A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
Forough Jahandideh, Jianping Wu,*
a Department of Agricultural, Food and Nutritional Science, Faculty of Agricultural, Life and Environmental Sciences, University of Alberta, Edmonton, AB, T6G 2P5, Canada
b Cardiovascular Research Centre, Faculty of Medicine & Dentistry, University of Alberta, Edmonton, AB, T6G 2S2, Canada
ABSTRACT
Insulin resistance leads to impaired glucose metabolism by disrupting both insulin secretion and sensitivity.Insulin resistance plays a key role in the pathophysiology of type 2 diabetes and metabolic syndrome. Reviews on the mechanisms of action of bioactive peptides on glucose homeostasis and insulin resistance are scarce.The recent discoveries of pathways and target cells in the management of glucose and energy metabolism have opened up new opportunities for identification of novel bioactive peptides on enhancing adipocyte differentiation and insulin signaling, glucose uptake, cholecystokinin receptor expression and activation,as well as insulin mimetics and incretin stimulants. Examples of food-derived bioactive peptides with glucoregulatory properties include Trp-Glu-Lys-Ala-Phe-Lys-Asp-Glu-Asp (WEKAFKDED), Gln-Ala-Met-Pro-Phe-Arg-Val-Thr-Glu-Gln-Glu (QAMPFRVTEQE), Glu-Arg-Tyr-Pro-Ile-Leu (ERKPIL), Val-Phe-Lys-Gly-Leu (VFKGL), Phe-Leu-Val (FLV), Val-Pro-Pro (VPP), Ile-Arg-Trp (IRW), Ala-Lys-Ser-Pro-Leu-Phe(AKSPLF), Ala-Thr-Gln-Pro-Leu-Phe (ATNPLF), Phe-Glu-Glu-Leu-Gln (FEELN), Leu-Ser-Val-Ser-Val-Leu(LSVSVL), Val-Arg-Ileu-Arg-Leu-Leu-Gln-Arg-Phe-Asn-Lys-Arg-Ser (VRIRLLQRFNKRS), and Ala-Gly-Phe-Ala-Gly-Asp-Asp-Ala-Pro-Arg (AGFAGDDAPR). However, as yet, clinical evidence on the efficacy of such bioactive peptides is rare but is inevitable to establish their applications against glucose intolerance and insulin resistance.
Keywords:
Adipose tissue
Bioactive peptides
Glucose homeostasis
Insulin resistance
Metabolic syndrome
1. Introduction
Metabolic syndrome (MetS) is a cluster of risk factors including hypertension, dyslipidemia, impaired glucose tolerance, and central obesity, which synergistically increases the risk of cardiovascular disease (CVD) and type 2 diabetes [1,2]. The prevalence of MetS is high and increasing rapidly worldwide. Individuals with MetS are essentially at twice the risk for developing CVD over the next 5 to 10 years and aboutfive times the risk for developing type 2 diabetes compared with those without the syndrome [3]. The high prevalence of MetS and its significant association with CVD has emerged this disease as both a public health concern and a clinical problem [2].
There are many unclear key aspects in delineation of risk factors that predispose an individual to MetS. The great variation in susceptibility and age of onset in individuals with similar risk profile suggests a major interaction between genetic and environmental factors. Obesity and insulin resistance play key roles in the pathophysiology of MetS [4].
Increased secretion of insulin by pancreatic β-cells(hyperinsulinemia) can maintain normal glucose levels in the initial stages of insulin resistance but when β-cell secretion fails to meet body insulin requirements, severe insulin resistance will lead to glucose intolerance and hyperglycemia [5]. Hyperglycemia also impairs insulin sensitivity. Therefore, in MetS, insulin resistance plays a key role in establishing a vicious circle leading to hyperinsulinemia and hyperglycemia further exacerbating insulin resistance. While insulin normally suppresses lipolysis in adipose tissue by inhibition of hormone-sensitive lipoprotein lipase [6], unrestrained lipolysis leads to excessive release of free fatty acids into the circulation in insulin resistance state. Increased circulating free fatty acids may also inhibit glucose uptake in peripheral tissues, resulting in further exacerbation of insulin resistance [5].
Bioactive peptides, released from their parent proteins mainly through enzymatic digestion or fermentation, have a great potential for the development of functional foods and/or nutraceuticals for the prevention and management of chronic diseases [7-12]. Indeed,bioactive peptides have shown benefits towards oxidative stress [13-17],inflammation [18-22], cancer [23,24], hypertension [25-32],hyperlipidemia [33,34], microbial infections [35,36], and bone health [37-40]. Bioactive peptides also have the potential to improve glucose homeostasis and insulin sensitivity through affecting different targets in the body including carbohydrate digestion, gut hormone release, insulin secretion and function, glucose uptake, and adipose tissue modification. In this review, we focus on the potential of bioactive peptides with beneficial effects on glucose metabolism towards carbohydrate digestion and glucose homeostasis. Given the key interactions between insulin resistance and adipose tissue dysfunction in the development of MetS, the biological functions of bioactive peptides on adipocyte differentiation, leading to smaller adipocytes for fat storage and reduced free fatty acids and triglycerides content in the circulation, liver, and muscle, are also discussed. Table 1 summarizes a few examples of representative bioactive peptides affecting different targets to modulate glucose homeostasis and insulin sensitivity.

Table 1Representative food-derived bioactive peptides with potential benefit on glucose metabolism and handling.
2. Targets towards carbohydrate digestion
During carbohydrate digestion,α-amylase, which breaks down long-chain carbohydrates, and intestinal brush borderα-glucosidase,which is required for the final step to release absorbable monosaccharides, are released. Therefore, these two enzymes are the targets for developing approaches for controlling blood glucose.
α-Amylase inhibitory peptides have been identified from a variety of food sources as reviewed elsewhere [41]. Peptides with inhibitory activities againstα-amylase were identified from pinto bean [42,43],cumin (Cuminum cyminum) seed [44], as well as rambutan (Nephelium lappaceumL.) and pulasan (Nephelium mutabile) seed proteins [45].α-Amylase inhibitory peptides have the capability to attach themselves to the catalytic and substrate binding sites of the enzyme and prevent theα-amylase from binding or hydrolyzing the substrate(carbohydrate polymers).α-Amylase inhibitory peptides may also attach to the starch and prevent it from digestion [45].
Theα-glucosidase inhibitors are a class of nonsystemic drugs that delay but do not prevent the absorption of ingested carbohydrates and prolong overall carbohydrate digestion time, causing a reduction in the rate of glucose absorption and eventually, leading to reduced postprandial blood glucose and insulin peaks [46]. Acarbose,miglitol, voglibose, and emiglitate are α-glucosidase inhibitors used for the treatment of postprandial hyperglycemia in diabetic individuals.Flatulence, abdominal cramping, vomiting, and diarrhea are the common side effects associated with the chronic use of these agents [47].Therefore, studies have been conducted to identify natural sources of α-glucosidase inhibitors. In vitro α-glucosidase inhibitory activity has been assessed and calculated as IC50value, which is the concentration of compound required to cause a 50% inhibition of the enzyme activity. Peptides with modest α-glucosidase inhibitory activity have been identified from many food proteins including but not limited to milk [48], whey [49,50], soy [51,52], rice bran [53],quinoa [54], and brewers’ spent grain [55]. Although in vitro α-glucosidase inhibitory effect of food-derived peptides has been reported in literature, it appears that α-glucosidase inhibitory activity depends on the α-glucosidase enzyme origin [50,56]. Therefore,further in vivo studies are necessary for validating the effectiveness of these peptides in physiological conditions.
