Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
Hui Teng, Yuewei Qin, Xioyun Fn, Hui Co,*, Yuting Tin,*, Lei Chen,b,*
a Guangdong Provincial Key Laboratory of Aquatic Product Processing and Safety, Guangdong Province Engineering Laboratory for Marine Biological Products,Guangdong Provincial Engineering Technology Research Center of Seafood, Key Laboratory of Advanced Processing of Aquatic Product of Guangdong Higher Education Institution, College of Food Science and Technology, Guangdong Ocean University, Zhanjiang 524088, China
b College of Food Science, Fujian Agriculture and Forestry University, Fuzhou 350002, China
ABSTRACT
The present study aimed at making a rational usage for European eel bone by-products by preparing Europen eel bone peptide chelated calcium (EBPC-Ca). Nutritional properties and bioactivity of EBPC-Ca were evaluated. Results showed that nutritional properties of calcium ions will cause intra- and inter-molecular folding and aggregation of peptide to uniformly form EBPC-Ca chelate. The chelated compound of EBPC and calcium ion triggered a strong apoptosis in heterogeneous human epithelial colorectal adenocarcinoma(Caco-2) in concentration- and time-dependent manners. Western blot analysis revealed that the EBPC-Ca induced apoptosis may be the result of a blocked autophagy flux through mitochondrial-dependent pathway.Additionally, the increase in FGF-23 protein expression inhibited the absorption of calcium ions and alleviated cell apoptosis. It was also found that the cell apoptosis occurs with significant increases in the levels of reactive oxygen species (ROS) and Ca2+ in the cells, indicating the anti-tumor potential of EBPC-Ca may involve multiple channels.
Keywords:
Europen eel bone
Peptide
Calcium
Nutritional properties
Caco-2
Apoptosis
1. Introduction
In seafood processing, there are a large number of by-products,including skin, offal, scale, and bone. Some of the by-products are currently processed into animal forage but most of them are disposed as waste. However, a comparison study on the chemical composition betweenfish meat and eel by-products revealed that these underused by-products are rich in protein and oil, which could be made into other high value-added commodities [1].European eel bones contain about 13.25% crude protein, 19.18% crude lipid, 5.63% crude ash, and 56.96% moisture [2]. Besides,fish bones account for a large part of the marine food industry.Therefore, if they are properly processed, these raw materials are able to be used in various products and materials.
As we know, calcium accounts for 1.5%–2.0% of total body weight, and among it, 99% is used to generate bones and teeth, which is an important trace element in the human body [3]. After the calcium enters the cell, it will control many cell activities, such as metabolism,contraction, growth and development of muscle, heart beating, cell division, brain thinking, blood coagulation, and endocrine activities [4].The deficiency of calcium can induce osteoporosis and even lead to illnesses such as high blood pressure, kidney stones and colon cancer [5]. An adequate intake of calcium through the diet can reduce the risk. However, due to lactose intolerance and dietary habits,the food intake of calcium such as milk and cheese products, is not enough for preventing the deficiency of calcium and other relative health problems [6]. Therefore, the research on calcium supplement products that can effectively absorb calcium has aroused great interests. Studies have found that certain food ingredients, especially protein and peptide have a positive effect on calcium absorption [7].Recently, peptides from certain enzymatic protein foods, such as whey protein [8], mackerel scale [9] and soybean protein [10] exhibited a good ability to chelate calcium, increasing the absorption and bioavailability of calcium. More interesting, a higher ability to chelate calcium by fish bones derived peptides was confirmed as well [11].Currently,fish bones are known as a by-product of processing with a low economic value. However, it contains 30% of collagen, which could be used as a good source for proteins and polypeptides [12].Therefore, many studies have been attempted to extract protein and peptides fromfish bones, and checked their binding ability to calcium [13].Nonetheless, rarely can found studies focused on the bio-toxicity and bioavailability of calcium chelatedfish bone peptides [11].
Caco-2 is a human colon cancer cell line, which has been extensively employed to evaluate the anti-tumor potential against CRC (bowel cancer and colon cancer) in vitro [14]. Apoptosis is a caspase-dependent programmed cell death, mostly accompanied by chromosome contraction, nuclear fragmentation, and inner phosphatidylserine (PS) conversion to the membrane surface, and apoptotic bodies. It has been studied that the combination of metal drugs and the desiredfishbone polypeptide has encouraging for antitumor potential, which could be developed as a new combination of chemotherapy for the treatment of CRC [15-17].
Thus, the present study extracted bone peptides from European eel, and chelated them with calcium. In order to well understand the nutritional composition and potential bioactivity of European eel bone peptide chelated calcium (EBPC-Ca), overall comparisons of mineral constituents and fatty acids between bone peptide meal and EBPC-Ca were conducted. And the abilities of EBPC-Ca on cell proliferation,apoptosis induction, as well as reactive oxygen species (ROS) burst in Caco-2 cells were analyzed and their anti-CRC potential was evaluated as well.
2. Meterials and methods
2.1 Preparation of EBCP
European eel bone is provided by Haixin Food Co., Ltd. (China),and was utilized to prepare for the sample of European eel bone peptide. Brie fly, the prepared bones were immersed into 5% NaOH for 1 h to remove the attached meat. The ratio for 5% NaOH and the sample was 1:2 (m/V), and the residue was washed repeatedly with chilled distilled water. The treated bones were dried at 60 °C for 6 h, and then crushed into powders. The eel bone meal was mixed with distilled water at a ratio of 1:8 (m/V), and then the mixture was hydrolyzed with pepsin (37 °C, pH 2.0, Beijing Solarbio Science &Technology Co., Ltd., China) for 2 h, and trypsin was added (37 °C,pH 7.0, Beijing Solarbio Science & Technology Co., Ltd., China) and coagulated at the same time. After that, lactase (8 000 U/g, 37 °C, pH 7.0,Shanghai Macklin Biochemical Co., Ltd., China) was added and the sample was hydrolyzed for 2 h. After hydrolysis, the mixture was heated at 100 °C for 10 min to inactivate the enzymes, and centrifuged at 7 830 r/min for 15 min under 4 °C. The sample for European eel bone collagen peptide (EBCP) was obtained after freeze-drying and used for further analysis.
