 24 h. Then, peptide T24 was added at a working concentration of 100 μg/mL, while the equivalent PBS group was set as the reference. After co-culturing for another 12 h, mRNA was extracted from the cells and RNA-Seq was performed by Biomarker Technologies Corporation Co., Ltd.,Beijing. The specific determination procedure was as follows: the total RNA was extracted using a TRIzol reagent, and each sample was mixed to form an RNA pool. Magnetic beads with oligo (DT)were used to enrich the mRNA, followed by reverse transcription to obtain dsDNA, for which the sequence end was repaired. Poly (a)was added and connected to a sequencing connector to prepare the sequencing library. The sequencing samples were enriched using PCR. The transcriptome library was constructed and sequenced using the Illumina HiSeq 2500 sequencing platform and PE125 sequencing method, respectively. The original image data obtained by sequencing were transformed into raw reads by base calling and thenfiltered to obtain clean reads. Then, the short-read assembling program Trinity was used for the de novo assembly of the transcriptome [27], and overlap groups were obtained by overlapping information assembly between sequences. Finally, TIGR gene indices clustering tools and using PHRED/PHARAP/CONSED software (http://www.pharap.org)were used to cluster and splice the transcripts to obtain a single gene cluster. The analysis items included sequencing assembly result analysis, FPKM statistical analysis, and SSR analysis. Using the BLASTX comparison tool, UniGene cells were compared with the protein database (E value ≤ 1 × 10-5). Functional annotation was carried out according to the similarity of the genes, and the protein with the highest sequence similarity with the given UniGene was determined; thus, the functional annotation information of UniGene was obtained. The protein databases included the non-redundant protein database (n), Swissprot protein database, cluster of original groups (COG), protein families database (Pfam), gene ontology (GO),and the Kyoto Encyclopedia of Genes and Genomes (KEGG).
2.8 Experimental protocol for UC mice
The laboratory animal facility was accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and all animal protocols used in this study were approved by the Institutional Animal Care and Use Committee of Tsinghua University (permission number: 14-XXH-1). C57BL/6J mice (8 weeks old, male) were kept under specific pathogen-free conditions at the animal facility at Tsinghua University. The mice were pretreated with PBS alone, received orally administered T24,and were subcutaneously injected with T24 for 7 days consecutively.There were 5–6 mice in each group. The dose of T24 was determined to be 30 mg/kg of body weight. After pretreatment, the mice were challenged with 3% DSS dissolved in Milli-Q water (Millipore Corp.,Bedford, MA, USA) for 8 days consecutively to induce experimental colitis. The mice were continuously treated with PBS alone, orally administered T24, and the subcutaneous injection of T24, under DSS induction to evaluate the preventive effects of T24 on UC induction.During DSS treatment, the mice were assessed daily for colitis development based on body weight change, gross rectal bleeding,stool consistency, and survival. After 15 days of treatment, the mice were sacrificed, and the overall disease severity assessment and hematoxylin-eosin (H&E) staining were performed as described in our previous study [24].
2.9 Immunohistochemistry
The mouse colonic tissues were embedded in paraffin and sliced into 4 μm sections that were placed on slides, which was followed by the deparaffinization and hydration of tissue slides through xylene and gradient ethanol treatment, respectively. Antigen retrieval was performed at 100 °C for 20 min in a citrate buffer (pH = 6.0). A peroxidase-blocking solution (Dako) was used to eliminate nonspecific staining. The tissue slides were incubated overnight at 4 °C with primary antibodies including phospho-mTOR (Ser2448) (Abcam,ab109268, 1:200), phospho-p70S6K (Thr421) (Invitrogen, MA5-38224, 1:100), phospho-p44/42 (Thr202/Tyr204) (CST, 4370, 1:250),NF-κB p65 (CST, 8242, 1:400), and Ki67 (CST, 12202, 1:400).After primary antibody incubation, the HRP rabbit/mouse secondary antibody solution (Dako) was applied to the tissue slides with 30 min incubation at room temperature (25 °C), and the color was developed using 3,3’-diaminobenzidine (Dako). Nucleus staining was performed using a hematoxylin solution (Sigma, 03971). The stained tissue slides were dehydrated using gradient ethanol and xylene treatment,then mounted with a mounting medium (Leica, 14070937891).Bright-field photographs of the tissue slides were obtained using a ZEISS Axio Scan.Z1 slice scanner. The relative expression of DAB(3,3’-diaminobenzidine) staining was scored and quantified according to previous literature [24].
2.10 Statistical analysis
The data are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed by student’s t tests for unpaired data and by one-way ANOVA analysis for multiple comparisons. A two tailed P value of < 0.05 was considered significant.In the experiments involving western blot, immunohistochemistry, and histological analyses, the data andfigures presented are representative of three or more independent experiments. GraphPad Prism 7 software was used for statistical analysis.