3. Targets towards glucose homeostasis
D-glucose is the primary source of energy for the cells. In order to ensure normal body function, blood glucose level needs to be controlled tightly in the body by a complex network of various hormones and neuropeptides released mainly from the brain, pancreas,liver, intestine, adipose, and muscle tissues. Intestinal absorption of glucose during the fed state, glycogenolysis (breakdown of glycogen),and gluconeogenesis (glucose production by liver during fasting state) are the three sources determining the level of circulating glucose. Indeed, the balance between the rate of glucose entering the circulation and the rate of uptake and metabolism by peripheral tissues determine the plasma glucose concentration in the body. The pancreas is the key player within this network by secreting insulin and glucagon hormones [57].
3.1 Insulin
Fasting glucose is around 4 mmol/L while after a meal the blood glucose level raises reaching to about 7 mmol/L [58]. In the fasting state, a low glucose level stimulates the secretion of glucagon, a hormone produced by the α-cells of the pancreas. Glucagon increases glycogenolysis and gluconeogenesis in liver leading to increase in blood glucose level [59]. After meal ingestion and the gastrointestinal digestion, nutrients are absorbed into the circulation via the hepatic portal vein. Therefore, liver plays a fundamental role in regulating postprandial glucose levels by controlling glucose production and storage [60]. Ingested nutrients also stimulate the release of incretins from intestinal endocrine cells. Incretin secretion together with the rise in blood glucose, stimulate insulin release from pancreatic β cells leading to glucose clearance from the blood. Insulin is secreted at a low, basal level in the non-fed state, while after a meal, insulin concentration raises making the cells take up more glucose [61].Insulin secretion is biphasic: the first phase occurring early after meal ingestion suppresses hepatic gluconeogenesis. Whereas,the second phase reaching a plateau within 1–2 h after the meal stimulates glucose uptake by skeletal muscle and adipose tissue.Although insulin does not stimulate glucose uptake in liver, it blocks glycogenolysis and gluconeogenesis, and stimulates glycogen synthesis, thus regulating glucose levels [60]. Fig. 1 summarizes glucose homeostasis in the body.
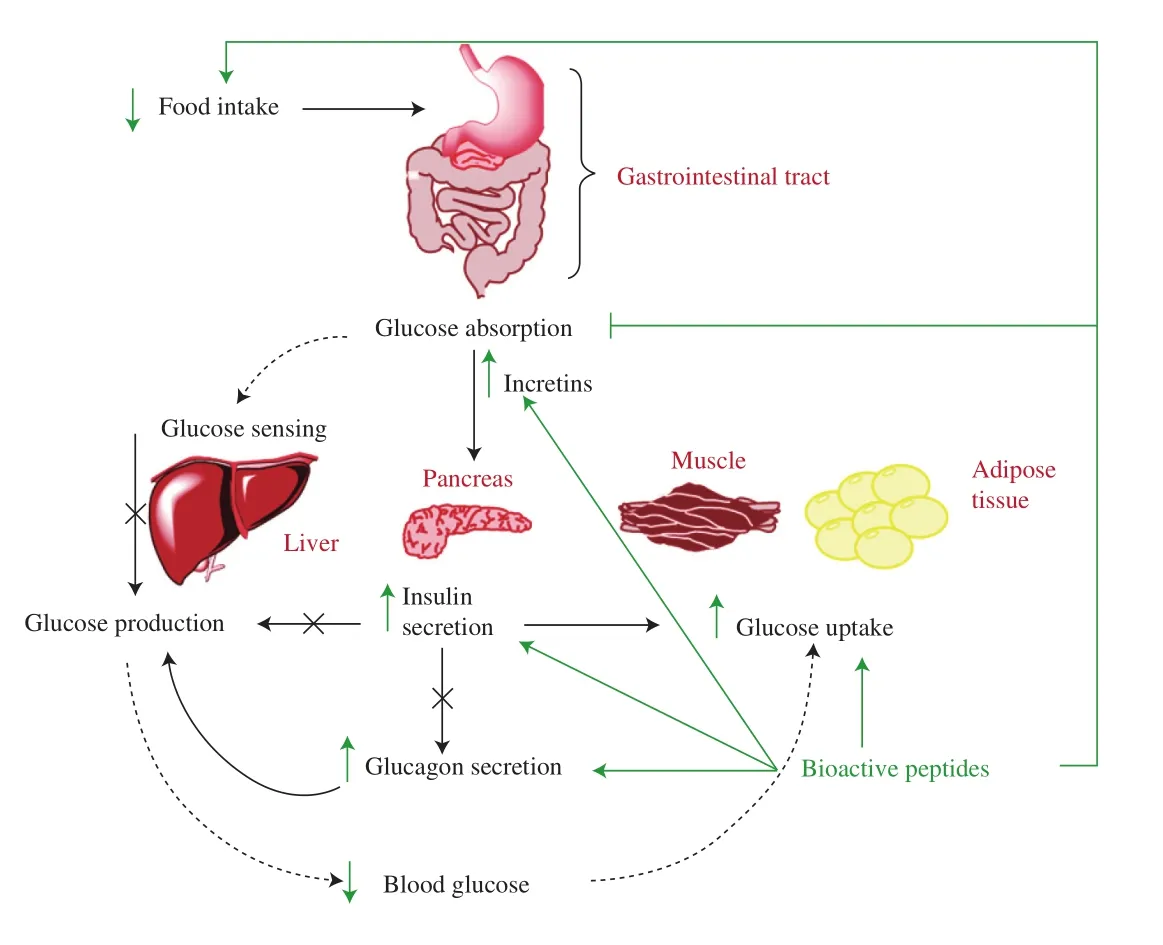
Fig. 1 Peripheral control of glucose homeostasis and potential mechanism of action of bioactive peptides on regulating blood glucose. Nutrients are absorbed into the circulation via the hepatic portal vein after digestion by the gastrointestinal tract. The liver plays a key role in regulating postprandial glucose levels by matching glucose appearance into the blood from meal ingestions (indicated by dashed arrows in thefigure) to tissue glucose usage.Ingested nutrients also stimulate the release of incretins which together with the rise in blood glucose stimulate insulin release from pancreatic β-cells.Insulin secretion suppresses hepatic glucose production on one hand and stimulates glucose uptake by insulin-sensitive tissues such as skeletal muscle and adipose tissue on the other hand. Insulin also indirectly regulates hepatic glucose production by inhibiting the release of glucagon from pancreatic α-cells. This change in insulin and glucagon levels maintains postprandial glucose levels within the normal range. Figure adapted from [60].