2.2 Preparation of EBPC-Ca
The lyophilized EBCP was dissolved in deionized water and CaCl2was added. The mixture was incubated in a water bath at 40 °C and pH 7.5 for 120 min. After the chelation reaction, absolute ethanol(9 times the volume of the solution) was added to the mixed solution to separate the chelate. After that, the mixture was centrifuged at 7 830 r/min for 30 min under 4 °C. The freeze-dried precipitate was the EBPC-Ca.
2.3 Material composition
Potassium content of the samples were determined by wet acid digested assay using flame emission spectrophotometry [18]. Calcium was quantitatively determined from the digest using the Atomic Absorption Spectrophotometer with appropriate hollow cathode lamps (Perkin Elmer Model 2280). Moisture content was determined after drying at 140 °C for 40 min, and crude fat was performed by Soxhlet extraction, ash content and crude protein were determined by incineration and Kjeldahl method, respectively [19].
2.4 Determination of amino acid scores
Total amino acids (TAA) was analyzed using high pressure liquid chromatography (QTRAP5500, AB SCIEXLTD, USA) equipped with an Elite amino acid chromatography column (4.6 mm × 250 mm, 5 μm).In short, the bone protein powder sample was hydrolyzed with 6 mol/L HCl at (110 ± 1) °C for 22 h in a sealed glass tube which wasfilled with nitrogen. At the same time, the same method is used to determine the content and composition of free amino acids (FAA) in the sample,but without acid hydrolysis.
2.5 Size distribution
The size distributions of the EBCP and EBPC-Ca complex were determined by a laser particle size analyzer (Malvern Instruments Ltd., Malvern, Worcestershire, UK). The sample of 1 mg/mL concentration in deionized water was passed through a 0.22 μmfilter membrane before the experiment. Then, approximately 1.5 mL of sample was added to the polystyrene pool (1 cm) and let sit for 2 min at 37 °C.
2.6 Ultraviolet-visible (UV-Vis) spectrum scan
UV-Vis molecular absorption spectroscopy is an effective method to evaluate the structural characteristics of substances through the shift and intensity changes of the ultraviolet absorption spectrum. The analysis was employed after dissolving the calcium-binding peptide in deionized water (0.5 mg/mL), and then added with CaCl2solution(conc. of 0, 50, 100, 500 and 1 000 mmol/L). The spectrum used a full-wavelength microplate reader SpectraMax190 (Molecular Devices Ltd., Silicon Valley, State of California, US) with a wavelength range of 200–400 nm. The blank calibration of full-wavelength micro-plate reader SpectraMax190 was completed with deionized water.
2.7 Cell culture conditions
Caco-2 cell was cultured in complete DMEM (Dulbecco modified Eagle’s medium, Hyclone, Logan, UT), in which supplemented with nonessential amino acid solution, L-glutamine, 10% fetal bovine serum (TransGen Biotech, Beijing, China), penicillin, and streptomycin (100 ng/mL; TransGen Biotech). And the cells were incubated at 37 °C with 5% CO2atmosphere.
2.8 Cell viability and proliferation assays
The proliferation effects of EBPC-Ca and CaCl2on Caco-2 cells were evaluated according to MTT (Methyl Thiazolyl Tetrazolium)assay [20]. The cells with a density of 5 × 105cells/well were seeded in a 96 well micro-titer plate and incubated with 5% CO2at 37 °C for 24 h. The prepared EBPC-Ca samples with different concentrations of 0.5, 1.0, 2.0, 4.0 and 8.0 mg/mL were added. After 20 h, the media was removed, and an aqueous of 100 μL MTT solution was added.Afterward, the plate was kept at the same incubation condition for another 4 h, and then 100 μL DMSO were added to dissolve the formed formazan crystals. The viability of the cells was evaluated by reading the absorbance at 570 nm using a Multimode plate reader(FluoSTAR Omega, BMG Labtech). The cell viability ratio was then calculated with reference to untreated control, and cell viability less than 70% was considered as toxic [21].
Ethidium bromide and Acridine orange (Himedia, India) staining were commonly employed for cells imaging. In the present study,Caco-2 cells with a density of 5 × 105cells/well were incubated with selected concentrations of calcium glucoheptonate for 24 h. And then they were washed with PBS,fixed in chilled methanol, stained with ethidium bromide and acridine orange, and the cell images were observed under fluorescent cell imager (ZOE, BioRad).
2.9 Flow cytometry to detect apoptosis
Annexin V-Alexa Fluor 488/PI Apoptosis Detection Kit(Yeasen Biotech Co., Ltd., Shanghai, China) was employed for the measurement. The cell suspension from the sample-treated culture was prepared by digesting with trypsin (without EDTA) and washing twice with pre-cooled PBS (pH 7.2). The cells were re-suspended in 100 μL binding buffer and then incubated with Annexin V-Alexa Fluor 488 and propidium iodide (PI) for 15 min at room temperature.Finally, 400 μL binding buffer was mixed and apoptosis analysis was carried out by using flow cytometer (Beckman Coulter, Inc.,Shanghai, China) [22].
2.10 Measurement level of cell ROS
The quantitative detection of intracellular reactive oxygen species was carried out by using Reactive Oxygen Species Assay Kit (Yeasen Biotech Co., Ltd., Shanghai, China). After the incubation, the detectable cell confluence can reach 50%–70%. Caco-2 cells were treated with EBCP-Ca (0, 0.5, 1.0, 2.0, 4.0 and 8.0 mg/mL) for 1.5 h. The cells were washed 3 times with PBS (pH 7.2), and then stained with 10 μmol/L DCFH-DA for 20 min at 37 °C. The absorption value was read by using fluorescence microscope (EVOS FL, Thermo Fisher Scientific,Waltham, Massachusetts, US) after washing with PBS thrice.