3. Results
3.1 Identification and characterization of anti-colitis peptides from AC-WGAF
As shown in Fig. 1, to explore the anti-colitis bioactive peptides in AC-WGAF, membrane ultrafiltration, recycling preparative HPLC, and MALDI TOF/TOF MS were applied sequentially. First,as shown in Fig. 2a, 4 components with different molecular weights(> 30 kDa, 10−30 kDa, 3−10 kDa, and < 3 kDa) were obtained from AC-WGAF. The component with the molecular weight of < 3 kDa significantly mitigated the decrease in ZO-1 expression under DSS treatment, which indicates that most of the active components of AC-WGAF were enriched in the fractions with smaller molecular weights. Therefore, the < 3 kDa component of AC-WGAF was selected as the raw material for further investigation. Second, 11 peaks (F1–F11)were eventually obtained from the < 3 kDa component of AC-WGAF.Notably, the F6 fraction showed the most remarkable effect among the 11 fractions (shown in Fig. 2b). To further identify the potential active peptide sequence, the F6 fraction was collected and subjected to MALDI TOF/TOF MS. Finally, 4 novel active peptides named T24(PVLGPVRGPFPLL), T26 (YQEPVLGPVR), T27 (WNMNMMTA),and T28 (CCNFCMSS) were identified. To explore the biological activity of the four active peptides, the cytotoxic characterization of the identified peptides was carried out using the CCK-8 assay in NCM460 cells (Fig. S1). None of the four identified active peptides exhibited significant cytotoxicity to NCM460 cells at a concentration of 20–200 μg/mL. These peptides are suitable for subsequent investigations.
3.2 Protective effect of identified active peptides on expression in tight junction proteins
To characterize the anti-colitis activity of the 4 targeted peptides on tight junction proteins in vitro, the expression of tight junction proteins ZO-1, claudin-1, and occludin under DSS induction was evaluated by Western blotting. As shown in Fig. 3, compared to the control group, there was a significant decrease in the expression of ZO-1, claudin-1, and occludin in NCM460 cells when treated with DSS alone. All 4 peptides alleviated the loss of ZO-1, claudin-1, and occludin. Among them, peptide T24 showed the most prominent protective function in NCM460 cells (P < 0.01); therefore, this peptide was selected for further investigation. As shown in Fig. S2, the protective effect of T24 on NCM460 cells without DSS induction was also investigated. T24 treatment at a concentration of 20–200 μg/mL remarkably increased the expression of tight junction proteins ZO-1,ZO-2, and claudin-4. With the increase in T24 concentration, the expression of ZO-1, ZO-2, and claudin-4 first increased, then decreased slightly. Meanwhile, the expression level of claudin-1 was slightly increased by T24 at lower concentrations. Thus, T24 exerted a protective effect by promoting the expression of tight junction proteins in colonic epithelial cells under DSS induction.

Fig. 2 Peptides screening and collection by ultrafiltration and recycling preparative HPLC. (a) Western blot analysis of ZO-1 in NCM460 cell line after 24 h of exposure to the 4 components with the molecular weight (> 30 kDa, 10−30 kDa, 3−10 kDa, and < 3 kDa) in AC-RWGA and AC-WGAF. The results of immunoblotting were quantified using the software image J. The diagrams represent the quantification result of the intensity of bands, calibrated to the intensity of the β-actin bands; (b) Chromatogram and Western blot analysis of ZO-1 in NCM460 cell line in NCM460 cell line after 24 h of exposure to the 11 components separated by chromatographic column. The results of immunoblotting were quantified using the software image J. The diagrams represent the quantification result of the intensity of bands, calibrated to the intensity of the β-actin bands.