Insulin also regulates lipid metabolism by increasing fatty acid synthesis, increasing esterification of free fatty acids, and decreasing lipolysis [62]. An increase in free fatty acids concentrations stimulates fatty acid oxidation while inhibiting carbohydrate oxidation and glycolysis [63]. Furthermore, insulin affects protein metabolism by increasing protein synthesis and decreasing proteolysis. Insulin also controls cell growth, proliferation, survival and differentiation [62].
3.1.1 Insulinotropic peptides
Proteins/peptides stimulating insulin production tend to reduce plasma glucose levels. Insulin secretion is sensitive to the composition as well as the concentration of plasma amino acids [64]. Food proteins are believed to enhance insulin secretion [65-67]. Ingestion of milk proteins especially whey has been shown to enhance insulin secretion [68].The essential amino acids Leu (L), Ile (I), Val (V), Lys (K), and Thr (T) showed the strongest correlation with insulin response [69].However, when a mixture of these amino acids was tested in healthy subjects in a follow-up study, despite a similar glycemic response to whey ingestion, no effects on insulin and incretin secretion were observed [70]. Whey protein hydrolysate exhibited a significant increase in glucose induced insulin secretion in a pancreatic β cell line in a dose-dependent manner [71]. Oral administration of whey protein hydrolysate to ob/ob mice for 8 weeks also improved blood glucose clearance, reduced hyperinsulinemia, and restored the pancreatic islet capacity to secrete insulin in response to glucose [71]. Boarfish muscle protein hydrolysate has also shown insulinotropic and antidiabetic effects both in cells and mice [72,73].
3.2 Insulin signaling
The effects of insulin on cell metabolism are mediated by insulin receptor expressed on most cells especially on major metabolic organs such as liver, adipose and skeletal muscle. The insulin receptor is a heterotetramer composed of two α-subunits localized on the outside of the cell and a transmembrane β-subunit dimer that are linked by disulfide bonds [58]. When insulin binds to the α-subunits of insulin receptor, autophosphorylation of the receptor β-subunits occurs which leads to the recruitment and tyrosine phosphorylation of insulin receptor substrate (IRS) proteins. This generates binding sites for Src homology 2 (SH2) domain proteins including phosphatidylinositol 3-kinase (PI3K), the Ras guanine nucleotide exchange factor complex known as growth factor receptor-bound protein 2/son of sevenless (GRB2/SOS), and SH2 domain containing protein tyrosine phosphatase-2 (SHP2) [74]. Indeed, the IRS proteins phosphorylation provides the basis for the subsequently associated downstream signaling through different pathways which mediate metabolic and mitogenic responses of insulin and glucose transport type 4 (GLUT4) translocation [58,75]. The two major signaling cascades,which mediate most metabolic and transcriptional effects of insulin in adipocytes, are the PI3K and the mitogen activated protein kinase(MAPK) pathways [76]. The PI3K pathway mediates the metabolic effects of insulin including glucose/lipid/protein metabolism and insulinstimulated glucose uptake. Recruitment of dimeric PI3K (p85/p110) to IRS produces membrane phosphatidylinositol 3,4,5-trisphosphate (PIP3)through phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2).PIP3 regulates the activation of phosphoinositide dependent kinase 1 (PDK1)which in turn phosphorylates and activates protein kinase B (PKB/Akt)at threonine and serine residues [74]. Akt activation mediates the effects of insulin on glucose transport through GLUT4 translocation to the plasma membrane, glycogen and protein synthesis, lipogenesis and suppression of hepatic glucose production through different downstream targets [62]. A second essential branch of the insulin signaling pathway, activated independently of PI3K/Akt, is the MAPK pathway. This pathway controls the mitogenic, growth and cell differentiation effects. Shc plays a critical role in mediating the mitogenic effects of insulin, primarily through activation of the GRB2/SOS/Ras/MAPK pathway however, compared with PI3K, is less defined. Phosphorylation and activation of the extracellular regulated kinase 1 and 2 (ERK1/2) plays a direct role in cell proliferation and differentiation via gene transcription regulation [76,77]. Insulin signaling cascade is shown in Fig. 2.
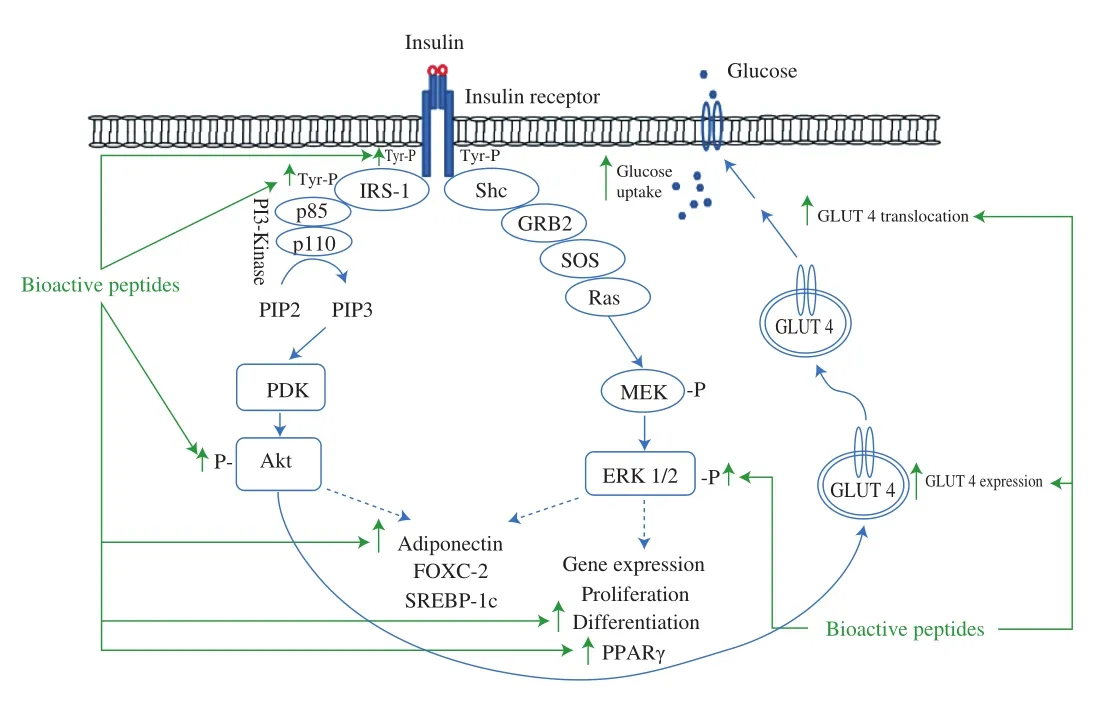
Fig. 2 The simplified insulin signaling pathway and reported targets for bioactive peptides on insulin sensitization and glucose uptake. Binding of insulin to its receptor triggers insulin receptor autophosphorylation and activation of the protein kinase that phosphorylates IRS proteins on tyrosine residues. These initial tyrosine phosphorylation reactions are the basis for conveying insulin signals to a complex network that ultimately mediates the specific biological effects of this hormone. IRS protein phosphorylation generates binding sites for PI3K, GRB2/SOS, and SHP2. Recruitment of PI3K to IRS phosphorylates PIP2 to PIP3 that leads to the activation of PDK and subsequently PDK activates the serine/threonine kinase Akt/PKB. Akt activation is important for the translocation of GLUT4 to the plasma membrane and glucose uptake. On the other hand, the MAPK pathway controlling the mitogenic, growth and cell differentiation is less well understood. Shc plays a critical role in mediating the mitogenic effects of insulin, primarily through activation of the GRB2/SOS/Ras/MAPK pathway. Phosphorylation and activation of ERK1/2 plays a direct role in cell proliferation and differentiation via gene transcription regulation. MEK, extracellular-signal-regulated protein kinase kinase. Figure drawn based on [74-76].