2.11 Cell cycle assay
The distribution of cells in different growth phases was measured by flow cytometer. PI is a double-stranded DNA fluorescent dye that can produce fluorescence after intercalating into double-stranded DNA, and the intensity of fluorescence is proportional to the doublestranded DNA content. Caco-2 cells were treated with different EBPC-Ca concentrations of 0, 2.0 and 8.0 mg/mL, respectively.After incubation for 48 h, the cells were washed with precooled PBS(pH 7.2) andfixed in 70% ethanol at 4 °C overnight. Then, PI (10 μg/mL)and ribonuclease (RNase) A (50 μg/mL; TransGen Biotech) were added to the cells, incubated at 37 °C for 30 min, and analyzed using flow cytometer.
2.12 Alizarin Red S staining for calcium uptake
Calcium uptake of EBPC-Ca was monitored through the incubation of Caco-2 cells in a six-well plate with selected EBPC-Ca concentrations for one day. After the incubation period, the treated cells were washed with PBS andfixed in 4% paraformaldehyde for 20 min at room temperature. And then they were stained with freshly prepared Alizarin Red (2% aqueous solution, pH 4.2), washed with PBS, and images of the stained cells were captured using an inverted microscope (Eclipse Ts2, Nikon Instruments Co., Ltd., Shanghai,China) under 10× magnification. The intensity of the stain uptake was used to assess the calcium accumulation by comparing with the untreated control [23]. Alizarin red stain was extracted from the cells with DMSO and quantified at 405 nm using a micro-plate reader.
2.13 Determination of mitochondrial membrane potential(ΔΨm)
Caco-2 cells (1.5 × 105cells/well) were seeded on 6-well plates and incubated for 1 day. And then, the cells were treated with 300 nmol/L AIPs for another 24 h. After adding with EBPC-Ca, the cells were washed with 50 mmol/L HEPES buffer thrice, harvested and stained with 5 μg/mL tetraethylbenzimidazoly-carbocyanine iodide (JC-1) (Thermo Fisher Scientific, USA) for 15 min at room temperature. After JC-1 treatment, the cells were centrifuged and analyzed using flow cytometer (Becton Dickinson and Company,Cytek FACSCalibur, USA).
2.14 Western blotting analysis
Caco-2 cells (1 × 106cells/well) were seeded on a 6-well plate and cultured for 7 days. After that, the cells were treated with calcium glucoheptonate and harvested by centrifugation (12 000 r/min for 30 min at 4 °C). The cell protein was extracted using lysis buffer(50 mmol/L Tris-HCl, 0.5% SDS, 250 mmol/L NaCl, 5 mmol/L EDTA, 50 mmol/L NaF) and quantified using BCA Protein Assay Kits (Beijing Solarbio Science & Technology Co., Ltd., Beijing,China). An equal amount of protein was resolved on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane (Beyotime, Shanghai, China). The membrane was blocked with 5% BSA and incubated with primary antibodies(rabbit anti-human-FGF-23 antibody, Sigma, USA) at 1:1 000 dilutions overnight at 4 °C. The membranes were also re-probed with 1:5 000 rabbit anti-β-actin antibody (catalog no. A2066; Sigma), and then incubated with 1:5 000 goat anti-rabbit HRP-linked secondary antibody (catalog no. 7074S; Cell Signaling, Danvers, MA, USA)for 2 h at room temperature. The immune-reactive proteins were visualized using Enhanced ECL Chemi-luminescent Substrate Kits(Pierce ECL western blotting substrate, Yeasen Biotech Co., Ltd.,Shanghai, China) under alpha chemi-luminescence gel imaging system. Protein expression levels were determined by densitometric analysis using Fluor Chem FC3.
2.15 Statistical analysis
Results were expressed as mean ± standard deviation. Two sets of independent data were compared by the unpaired Student’st-test. The analysis of variance (ANOVA) for multiple sets of data was employed by the single factor analysis of Tukey’s multiple comparison tests.Significance was checked by Origin 9.1 (OriginLab Software,North Carolina, Massachusetts, USA), and the data was considered significant whenP< 0.05.
3. Results
3.1 Component analysis
Table 1 shows the basic ingredients analysis of European eel bone powder (EB), EBCP and EBPC-Ca. EB contains(38.48 ± 0.31)% protein, (16.89 ± 1.47)% fat, (6.86 ± 0.23)% moisture,and (61.26 ± 0.62)% ash. Moreover, it is rich in calcium ((7.56 ±0.45)%) and phosphorus ((4.27 ± 0.03)%), giving a molar ratio of calcium to phosphorus of 1.77, which is similar to that of mammals(1.68) and humans (1.69) [24]. After been enzymatically hydrolyzed into polypeptide (EBCP), its contents of calcium and phosphorus significantly decreased to (0.39 ± 0.02)% and (0.21 ± 0.01)%,respectively, indicating that calcium and phosphorus may be decomposed during enzymatic hydrolysis. Impurity has a little effect on the chelation reaction of eel bone polypeptide and calcium ions. The calcium content of EBPC-Ca is (9.45 ± 0.03)%, which is significantly higher than that contained in EBCP, suggesting a good chelation effect of the eel bone polypeptide on calcium ions.
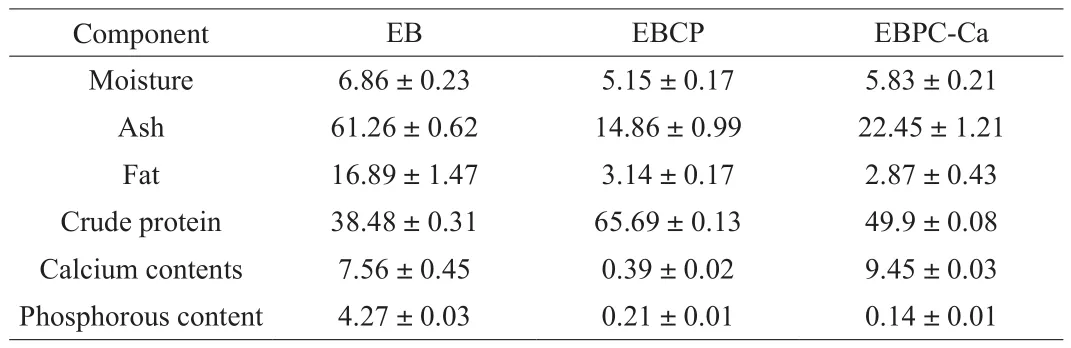
Table 1Analysis results of basic components (%) in EB, EBCP and EBPC-Ca.