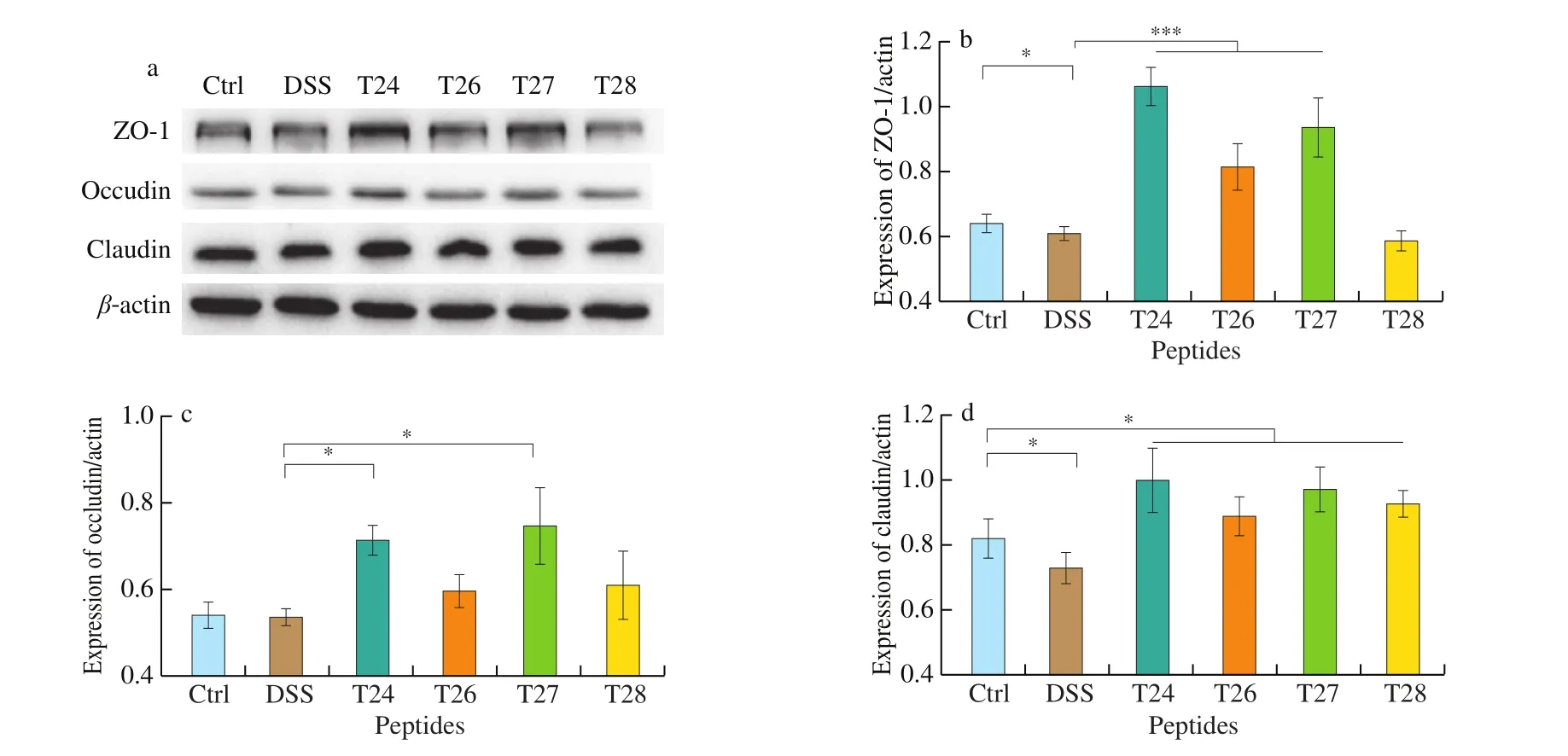
Fig. 3 Western blot analysis of ZO-1, claudin-1 and occludin in NCM460 cell line after 24 h of exposure to the identified four bioactive peptides derived from the component with the molecular weight of < 3 kDa in AC-WGAF. The results of immunoblotting were quantified using the software image J. The diagrams represent the quantification result of the intensity of bands, calibrated to the intensity of the β-actin bands. The values are expressed as mean ± SD (n = 3). *P < 0.05,***P < 0.001, the symbols indicate the differences from the controls.
3.3 Effect of T24 on the epithelial barrier integrity of NCM460 cell monolayers under DSS induction
To further evaluate the protective function of these peptides in cell tight junctions, we adopted NCM460 cell monolayers with 4 000 Da FITC-dextran as an in vitro model to examine epithelial barrier integrity. As demonstrated in Fig. 4, DSS treatment significantly aggravated the permeability of NCM460 cell monolayers, which was indicated by an observed greater increase in the fluorescence signal of FITC-dextran passing through the cell monolayers compared with PBS controls (P < 0.001). It was notable that the peptide T24 treatment at concentrations of 200 μg/mL and 1 mg/mL notably ameliorated the epithelial barrier permeability,while peptide T26 treatment significantly mitigated the cell monolayer permeability only at a concentration of 200 μg/mL. Thesefindings were in concordance with the protective effect of peptides in alleviating tight junction loss in NCM460 cells in vitro (Fig. 2). These results indicated that T24 effectively maintained or restored the epithelial barrier integrity of NCM460 monolayers under DSS treatment.
3.4 Transcriptome analysis of NCM460 cells in response to T24
To reveal the underlying mechanism of T24 in exerting biological activities, NCM460 cells were treated with T24 or PBS in a reduced serum medium without DSS treatment for 24 h, followed by total RNA extraction, cDNA library construction, and sequencing. The assembly and analysis of the transcriptome are shown in Table S1.The datasets of NCM460 cells treated with T24 were compared with those of the control group to analyze the differential gene expression.The comparison results are summarized in a volcano map, as shown in Fig. 5a. As a result, 302 transcripts were differentially expressed,116 of which were upregulated and 186 were downregulated. Pathway enrichment analysis of differential genes was carried out using an R package, clusterProfiler that automates the process of biologicalterm classification and the enrichment analysis of gene clusters.Hypergeometric analysis was adopted to find the KEGG pathway,which was significantly enriched compared to the whole genome background. As shown in Fig. 5b, the mitogen-activated protein kinase (MAPK), Hippo, and pluripotent stem cell signaling pathways were the top three most evident differentially expressed genes.