One of essential defects in type 2 diabetes also the defining feature of the MetS, is insulin resistance [78-80]. Insulin resistance is a pathologic state in which target cells fail to respond to normal levels of circulating insulin [75]. Therefore, insulin is unable to provide normal glucose and lipid homeostasis. Consequently, higher concentrations of insulin are needed in order to maintain normal glucose levels [6]. In MetS, insulin resistance is linked predominantly to a cluster of disorders including lipid and glucose metabolism,hypertension, and vascular in flammation. Antihypertensive peptides have shown potential in alleviating other complications of the MetS due to the interplay between the renin angiotensin system and insulin resistance [81].
3.2.1 Peptides enhancing insulin sensitivity
Enhanced insulin sensitivity is another major mechanism by which proteins and bioactive peptides ameliorate insulin resistance in the context of MetS. Fish is a potent source of biologically active proteins and peptides with beneficial effects on glucose uptake and insulin resistance. High-fat feeding has been reported to induce whole body and skeletal muscle insulin resistance in rats. Feeding cod protein to rats however, prevented the development of insulin resistance in these rats whereas casein and soy protein feeding did not. Higher rate of insulin-mediated muscle glucose disposal was also observed in cod protein fed rats [82]. The beneficial effect of sardine protein on glucose tolerance and insulin resistance has also been reported in fructose-induced MetS rats [83].
In addition to proteins, fish-derived peptides have also the potential to enhance insulin sensitivity. The effect of peptides derived from differentfish sources on glucose tolerance and insulin resistance has been studied in rats. Bonito, herring, mackerel, or salmon fish protein hydrolysates at 20% (m/m) of the feed were tested in rats fed a high fat-high sucrose diet. Rats fed salmon protein hydrolysate gained less body weight as well as epididymal adipose tissue compared to casein fed group. Although none of the fish protein hydrolysates were effective on improving glucose tolerance, interleukin 6 (IL-6)and tumor necrosis factor α (TNF-α) expression in visceral adipose tissue of rats reduced in all groups. Moreover, the whole-body insulin sensitivity was improved in the salmon protein hydrolysate fed group as measured by the hyperinsulinemic-euglycemic clamp technique [84].Since, insulin sensitivity was not assessed for the otherfish protein hydrolysate groups; it is not known whetherfish source is a crucial factor for the observed effects. Egg and soy are other protein sources containing peptides with insulin sensitizing properties [85].Recently, we showed insulin mimetic and sensitizing effects of egg white hydrolysate, prepared by thermolysin and pepsin, through cell and animal studies. Treatment of 3T3-F442A preadipocytes with egg white hydrolysate enhanced insulin effects on the upregulation of Akt phosphorylation (insulin sensitizing) as well as increased ERK1/2 phosphorylation to a level similar to that of insulin (insulin mimetic) [86]. Insulin mimetic effects of the egg white hydrolysate on ERK phosphorylation was mediated through insulin receptor β phosphorylation [86]. Treatment of insulin resistant muscle cells with egg white hydrolysate or Ile-Arg-Trp (IRW), the ovotransferrinderived tripeptide, reversed insulin resistance through increased phosphorylation of insulin receptor substrate-1 (IRS-1) (at tyrosine residue) and Akt, as well as decreased IRS-1 serine residue phosphorylation [87]. Insulin sensitizing effect of the egg white hydrolysate was further studied in an insulin resistant rat model. Oral administration of the egg white hydrolysate to high-fat diet fed rats improved glucose and insulin tolerance, as well as potentiated insulininduced Akt phosphorylation in muscle and adipose tissue of these rats [88]. Ile-Pro-Pro (IPP) and Val-Pro-Pro (VPP) are other bioactive peptides with insulin sensitizing effects associated with upregulation of Akt phosphorylation in normal and insulin-resistant adipocytes [89].
3.3 Targets towards glucose uptake
Glucose is transported into various tissues by either Na+-dependent glucose cotransporters that are not regulated by insulin, or by a family of specialized transporter proteins called glucose transporters(GLUTs). GLUT1 is ubiquitously expressed and probably mediates basal glucose uptake. GLUT2, is expressed in the liver, kidney,intestine and β-cells of the pancreas. GLUT3 together with GLUT1 is involved in non-insulin mediated uptake of glucose into the brain. GLUT4 is the only glucose transporter that is regulated by insulin and is responsible for glucose uptake in muscle and adipose tissue [61]. Upon insulin stimulation, the translocation of GLUT4 from intracellular sites to the cell surface occurs. Insulin’s primary actions are on muscle, adipocytes, and liver cells. Skeletal muscle is the major organ for the insulin-induced glucose disposal followed by the adipose tissue.
3.3.1 Peptides affecting glucose uptake
Free amino acids as well as bioactive peptides have been reported to enhance glucose uptake in rodents. Leu was shown to increase glucose uptake in soleus muscle of normal SD rats via PI3K and protein kinase C (PKC) dependent pathways [90]. Ile is another amino acid with reported beneficial effects on glucose uptake. Single oral administration of Ile to SD rats significantly reduced plasma glucose level at 30 and 60 min in an oral glucose tolerance test. Plasma insulin levels at 30 and 60 min after glucose bolus were also lower than that of the control group, which reveals that insulin secretion was not affected by Ile. The signaling pathway analysis in C2C12 myotubes suggested that similar to Leu, PI3K and PKC were involved in the enhancement of glucose uptake by Ile [91]. Feeding Wistar rats with commercial whey protein (Hilmar product 8000) or whey protein hydrolysate (Hilmar product 8350) enhanced GLUT-4 translocation and glycogen synthesis in liver, heart and skeletal muscle of rats.Enhanced muscle Akt phosphorylation in rats fed with whey protein hydrolysate can be partially responsible for the observed enhanced GLUT-4 translocation to the plasma membrane [92]. Pre-incubation of muscle cells with egg white hydrolysate or IRW recovered TNF-induced impaired glucose uptake and GLUT4 abundance on muscle cell membrane through activation of IRS-1 tyrosine residue and Akt,and decreased activation of IRS-1 serine residue [87]. VPP treatment of insulin resistant adipocytes also enhanced GLUT4 expression in adipocytes and restored glucose uptake in these cells [89].