3.2 Comparison of free fatty acid (FFAs) of EB, EBCP and EBPC-Ca
The function of a protein or peptide mainly depends on its amino acids composition. The amino acid compositions of EB, EBCP and EBPC-Ca are shown in Table 2. It was observed that amino acids greatly changed in European eel bone sample after hydrolyzation.Moreover, cysteine, glutamic acid, serine, threonine and alanine in eel bone polypeptide were 133.11, 20.07, 69.99, 56.60 and 46.69 mg/g,respectively, which are relatively higher than other amino acids.After chelating with calcium ions, EBPC-Ca contains higher contents of serine (171.67 mg/g), threonine (136.70 mg/g), glutamic acid(52.45 mg/g), and alanine (127.56 mg/g). These 4 amino acids had been considered as the main sites for calcium binding that may relate to the ability of peptides to chelate calcium [25,26]. As shown in Table 2, the contents of positively charged amino acids and negatively charged amino acids in EBCP are 49.08 and 32.42 mg/g, respectively.After chelation of calcium ions (EBPC-Ca), negatively charged amino acids and positively charged amino acid contents were increased to 83.09 and 87.22 mg/g, respectively. Beside, calcium chelated polypeptide (264.41 mg/g) had a higher content of hydrophobic amino acids than eel bone polypeptide (111.61 mg/g). The results showed that the carboxyl groups of negatively charged amino acids (Glu and Asp), the residues of positively charged amino acids (Lys, Arg and His) and hydrophobic amino acid residues are critical in the peptide calcium binding process.
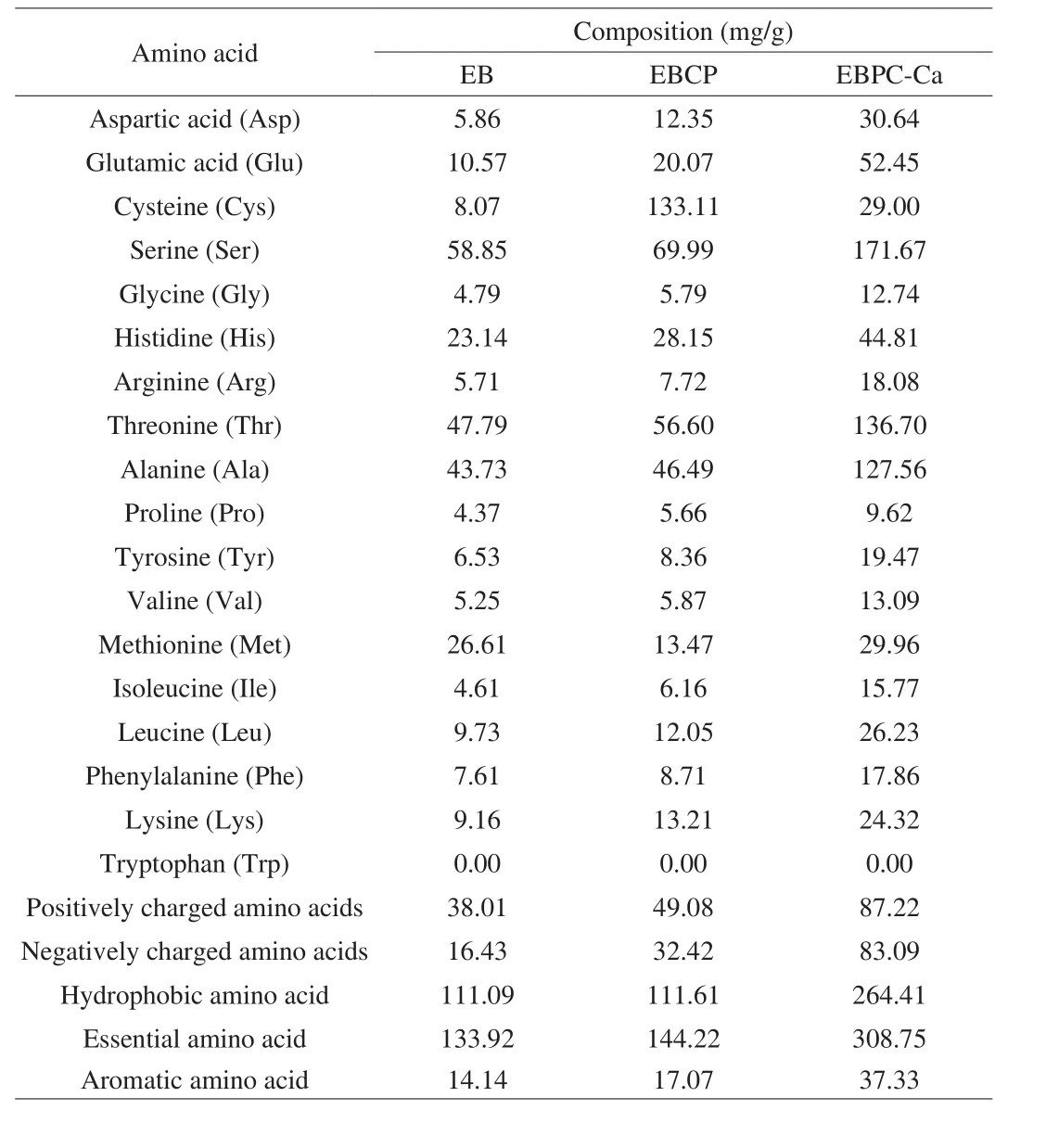
Table 2Comparison of amino acid compositions in EB, EBCP and EBPC-Ca.