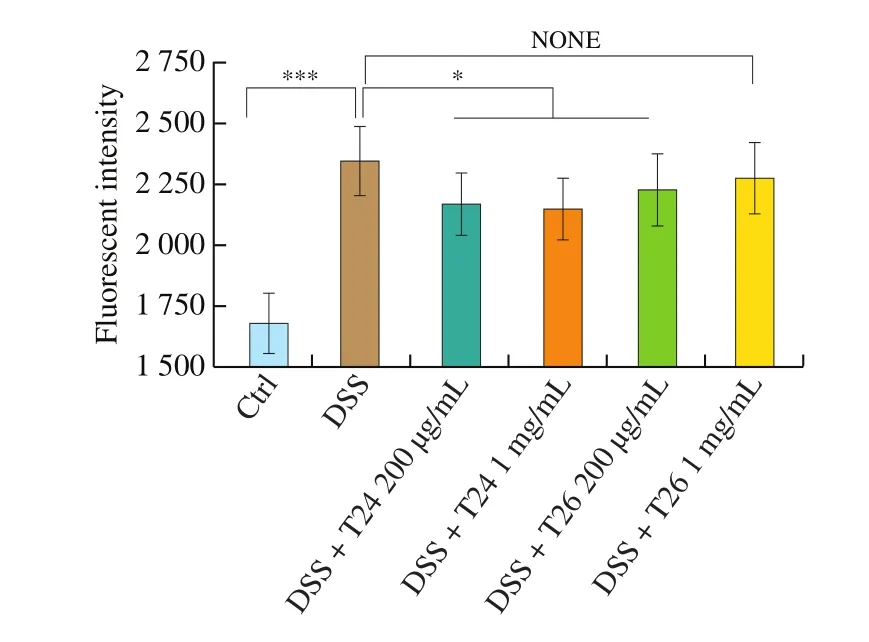
Fig. 4 Permeation of FITC-dextran in NCM460 cell monolayers. Permeation of FITC-dextran to the basolateral side was reported as a percentage of FITC-dextran applied to the apical side. Error bars represent 1 standard deviation of the mean. The values are expressed as mean ± SD (n = 3). *P < 0.05, ***P < 0.001,the symbols indicate the differences from the controls.
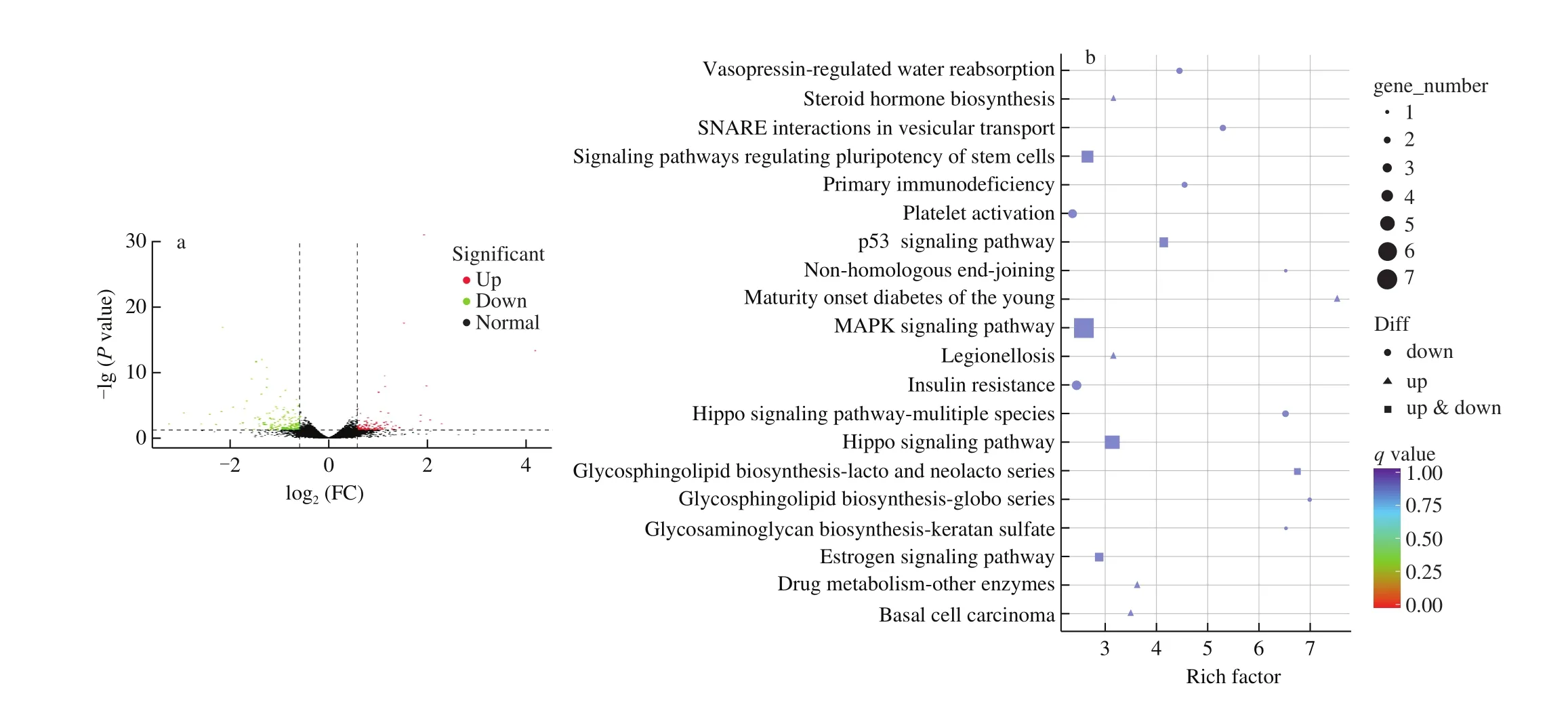
Fig. 5 NA-seq analysis of T24 treatment in NCM460 cells. (a) Volcano plot representing all expressed transcripts; (b) the enrichment scatter diagram of differential expression gene KEGG pathway, and each line in thefigure represents a KEGG pathway.