3.4 MP-activated protein kinase (AMPK) pathway
Apart from the insulin-dependent pathway for glucose uptake,insulin-independent pathways also contribute to glucose uptake by cells. AMP-activated protein kinase (AMPK) is one of these pathways involved in insulin-independent glucose uptake, enhanced insulin sensitivity, and glucose homeostasis. Physiological, pharmacological,natural, and hormonal stimuli can activate AMPK [93]. Although the net effect of AMPK activation is to restore energy balance through inhibition of energy consuming processes and promoting energyproducing processes, it is also involved in glucose homeostasis process. Increase in AMPK activity in skeletal muscle results in enhanced glucose transport rate. Fig. 3 depicts insulin dependent and independent pathways for glucose uptake in muscle.
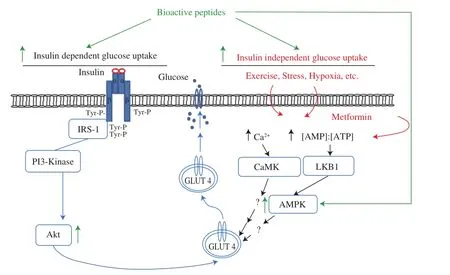
Fig. 3 Insulin-dependent and independent glucose uptake in muscle.Glucose uptake in skeletal muscle occurs through both insulin dependent and -independent pathways under different physiological conditions. Stimuli(exercise, hypoxia, stress etc.) activate several signaling proteins (e.g., AMP-activated protein kinase (AMPK), Ca2+/calmodulin-dependent protein kinase(CaMK), etc.). An increase in AMP/ATP ratio activates AMPK, while the increase in Ca2+ concentration activates CaMK which subsequently induce glucose transport by enhancing translocation of the GLUT4 to the cell membrane. The signaling mechanism of AMPK and glucose transport remains largely undefined. For insulin-dependent glucose uptake see text (section 3.2).Bioactive peptides have been reported to increase glucose uptake through both insulin dependent (as discussed before) and independent pathways (mainly through activation of AMPK). Akt, protein kinase B/Akt; IRS-1, insulin receptor substrate-1; LKB1, liver kinase B1; PI3K, phosphatidylinositol 3-kinase. Figure adapted from [94-96].
3.4.1 Peptides activating AMPK pathway
While AMPK is dysregulated in animals and humans with MetS or type 2 diabetes, physiological or pharmacological activation of AMPK induces insulin-independent glucose uptake and insulinsensitizing effects. AMPK is an energy-sensing serine/threonine kinase that is activated by two distinct signals: i) Ca2+-dependent pathway and ii) AMP-dependent pathway [97]. Therefore, conditions that can alter the intracellular AMP/ATP ratio and calcium concentration can affect AMPK. Numerous physiological (such as exercise, fasting, and calorie restriction), pharmacological (such as metformin and AICAR), natural (resveratrol and berberine), and hormonal (such as adiponectin, IL-6 and leptin) activators of AMPK have been reported [93]. Activation of AMPK stimulates glucose uptake in skeletal muscle, fatty acid oxidation in adipose tissue, and reduces hepatic glucose production [98]. Overall, AMPK activation improves insulin sensitivity and glucose homeostasis.
The fact that the AMPK-mediated glucose uptake is shown to be functional in muscle cells from patients with type 2 diabetes while insulin-induced glucose uptake is impaired [99], further highlights the importance of this pathway. Metformin, the potent antihyperglycemic drug from the biguanide family is the first line oral therapy for type 2 diabetes. The main effect of metformin is to acutely reduce hepatic glucose production, mostly through inhibiting mitochondrial respiratory-chain complex 1. The reduction in liver energy status further activates AMPK providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program [100,101].
Although protein hydrolysates and bioactive peptides have shown potential effects on AMPK activation, insulin independent pathways for glucose uptake are less explored compared to the insulin dependent pathways. The low molecular weight (300–500 Da)fractions of soybean peptides separated by the electrodialysis with an ultrafiltration membrane have been reported to improve glucose uptake in L6 muscle cells in the presence of insulin. These charged peptides also activated AMPK in muscle cells, however, no increase in glucose uptake was observed in the absence of insulin in these cells [102]. Soy glycinin-derived peptides; Ile-Ala-Val-Pro-Gly-Glu-Val-Ala (IAVPGEVA), Ile-Ala-Val-Pro-Thr-Gly-Val-Ala(IAVPTGVA), and Leu-Pro-Tyr-Pro (LPYP), have been reported to modulate glucose metabolism in HepG2 cells by activation of GLUT1 and GLUT4 through Akt and AMPK pathways stimulation [103].Egg white hydrolysate and IRW reversed the impaired glucose uptake through down-regulation of the p38-mitogen-activated protein kinase(p38) and c-Jun N-terminal kinases (JNK) 1/2 in TNF-α-treated skeletal muscle cells [82].
3.5 Satiety and gut hormones
The gastrointestinal tract, the body’s largest endocrine organ,plays a significant role in appetite control and energy regulation by secreting different regulatory peptide hormones acting on a variety of tissues to in fluence physiological processes. Most of these hormones are sensitive to gut nutrient content. Changes in circulating gut hormone levels affect short-term feelings of hunger and satiety [104].Because of their significant role in appetite control and energy regulation, gut hormones have been considered as new therapeutic approaches for obesity or type 2 diabetes treatment [105]. The major peripheral peptides known to control satiety include anorexigenic(appetite suppressing) hormones such as cholecystokinin (CCK),glucagon-like peptide 1 (GLP-1), and peptide YY (PYY) which play a significant role in the regulation of energy homeostasis [106].CCK is mainly secreted by I-cells located in the duodenum and jejunum following the ingestion of fat or protein. CCK inhibits food intake and gastric emptying, while stimulates pancreatic secretion and gall bladder contraction [107]. CCK acts via two types of G-protein coupled receptors. The CCK receptor-type 1 (CCK-1R), widely expressed in different cell types of the gastrointestinal tract, is the receptor implicated in the regulation of food intake [108]. Activation of CCK-1R signaling by CCK analogs has been shown to beneficially affect calorie intake, glucose tolerance and insulin sensitivity in mice [105].Developing CCK-1R activating peptides, therefore, appear to be another promising approach to develop functional foods with satiety suppressing effects. The gastrin/CCK-like molecules were identified for the first time from marine byproducts such as shrimp waste (Pandalus borealis), cod (Gadus morhua) head, and head and viscera of sardine (Sardina pilchardus) obtained after hydrolysis or autolysis [109]. This kind of peptides was identified in a variety of other marine byproducts [110,111], indicating the potential of these sources for the production of bioactive peptides with value added properties. The gastrin/CCK like peptides identified in cod muscle and shrimp (Penaeus aztecus) head hydrolysates were shown to bind to CCK receptors in vitro [110]. Soy, potato and casein hydrolysates have also been shown to directly stimulate CCK-1R expressing cells and CCK release in vitro [112]. Identification of such peptides from food proteins can either enhance CCK-1R signaling through binding to receptor, or enhance CCK-1R expression, which opens up a new venue in the obesity/hyperglycemia treatment due to the potential therapeutic effects of CCK-1R activation on obesity, hyperglycemia and related metabolic disturbances [113].