3.3 Size distribution
The particle size, which depends on the volume of particles, is an important parameter to evaluate the physical character of composite materials. The typical particle size distributions of EBCP and EBPC-Ca complex nanoparticles are shown in Fig. 1A. The average sizes for EBCP and EBPC-Ca were (295.8 ± 13.4) nm (PDI: 0.413 ± 0.011)and (369.1 ± 15.7) nm (PDI: 0.551 ± 0.008), respectively. Compared to EBCP, the particle size of EBPC-Ca complex was partially larger,which indicated the occurring of structural folding between EBCP and calcium ion. A good agreement was confirmed in the UV-Vis absorption spectrometry (Fig. 1B), validating that the formation of the EBPC-Ca complex was able to induce the aggregation and folding changing of peptides. Moreover, changes in PDI further indicated the mono-dispersity of EBPC-Ca. Previously published works also revealed the similar phenomenon for a peptide-calcium complex [26,27].
3.4 UV-Vis absorption characters
The formation of organic ligand and metal ions complexes could cause the appearance of the shift/disappearance of original absorption peaks or new absorption peaks. The UV-Vis spectrum of EBCP and its chelated calcium showed a significant difference (Fig. 1B). The maximum absorption peak of EBCP was observed near 210 nm, which usually corresponds to specific amide bonds, carboxyl, and carbonyl in the peptide. The absorption band around 280 nm was corresponded to the absorption of aromatic amino acid residues. It is worth noting that the intensities of absorption bands at approximately 210 and 280 nm were red-shifted and significantly increased, with the increase of CaCl2concentration. The UV absorption intensity of EBCP was 2.12,however, the value decreased to 1.90 after the chelation reaction,which indicated that carbonyl oxygen and amino nitrogen in the peptide bond was combined with calcium ions and amide bond was changed as well. The increase in the absorption intensity of human collagen calcium complex could lead to a red-shifted phenomenon of the maximum absorption. Thus, it may indicate that the carbonyl oxygen and amino nitrogen in the peptide bond combine with calcium ions and change the electron cloud structure of the amide bond [28].
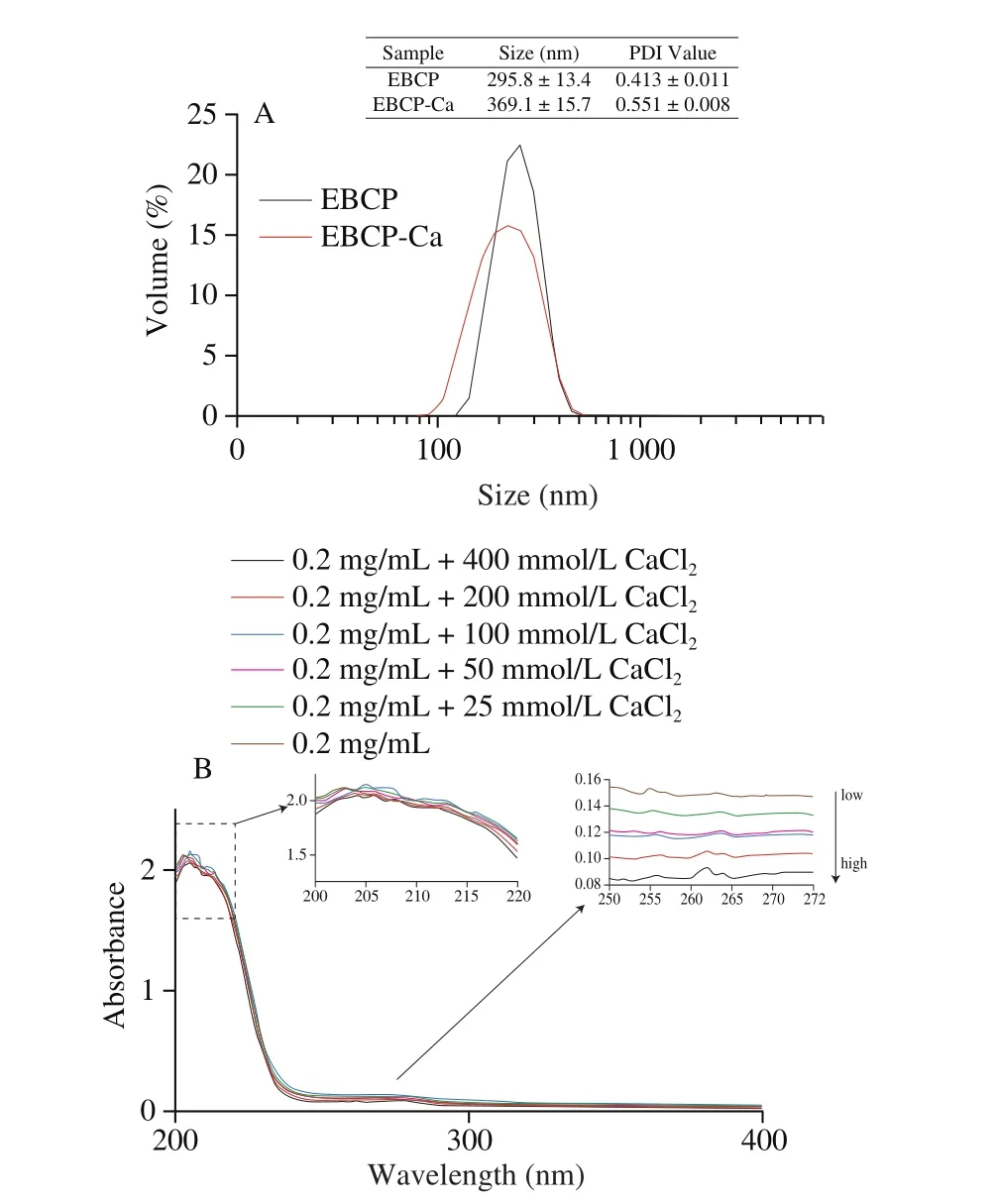
Fig. 1 Size distribution (A) and UV-Vis spectra (B) of EBCP and the EBPC-Ca complex.