3.5 Effect of T24 on the phosphorylation of various MAPKs
Based on the transcriptome analysis, we further applied western blotting to verify the regulatory effect of T24 at various concentrations on the phosphorylation of extracellular signal-regulated kinase (ERK),p38, and c-Jun amino-terminal kinase (JNK), the three major MAPKs.As shown in Fig. 6, the phosphorylation of ERK was significantly increased, while the phosphorylation of p38 was notably inhibited by the elevated concentrations of T24 in normal NCM460 cells without DSS treatment. The regulatory effect of T24 on JNK phosphorylation was not pronounced, as shown in Fig. S3.
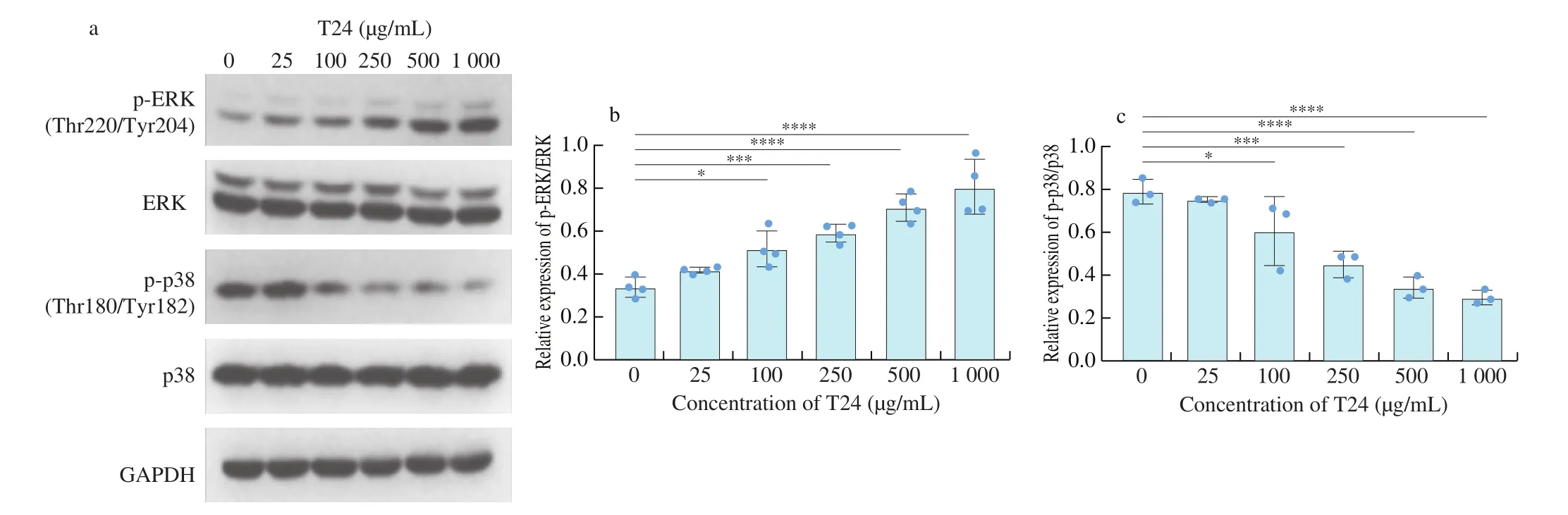
Fig. 6 Effect of T24 on phosphorylation of MAPK pathway important protein (ERK/p38) in NCM460 cells. (a) Representative images of Western blotting.(b, c) Statistical results of relative phosphorylation level in ERK and p38 signaling pathway, respectively. The values are expressed as mean ± SD (n = 3). *P < 0.05,***P < 0.001, ****P < 0.000 1, the symbols indicate the differences from the controls.

Fig. 7 In vivo characterization of oral administration and hypodermic injection of T24 in DSS-induced mouse colitis model. (a) Bodyweight curve. (b) Colon length. (c) Spleen index. (d) IL-6 concentration in mouse serum. (e) Histology score of H&E staining in colonic tissues. (f) Representative image of H&E staining in colonic tissues, scale bar, 100 μm. The values are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, the symbols indicate the differences from the controls.