GLP-1, and glucose-dependent insulinotropic polypeptide (GIP)are the incretin hormones secreted from the enteroendocrine cells (K and L cells respectively) after meal ingestion [114]. These hormones involve in the augmentation of postprandial insulin secretion [115].Given this important physiological function, there has been major interest in the potential for incretin-based pharmaceuticals as antidiabetic drugs. Indeed, GLP-1 agonists have been suggested as effective treatments for hyperglycemia and type 2 diabetes [116].
3.5.1 Satietogenic peptides
Dietary proteins are believed to induce satiety feeling and thermeogenic effects. The normal protein intake should account for 10%–15% of energy intake when in energy balance and weight stable [117].High protein diets are considered as meals with 20%-30% of energy from protein when consumed in energy balance. Ad-libitum intake of high protein diets induces satiety and causes significant weight loss in animals and human subjects [118]. Mechanisms explaining proteininduced satiety are not fully understood, but increases in concentrations of satiety hormones, energy expenditure, and amino acid concentration have been suggested as potential mechanisms [118].
Although high protein diets have shown satiety-induced effects,the amino acid composition of ingested proteins may have a major role in this process [119]. This means that the high amounts of proteins per se may not be the key factor for the satietogenic effects while the release of bioactive fractions and specific free amino acids during protein digestion has a role in this phenomenon. The satiating effect of milk has been shown in a 6-month trial with an energy-restricted diet in which the group receiving milk supplementation showed a decreased desire to eat and hunger versus the placebo group [120].The satiating effect of whey protein has been attributed to the high concentration of branch chain amino acids, particularly L-Leu,existence of certain peptides or the release of satiety hormones [121].In a comparative study of casein and whey, as the major proteins of milk, on gastrointestinal hormone secretion and appetite, a greater subjective satiety after whey load was found. Moreover, a higher increase in postprandial plasma amino acid concentration, and higher levels of CCK, GLP-1 and GIP was observed in the group received whey compared to casein [122]. This clearly highlights the importance of the type of protein and confirms that the appetite response depended on the postabsorptive increase in plasma amino acids and gut hormones. Whey protein but not the commercial whey protein hydrolysate consumed before a meal reduced food intake, and postmeal blood glucose and insulin response in a dose-dependent manner in healthy young adults [123]. The authors have attributed both insulinotropic and non-insulinotropic mechanisms for the observed effects of whey protein on blood glucose whereas, whey protein hydrolysate only showed insulinotropic effects without pronounced effects on post-meal blood glucose levels. Delayed gastric emptying has been suggested as the possible non-insulinotropic mechanism due to the fact that whey proteins but not the mixture of branchedchain amino acids have been reported to enhance CCK and GLP-1 release [70]. This indicates that non-insulinotropic mechanisms arise from the digestion of intact proteins but not the protein hydrolysate.The commercial whey protein hydrolysate contained 40% free amino acids and short peptides of up to 10 amino acids, 27% of peptides of 10–200 amino acids, 16% of 50–200 amino acids, and 17% with more than 200 amino acids as reported by the manufacturer [123].Indeed, neither short peptides and free amino acids nor larger protein fragments could induce gut hormone release to affect the post-meal glucose levels in healthy adults in this study. Apart from this study, however, there are numerous reports on the effects of protein hydrolysates on gut hormone release and satietogenic effects.Pepsin hydrolysates of porcine meat enhanced CCK release from enteroendocrine cell line STC-1 and further induced satiety in SD rats in a dose dependent manner [124]. Marine-derived proteins and bioactive peptides also have strong stimulating effects on GLP-1 and CCK release exerting satiety effects. Cudennec and co-workers initially demonstrated that blue whiting (Micromesistius poutassou)muscle hydrolyzed with a mixture of commercial enzymes enhanced CCK secretion in STC-1 cell line [125]. This marine hydrolysate mainly consisted of short peptides in range of 1 000 Da. In a follow up study by the same group, satiety inducing effects of this treatment was confirmed in animals. The administration of blue whiting hydrolysate to SD rats reduced the short-term food intake correlated to an increase in the CCK and GLP-1 plasma levels. Chronic administration of this marine hydrolysate also decreased the body weight gain in these rats [126].
3.5.2 Incretin mimetic peptides
Strategies based on the enhancement of endogenous GLP-1 secretion are promising for the prevention of hyperglycemia.Previous reports have documented that amino acids, particularly glutamine (Glu), stimulated GLP-1 secretion and increased plasma GLP-1 level in humans [127,128]. The low molecular weight fraction of wheat protein (gluten) hydrolysate, rich in glutamine residues,has been reported recently to increase GLP-1 secretion followed by enhanced insulin secretion, and to improve glucose tolerance in SD rats [129]. Peptones are commercially available protein hydrolysates with a mixture of different length peptides as well as free amino acids [114]. Meat peptones have been reported to stimulate GLP-1 secretion in NCI-H716 cell line (a human intestinal cell model) [130]and murine primary colonic cultures [131]. Although the active components of meat peptone on GLP-1 secretion were not identified,Reimer showed that the commercial mixture of essential amino acids(Sigma) increased GLP-1 secretion in NCI-H716 cells [130].
3.5.3 Dipeptidyl peptidase IV (DPP-IV) inhibitors
Both GIP and GLP-1 are rapidly degraded after release into the circulation (half-lives < 7 and 2 min, respectively) due to the action of the ubiquitous enzyme DPP-IV [105]. This issue however has been overcome through developing long-acting enzymatic resistant incretin analogues [104] or inhibition of DPP-IV which results in an increased and prolonged insulin response. DPP-IV inhibitors are a class of oral antihyperglycemic agents to treat patients with type 2 diabetes [132].Thefive available DPP-IV inhibitors in the market include sitagliptin,vildagliptin, saxagliptin, linagliptin, and alogliptin, which are small molecules used orally with similar overall clinical efficacy and safety profiles in patients with type 2 diabetes [133]. Recently, many studies revealed the potential of food proteins as the precursor for DPP-IV inhibitory peptides. Hydrolysate and peptides from different proteins including fish [134-137], amaranth [138], soy and lupin [139-141],and milk [49,142] exerted DPP-IV inhibitory activity in vitro.Peptides GPHypGPAG derived from porcine skin gelatin, GPAE, and GPGA derived from salmon skin are some examples of the potent DPP-IV inhibitory peptides with IC50values less than 50 μmol/L [143].Bioactive peptides may exert their DPP-IV inhibitory effect through competitive, uncompetitive, noncompetitive and mixed-type modes.by binding either at the active site and/or outside the catalytic site of DPP-IV enzyme [144]. In vivo experiments are required to assess the activity and efficacy of the peptides/protein hydrolysates with in vitro DPP-IV inhibitory activity on blood glucose regulation.