3.5 Effect of EBPC-Ca on the proliferation of Caco-2 cells
The proliferative effect of EBPC-Ca (0.125–8 mg/mL) was tested using MTT assay and the results are shown in Fig. 2A. Comparing to the control of calcium chloride, EBPC-Ca in the same concentration level displayed a higher proliferation effect on Caco-2 cells. No significant decline in survival rate was found as well when EBPC-Ca concentration less than 2 mg/mL, but a remarkable reduction was checked at the highest tested concentration of EBPC-Ca (8 mg/mL).
Thus, EBPC-Ca of 2 and 8 mg/mL were selected for further studies. Fig. 2B shows the cellular morphology of Caco-2 cells treated with EBPC-Ca, in which a significantly higher number of viable cells were detected and the cytotoxicity was negligible.
3.6 Apoptosis induction in Caco-2 cells
The use of PI in conjunction with Annexin V-FITC is able to distinguish early and late phase of apoptotic cells and discriminate dead cells as well. Specifically, the cells stained only by Annexin V-FITC are considered as early apoptotic stage, and those stained by both Annexin V-FITC and PI are in late apoptotic stage. The lower left part of Fig. 2C represents normal cells, the upper left shows debris,the lower right displays early apoptotic cells, and the upper right displays late apoptotic cells. The ratio for untreated cells was 75.98%,which was significantly higher than that of lipopeptide-treated cells(64.93%). The apoptotic ratio for all lipopeptide treated cells was 31.07%, which was much higher than untreated cells (21.97%).Hence, it was found that the high concentrations of EBPC-Ca significantly promoted the apoptosis of Caco-2 cells. Previously studies revealed the incubation with polyphenol compounds and metal complexes for 24 h can induce Caco-2 cell death through a form of programmed necrosis called necrosis, which is independent of caspase [29]. Interestingly, EBPC-Ca combined treatments for 24 h did not either change the quantities of cell or activated receptorinteracting protein kinase 1 (RIP-1), which is one of the key regulators of necrosis [30].
3.7 Induction of ROS burst in Caco-2 cells
Oxygen free radicals and their derivatives (ROS) are known as strong oxidants in aerobic organisms. DCFH-DA could be hydrolyzed by intracellular esterase to produce DCFH, and the reactive oxygen species in cells can then oxidize non-fluorescent DCFH to produce fluorescent DCF. Thus, the intracellular ROS level is able to be detected by DCF fluorescence, and higher fluorescence intensity stands for higher ROS level. As shown in Fig. 2D, the fluorescence of EBPC-Ca (0, 2 and 8 mg/mL) treated cells was very significantly stronger than that by untreated cells (P < 0.001). In the presence of ROS inhibitors, the viability of cells treated with EBPC-Ca was higher (Fig. 2D), indicating that the inhibitory effect of EBPC-Ca on cell viability might be partly caused by the ROS increase.
In this study, it was also checked that the EBPC-Ca can induce ROS bursts, causing oxidative damages to cell membrane, proteins,and the DNA. This result is consistent with the previously reported work for ROS bursts effect of surfactin on MCF-7 cells. ROS has been clearly verified effect in regulating intrinsic apoptotic pathway [31],which indirectly promotes the formation of apoptotic bodies. When ROS burst promoted cell apoptosis, the inhibitory effect of EBPC-Ca on cell viability was not eliminated, indicating that the inhibitory effect of EBPC-Ca on Caco-2 cells may be related to the apoptosis experiment.
3.8 Inhibition effect on the Caco-2 cell cycle
The cell viability results in Fig. 2A indicated Caco-2 cell cycle might be affected by EBPC-Ca. Therefore, cell cycle was confirmed using flow cytometer and the result was shown in Fig. 2E. It was found that the control (without treatment) maintained a quite stable growing throughout, but the portion of cells in the G1 phase significantly increased after EBPC-Ca treatment, meanwhile, a significant decline was checked in S phase. An aneuploidy peak was also noted in lipopeptidetreated cells (Fig. 2E), which may indicate apoptosis.
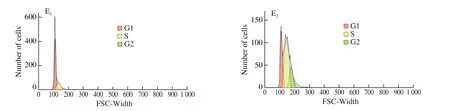
Fig. 2 (Continued)
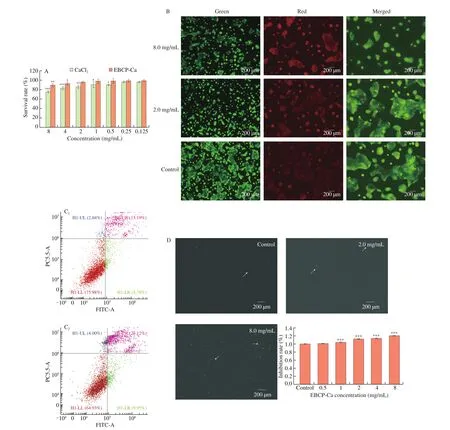
Fig. 2 Effect of EBPC-Ca on the proliferation of Caco-2 cells. (A) MTT assay. (B) Photomicrographs of live/dead stained Caco-2 cells treated with selected concentrations of EBPC-Ca in comparison to untreated control (Scale bar 100 μm). The green fluorescence represents the nuclei stained with acridine orange indicating viable cells and red fluorescence represent the nuclei stained with ethidium bromide. Cell distribution diagram of Annexin V-FITC/propidium iodide staining (C). ROS detection by the DCFH-DA analysis and the inhibitory effect of EBPC-Ca on cell viability (D). Flow cytometry to detect cell cycle of untreated cells and cells treated with 8 mg/mL EBPC-Ca (E). The higher red peak indicates the number of cells in the G1 phase, whereas the lower red peak represents the number of cells in the G2/M phase. The number of cells in the S phase is that between the two red peaks. Values are mean ± standard error of at least 3 independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001.
Shen et al. [32] found that the inhibitory activity of necroptosis could cause cell death through apoptosis. Therefore, the tendency for apoptotic cell rate and cycle (Figs. 2C and 2E) may be relative to the destruction of the necrotic process caused by the simultaneous administration of EBPC-Ca. Consistently, autophagy seems to play an important part in inhibiting or promoting other forms of cell death,due to its dual cytoprotective and cytotoxic properties.