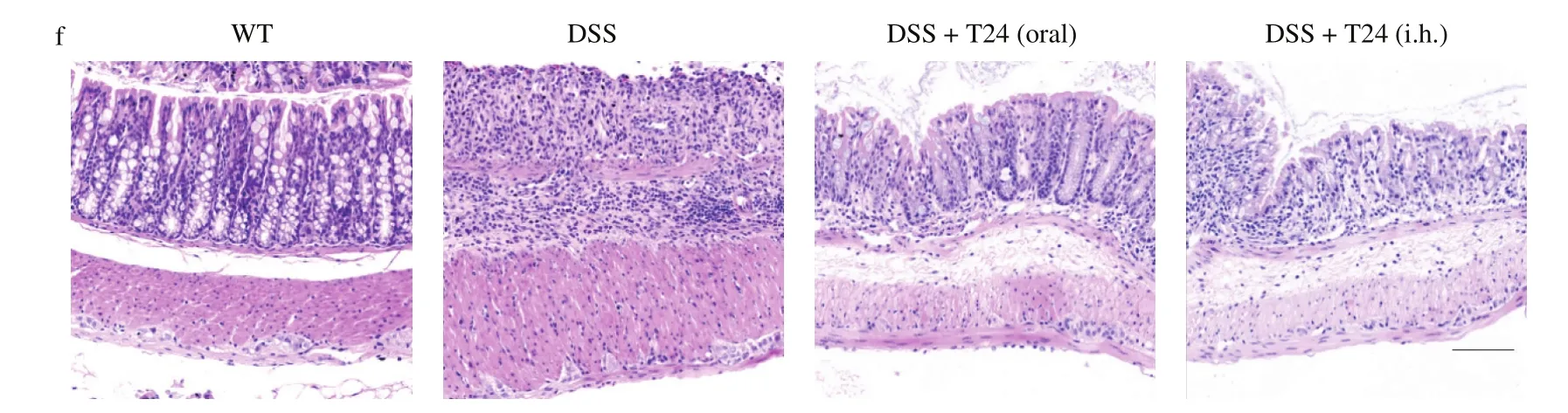
Fig. 7 (Continued)
3.6 Anti-colitis activity of T24
Based on the in vitro investigation described above, DSS-induced UC mice were employed to further assess the in vivo anticolitis activity of T24. The mice were pre-treated with T24 through oral administration or subcutaneous injections for a consecutive 7 days, followed by 3% DSS induction for another 7 days. Several symptomatic colitis parameters of UC, including body weight change,spleen index, serum IL 6 expression, and histological evaluation of colonic tissues, were examined. As shown in Fig. 7, DSS-treated mice showed greater symptoms of diarrhea, gross rectal bleeding, and significant body weight loss (P <0.001) with a marked increase in serum IL 6 expression (P < 0.001), increased spleen index (P < 0.01),and colon length shortening (P < 0.05), compared with vehicle-treated control mice. Both the oral administration and subcutaneous injection of T24 in UC mice significantly reduced the serum IL-6 expression and spleen index. In UC mice with T24 administered orally, the reduction in weight loss and increase in colon length were not significant. In contrast, the subcutaneous injection of T24 in UC mice significantly ameliorated body weight loss (P < 0.05) and mitigated colon length shortening (P < 0.05). Overall, the subcutaneous injection of T24 greatly alleviated the disease progression in UC mice, compared to the oral administration of T24.
3.7 Effect of T24 on cell proliferation via elevating the expression of ERK and mTOR
Section 3.5 discussed the transcriptome analysis of the NCM460 cells, which showed that T24 exerted a substantial impact on signaling pathways, including the MAPK, Hippo, and stem cell regulatory pathways, which are closely related to cell proliferation [28-30].To further analyze how T24 exerts protective effects against colitis,the expression of phospho-ERK, phospho-mTOR, phospho-p70S6K,NF-κB p65, and Ki67 in model mouse colonic tissues was evaluated by immunohistochemistry. As shown in Fig. 8, the expression of phospho-ERK, phospho-mTOR, phospho-p70S6K, and Ki67 was remarkably reduced in DSS-induced UC mice compared with that in the control group. Meanwhile, the activation of NF-κB p65 was observed in DSS-induced UC mice, indicating the presence of inflammatory response in colonic epithelia in vivo [31]. The expression of these factors in UC mice was dramatically reverted by the oral administration and subcutaneous injection of T24 under DSS induction. Thesefindings suggest that T24 possesses the ability to activate the expression of ERK, mTOR, and Ki67 in vivo. Since the phosphorylation of mTOR at Ser2448 indicated the activation of mTORC1 complex, followed by the phosphorylation of downstream effector p70S6K at Thr 421. The activation of mTOR-p70S6K signaling pathway was correlated with cell proliferation, which is related with the elevated expression of Ki67, a well-known marker of cell proliferation. Ourfindings indicate that T24 might restore the colonic epithelial barrier in UC mice by promoting colonic epithelial proliferation through the activation of ERK and mTOR-p70S6K signaling pathways in vivo. The alleviated expression of NF-κB p65, a crucial indicator of in flammatory response, in T24 treated UC mice also suggested the disease progression of UC is relieved in T24 treated mice, at least in the aspect of mitigating colonic in flammation.