4. Obesity and insulin resistance
Obesity, one of the major underlying causes of the MetS, is increasing rapidly worldwide largely due to the sedentary lifestyle and positive energy balance conditioned by environmental and genetic factors. Obesity is defined by WHO as a body mass index(BMI) ≥ 30 kg/m2[117]. Obesity is accompanied by high plasma levels of nonesterified fatty acids that cause insulin resistance in skeletal muscle and overload the liver with lipid, producing fatty liver and atherogenic dyslipidemia [3]. The pathophysiology of insulin resistance involves a complex network of signaling pathways shown in Fig. 4.
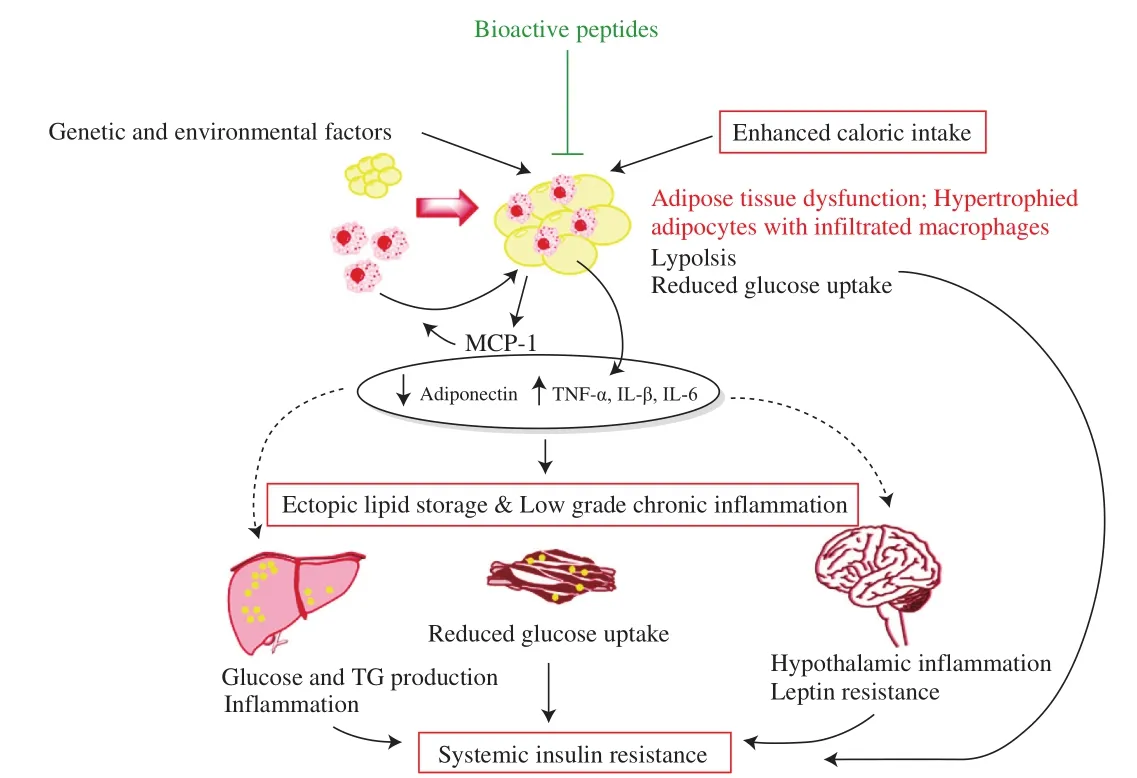
Fig. 4 Pathophysiology of insulin resistance. Genetic, environmental and/or positive energy balance leads to weight gain and increased visceral adiposity. Enhanced visceral adiposity results in formation of hypertrophic and dysfunctional adipocytes with a pro-in flammatory nature. Adiponectin production is diminished and secretion of pro-in flammatory adipokines is increased. Recruitment of macrophages with pro-in flammatory phenotype through monocyte chemoattractant protein-1 (MCP-1), and infiltration through the adipose tissue further exacerbate the pro-in flammatory state. This lowgrade chronic in flammation in adipose tissue as well as other organs induces insulin resistance locally and systematically through inhibiting signaling pathways. Moreover, the loss of lipid buffering capacity and sustained levels of pro-in flammatory factors results in ectopic fat deposition in non-adipose tissues especially liver and skeletal muscle further triggering systemic insulin resistance. Figure adapted from [147,148].
Excessive fat, which is particularly deposited in visceral organs,generates a chronic low-grade in flammation state that contributes to the development of various metabolic disorders and CVDs. Moreover,fat accumulation in the liver may stimulate hepatic cytokine production. Jang and co-workers reported the anti-obesity effects of an isoflavone-free peptide mixture derived from black soybean(Rhynchosia volubilis) in mice [145]. This treatment reduced food intake through activation of the leptin-like signaling in hypothalamus,and reduced body weight gain in mice fed a high fat diet for 13 weeks. Interestingly, identification of a heptapeptide IPPGVPY in the plasma 30 min after oral administration of 1 g black soybean peptide mixture suggests the potential role of absorbed peptides in the observed physiological effects in vivo [145]. Development of obesity is concomitant with increase in fat cell size (adipocyte hypertrophy)and number (adipocyte hyperplasia). Adipose tissue has a crucial role in maintaining energy homeostasis and insulin sensitivity in the body. White adipose tissue (WAT) is widely distributed in the body and represents the primary site of fat metabolism and storage.White adipocytes are found in WAT and are on average 60–100 μm in diameter. White adipocytes have the capacity for lipid storage.Mature white adipocytes store triglycerides in a single large lipid droplet occupying the center of the cell and accounts for 85%–90% of the mass of the cell. Although they have variable volume, mature white adipocytes are large cells and their size can change dramatically depending on the quantity of triglycerides they accumulate [146].White adipocytes have been referred as “adipocytes” in this paper.