3.9 Increased Ca2+ accumulation in Caco-2 cells
Ca2+is a second death signal or messenger, which mediates apoptosis by activating some nucleases and protein kinases associating with apoptosis induction [33]. According to the staining intensity of Alizatin Red, cell accumulation ability of calcium was quantified. It was found that the accumulation of calcium enhanced with the increase of sample dose, as indicated by the intense of red coloration (Fig. 3). Comparing to the control, the sample treated group showed higher calcium uptake and retention rate, and calcium absorption was increased by 100% at the EBPC-Ca concentration of 1.0 mg/mL (Fig. 3).
The mechanism of EBPC-Ca for Caco-2 inhibition is supposed to be related to the differences of mitochondrial potential, the increase of Ca2+concentration, and the activation of FGF-23. Mitochondria,known as Ca2+pool, are the energy source for the whole cell and help to regulate the normal operation of cells [32]. When Ca2+enters the mitochondrial matrix, abnormal functions would be caused, leading to ROS breakdown [34]. And this imbalance between Ca2+homeostasis and mitochondria triggers cell apoptosis. A similar work had reported the lipo-peptide from Fengycin was able to killed cancer cells through inducing the accumulation of ROS, uptaking Ca2+, and activating caspase-12 pathway [35].
3.10 Decreased mitochondrial membrane potential in Caco-2 cells
The prerequisite for supplying energy to cells is a normal mitochondrial membrane potential, and a decrease in mitochondrial membrane potential induces early apoptosis. Aggregated JC-1 reveals red fluorescence, indicating a higher mitochondrial membrane potential. In a contrast, monomeric JC-1 dye of fluorescent green suggests a lower potential for mitochondrial membrane. As shown in Fig. 4A, the most of EBPC-Ca treated cells fluoresced green,whereas the control (untreated cells) displayed red, indicating the mitochondrial membrane potential of Caco-2 cells was decreased by EBPC-Ca. And meanwhile, we deduced the permeability of mitochondrial membrane was increased by EBPC-Ca as well.
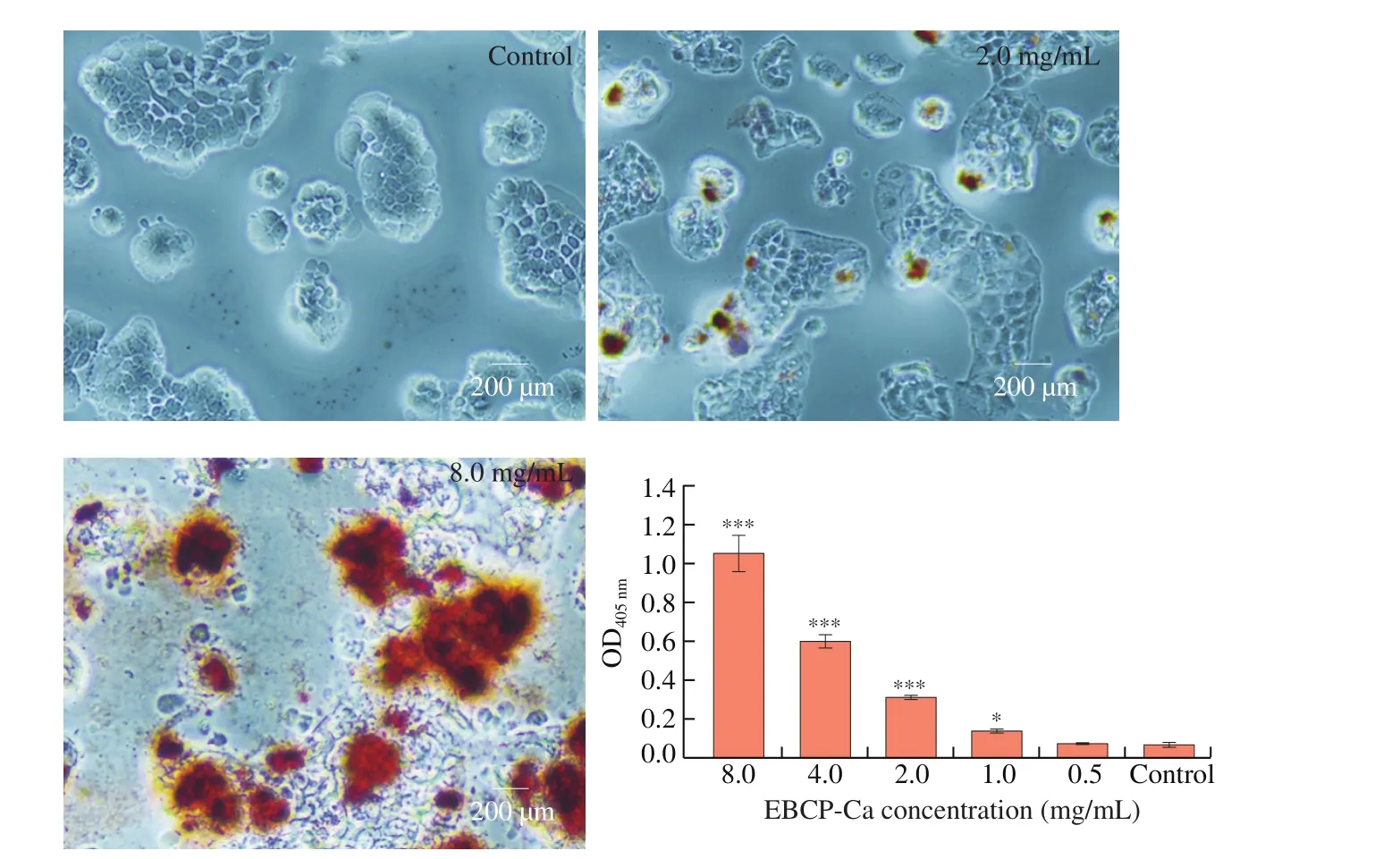
Fig. 3 Quantification and visualization of calcium uptake and retention rates in Caco-2 cells. Values are mean ± standard error of at least 3 independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001.