8. Discussion
Enzymatic hydrolysis of proteins during fermentation leads to the release of some peptides with potential biological functions [32]. In this study, a novel peptide, T24 (PVLGPVRGPFPLL), was identified from the alcohol-soluble components in the fermentation products of a mixed culture of four Lactobacillus spp. (AC-WGAF) using NCM460 cells as an in vitro model for the DSS treatment. Peptide T24 exhibited prominent protective anti-colitis effects by preventing tight junction protein decrease and alleviating epithelial barrier permeability against DSS stimulation in vitro. Correspondingly, in vivo studies suggested that peptide T24 also exhibited remarkable protective effects on DSS-induced UC mice in terms of ameliorating bodyweight decrease, preventing colon length shortening, mitigating mucosal in flammation, and inhibiting pro-inflammatory cytokine expression, suggesting the therapeutic potential of peptide T24 for UC treatment. Furthermore, the administration route of T24, including oral administration and subcutaneous injection, was investigated to evaluate the anti-colitis activity of T24 in vivo. Interestingly, in terms of the apparent data including body weight change, colon length, and serum pro-inflammatory cytokines, the subcutaneous injection of T24 exerted better efficacy than the oral administration of T24. This phenomenon might be attributed to T24 partial digestion by proteases in the gastrointestinal tract after oral administration, whereas the subcutaneous injection of T24 evaded the gastrointestinal digestion.However, in terms of activating the signaling pathways, including phospho-ERK, phospho-mTOR, and Ki67 in the colonic tissue,orally administered T24 exhibited better performance than that of subcutaneously injected T24, which might be related to the various interplays among T24, colonic microbiome, and colonic epithelial cells. Orally administrated T24 is predicted to regulate colonic microbiome composition and related microbial metabolites in DSS-induced UC mice, which needs to be further verified in subsequent experiments.
The loss of colonic epithelial barrier integrity is a characteristic feature of UC; hence, restoring altered barrier functions is a key factor in UC treatment. Dysregulated cell apoptosis in the colonic epithelium,which results in an intestinal barrier defect, is elevated in patients with both CD and UC [33]. Therefore, it is important to inhibit colonic epithelium apoptosis, increase colonic cell proliferation, and promote the expression of tight junction proteins to restore colonic epithelial barrier integrity and promote the sustained resolution of inflammation [34,35]. As shown in Figs. 3 and S2, T24 effectively increased the expression of tight junction proteins and maintained the integrity of colonic epithelial monolayers. Furthermore, as shown in Fig. 8, T24 treatment promoted the expression of Ki67 as well as cell proliferation in the colonic epithelium, which was consistent with the in vitro studies of cell viability shown in Fig. S6. Thesefindings indicate that T24 may alleviate intestinal mucosal injury and UC by promoting cell proliferation in the colonic epithelium.
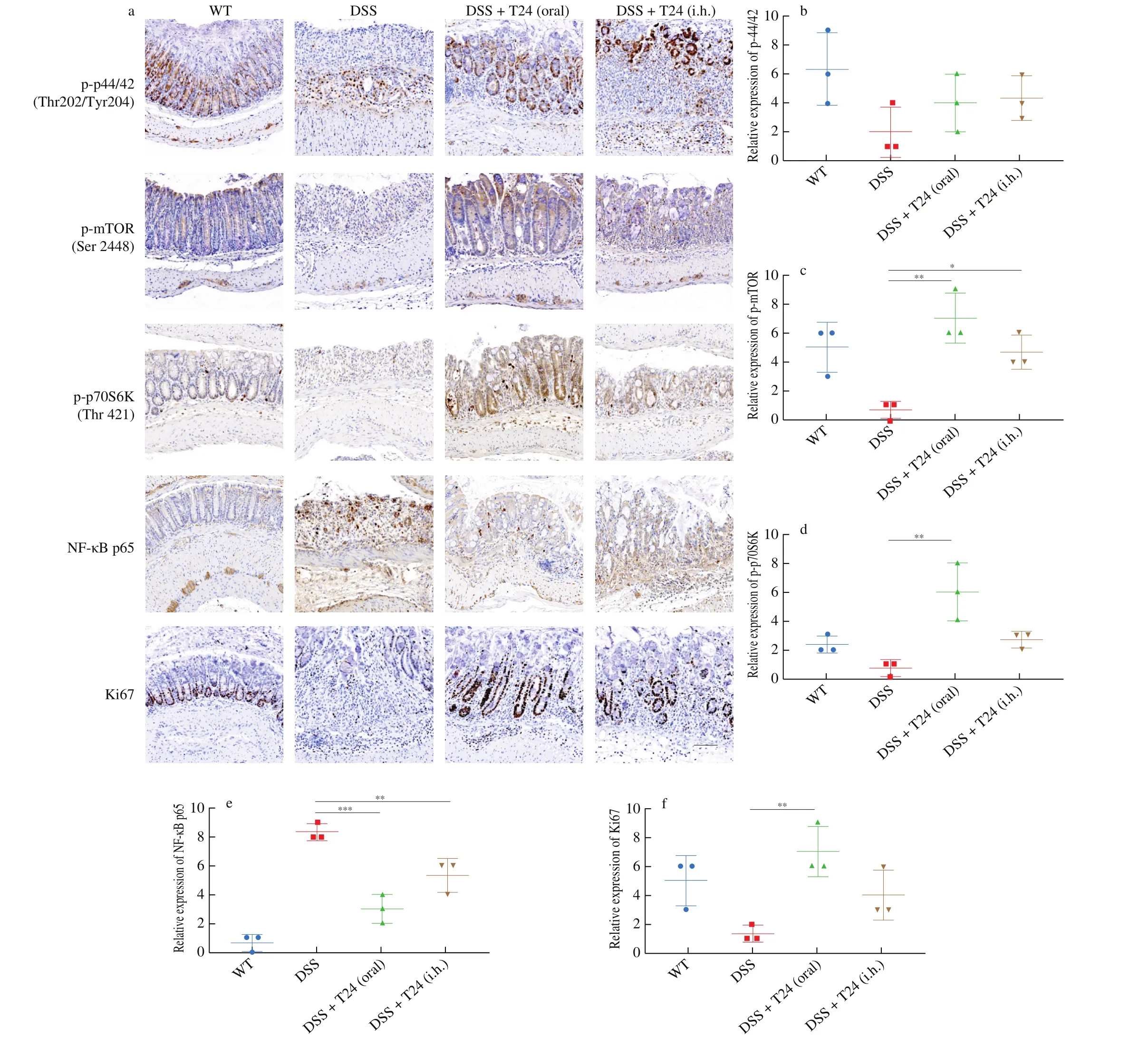
Fig. 8 In vivo analysis of T24 in activating cell proliferation related signaling pathway and marker in UC mice. (a) Representative image of immunohistochemistry and (b-f) statistical results of relative expression in phospho-p44/42, phospho-mTOR (Ser 2448), phospho-p70S6K, NF-κB p65,and Ki67, respectively in mouse colonic tissue sections, scale bar, 100 μm.