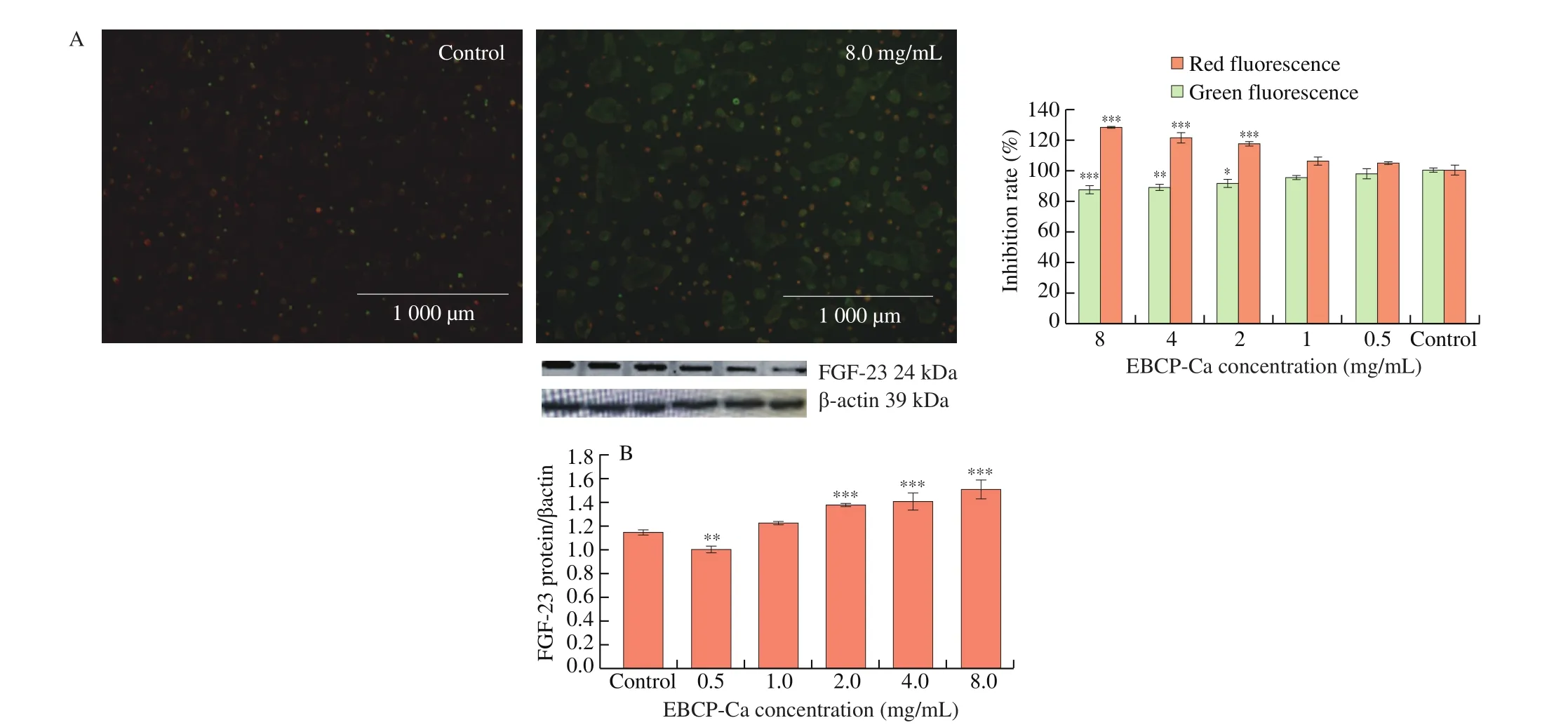
Fig. 4 Mitochondrial membrane potential detected by JC-1 dye (A). Changes in the expression pattern of FGF-23 in Caco-2 cells treated with EBPC-Ca for seven days (B). Western blot for EBPC-Ca respectively. Values are mean ± standard error of at least 3 independent experiments.* P < 0.05; ** P < 0.01; *** P < 0.001.
3.11 The effect of EBPC-Ca on gene and protein expressions of Caco-2 calcium uptake
Fig. 4B shows the western blot results of the expression level of FGF-23. Comparing to the control, the expression level was significantly higher when EBPC-Ca concentrations more than 2.0 mg/mL,indicating higher concentration of EBPC-Ca could enhance the absorption characteristics of Caco-2 cells.
Since uncontrolled calcium absorption is undoubtedly harmful,intestinal epithelial cells should be equipped with mechanisms to prevent excessive calcium intake in the body. Studies have found that CaSR can detect calcium and calcium super-absorption in a long-term exposure to high root tips, which in turn increases the expression of FGF-23 to inhibit calcium transport [36].
4. Conclusions
The present study found that the EBPC-Ca could inhibit the proliferation of human colorectal adenocarcinoma cells(Caco-2) in both concentration- and time-dependent manners. The results showed that the addition of EBPC-Ca significantly enhanced the anti-proliferative activity, but the cell viability and drug exposure time were remarkably reduced. The chelated compound of EBPC and calcium ion triggered a strong apoptosis, which may be the result of a blocked autophagy flux. Regarding unpaired autophagy,the significant pro-oxidation effect of drug combination caused a destruction of mitochondrial function, leading to cell apoptosis.Thus, it was found that the pathway of cell death was related to mitochondrial dysfunction as induced by ROS accumulation, Ca2+concentration, abnormal metabolism, and protein expression, and those factors promote interactively and ultimately leading to cell death. Overall, EBPC-Ca has shown a potential for anticancer drug development.
Con flicts of interest
The authors declare that there are no con flicts of interest.
Acknowledgments
This work is supported by National Natural Science Foundation of China (NSFC, Grant No. 31801459, Grant No. 32072209); China Postdoctoral Science Foundation Funded Project (2020M682073),the Science and Technology General Projects of Fujian Province(2019J01393). We are grateful to the Program of Innovative Research Team in Science and Technology in Fujian Province University([2020]12), for providing the equipment used in this research.
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas
- Protective effects of tilapia (Oreochromis niloticus) skin gelatin hydrolysates on osteoporosis rats induced by retinoic acid