MAPKs are key nodes that play a critical role in the regulation of cell proliferation, and the three major subfamilies of MAPK proteins,ERK, JNK, and p38 MAP kinases, can regulate cell proliferation and apoptosis [36,37]. The activation and translocation to the nucleus of ERK1/2 and its downstream protein RSK can activate a variety of transcription factors, thus leading to effector protein synthesis and cell proliferation [36]. However, the p38 MAPK family, which is activated by cellular stress, plays a major role in suppressing cell proliferation;therefore, the contour balance of ERK and p38 may play an important role in regulating cell growth [38]. As shown in Fig. 6, the addition of T24 significantly activated ERK phosphorylation and suppressed p38 phosphorylation in vitro, thus promoting the balance in cell proliferation. mTOR is an evolutionarily conserved serine/threonine kinase that modulates a diverse class of key cellular processes,including protein synthesis and autophagy [39]. Activation of the mTOR pathway can lead to the activation of downstream substrates,such as S6 kinase-1 (S6K1) and eIF4E binding protein-1 (4E-BP1),and directly regulate the synthesis of downstream proteins [40].In this study, we demonstrated that in NCM460 cell lines, T24 could activate mTOR expression in the colon of experimental mice. DSS led to a decrease in phosphorylated mTOR abundance and then a decrease in phosphorylated P70S6K1 abundance, which indicated that DSS inhibited the mTOR signaling pathway and protein synthesis. Overall, T24 may play a protective role by upregulating the MAPK and mTOR signaling pathways, and activating protein synthesis and cell proliferation, thus alleviating tight junction loss and experimental colitis.
To study the relationship between peptide sequence and biofunction, the sequence of T24 was blasted on the website of the National Center for Biotechnology Information (NCBI), and T24 showed a 92% identity with the β-casein of milk. We then searched for the sequence and function of peptides similar to the T24 sequence(PVLGPVRGPFPLL) and compared them. A similar peptide(EPVLGPVRGPFP) isolated from the β-casein fermentation of Lactobacillus animalis DPC6134 exhibited strong ACE-inhibitory activity with an IC50value of 790 μmol/L [41]. Other reported peptides with highly-homologous sequences to T24, such as YQQPVLGPVR,VLGPVRGPFP, YQEPVLGPV, YQEPVLGPVRGP, and VRGPFPIIV, exerted ACE-inhibitory and antimicrobial activities [42],which suggested that peptides with similar sequences could possess prominent physiological functions. In our study, the sequences of T24 and T26 were also similar, but they have different functions in alleviating the decrease in tight junction proteins. The underlying reason might be attributed to the difference in the terminal amino acid sequence, which could potentially alter the biological activity of peptides. The relationship between the specific amino acid sequence of T24 and its physiological functions requires further investigation.Intriguingly, most of the peptides with highly-homologous sequences to T24 were found in dairy products fermented using Lactobacillus sp.,which indicated that peptides with similar sequences had certain similarities in function and that these peptides can be produced by fermentation in the future.
Declaration of competing interest
The authors declare that they have no known competingfinancial interests or personal relationships that could have appeared to in fluence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Plan, China (2016YFD0400203-4) and the Shenzhen Science and Technology Innovation Commission(KCXFZ20201221173207022).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.06.003.
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas
- Protective effects of tilapia (Oreochromis niloticus) skin gelatin hydrolysates on osteoporosis rats induced by retinoic acid