4.1 Adipocyte differentiation
Adipocytes derive from multipotent mesenchymal stem cells.These cells have the capacity to differentiate into myocytes,chondrocytes, osteocytes and adipocytes [149]. Normal adipocyte differentiation is fundamental for generation of insulin sensitive adipocytes with enough capacity to store fats. Adipogenesis has mainly been described in 2 major phases: determination and terminal differentiation. The initial phase results in the conversion of the stem cells to pre-adipocytes. The pre-adipocytes then go through terminal differentiation to obtain the characteristics of mature adipocytes by acquiring the necessary characteristics for lipid transport and synthesis, insulin sensitivity and the secretion of adipocyte-specific proteins [149]. Peroxisome proliferator-activated receptor-γ (PPARγ)and CCAAT/enhancer binding protein (C/EBP) are the transcription factors playing a crucial role in the complex transcriptional cascade during adipocyte differentiation. Growth and differentiation of preadipocytes is highly controlled by communication between individual cells or between cells and the extracellular environment. Various hormones and growth factors affect adipocyte differentiation in a positive or negative manner by transferring external growth and differentiation signals to differentiating adipocytes [149]. Insulin is the key hormone enhancing adipocyte differentiation. Adipocyte differentiation especially the molecular regulation of terminal differentiation has been extensively characterized by using murine cell lines such as the 3T3-L1, F442A, and ob1771 pre-adipocytes that under appropriate hormonal control can differentiate into mature white adipocytes. Having morphological similarity tofibroblastic preadipose cells, these pre-adipocyte cell lines exhibit the characteristics of adipocytes presenting within the adipose tissue after differentiation.
Metabolically normal adipocytes are small in size, sensitive to insulin and secrete insulin sensitizing hormones such as adiponectin.Impaired differentiation of new adipocytes under metabolic disorders on the contrary, leads to the enlargement of existing adipocytes(hypertrophy) and spillover of fat into other insulin sensitive tissues.Hypertrophic adipocytes are inflamed, insensitive to insulin and increasingly express harmful adipokines affecting other organs in the body and eventually development of insulin resistance locally and systematically [146,150]. Therefore, enhanced differentiation of pre-adipocytes into mature adipocytes and restoration of insulin actions either by improving insulin sensitivity or by using compounds mimicking insulin functions is a key strategy for controlling MetS and its complications.
4.1.1 PPARγ; the master regulator of adipogenesis
The terminal adipocyte differentiation involves a cascade of transcriptional changes that has been extensively studied. In the initial stage of adipogenesis, the transient induction of two members of the C/EBP family of transcription factors including C/EBPβ and C/EBPδ occurs dramatically. They both directly induce expression of PPARγ and CCAAT/enhancer binding protein α(C/EBP-α). PPARγ and C/EBPα are considered as the key regulators of adipocyte differentiation. They appear to act cooperatively in adipocyte differentiation by activating the expression of each other and regulating the expressions of other adipocyte specific genes.Despite the importance of C/EBPs in adipogenesis, these transcription factors cannot function efficiently in the absence of PPARγ [149].PPARγ is most specific to adipogenic differentiation and is induced before transcriptional activation of most adipocyte genes. PPARγ has been shown to be both necessary and sufficient for fat formation [151].Over expression of PPARγ can induce adipogenesis in mouse embryonic fibroblasts lacking C/EBPα, but C/EBPα cannot rescue adipogenesis when PPARγ is not expressed, showing that PPARγ is the master regulator of adipogenesis [149]. PPARγ is also necessary for the function of mature adipocyte. PPARγ exists as two protein isoforms: PPARγ1 and PPARγ2, with the latter containing an additional 30 amino acid residues at its N-terminus [152]. Both isoforms promote differentiation and are highly expressed in adipose tissues. However, PPARγ1 is found in many different tissues while expression of PPARγ2 is predominantly localized to adipocytes [149].The outcome of enhanced differentiation in pre-adipocytes is the generation of smaller adipocytes which are more insulin sensitive than larger cells and represent a new pool that can enhance the absorption of circulating free fatty acids and triglycerides, thereby limiting their ectopic deposition into peripheral tissues, lipotoxicity and insulin resistance [146,153]. In addition to the primary role in adipocyte differentiation, PPARγ also upregulates genes involved in glucose uptake in adipose tissue and controls the expression of adipokines such as adiponectin, leptin, TNF-α, and resistin that communicate with other organs to affect whole-body insulin sensitivity [154].Therefore, distinct signaling pathways and target tissues that physiologically complement each other may be involved in achieving the insulin-sensitizing effects of PPARγ [152,155].
Thiazolidinediones (TZDs), the highly effective oral medications for type 2 diabetes, have been introduced for the treatment of type 2 diabetes since the late 1990s. TZDs are potent activators of PPARγ [154,156]. Since PPARγ is mainly present in adipocytes, the systemic insulin sensitization effects of TZDs are believed to occur through affecting adipose tissue. One of the proposed mechanisms of TZDs effects is the enhanced lipid storage ability of adipocytes,since TZDs enhance adipocyte differentiation and generate smaller adipocytes. This increases the triglyceride content of adipose tissue while reduces the free fatty acids and triglycerides in the circulation,liver and muscle, reduces lipotoxicity in muscle and liver which eventually improves whole body insulin sensitivity [152,154,157].
4.1.2 Peptides promoting adipocyte differentiation
The various effects of PPARγ on insulin sensitivity and glucose homeostasis in different tissues obviously illustrate the promise for multiple and potentially safe routes for therapeutic interventions [152]. Dysregulation of PPARγ activity by in flammatory cytokines may be involved in the pathogenesis of proinflammatory diseases such as insulin resistance and atherosclerosis[158,159]. Insulin, on the other hand, has been shown to increase PPARγ expression [160]. The beneficial effect of a soy protein peptic hydrolysate on adipocyte differentiation has been recently reported in 3T3-L1 cells [161]. The soy protein hydrolysate stimulated adipocyte differentiation through the upregulation of PPARγ expression. The expression and secretion of adiponectin and insulin sensitivity was also enhanced on 3T3-L1 cells treated with the soy protein hydrolysate [161].Interestingly, FLV, a soy-derived peptide, has been reported recently to prevent adipose inflammation and insulin resistance in another study [162]. This peptide, transported into adipocytes through the peptide transporter PepT2, inhibited the release of inflammatory molecules (TNF-α, MCP-1, and IL-6) from both TNF-stimulated adipocytes and co-cultured adipocytes/macrophages. Furthermore,FLV enhanced insulin responsiveness and increased glucose uptake in adipocytes [162]. Our group has recently reported the beneficial effects of egg white hydrolysate on adipocyte differentiation through cell-based and animal studies. Incubation of pre-adipocytes with egg white hydrolysate promoted adipocyte differentiation as shown by increased lipid accumulation and release of adiponectin, as well as upregulation of PPARγ and C/EBP-α in 3T3-F442A cells [86]. In vivo administration of egg white hydrolysate also reduced adipocyte size and increased PPARγ2 protein abundance in adipose tissue of insulin resistant rats [88]. Further purification of egg white hydrolysate led to identification and validation of four bioactive peptides;WEKAFKDED, QAMPFRVTEQE, ERYPIL, and VFKGL from ovalbumin with PPARγ stimulatory activity in adipocytes [163].
5. Conclusions
Development of bioactive peptides against different aspects of MetS especially on glucose homeostasis and insulin resistance is a growing researchfield. The recent discoveries of pathways and target cells in the management of glucose and energy metabolism have opened up new opportunities for identification of novel bioactive peptides on enhancing adipocyte differentiation and insulin signaling,CCK receptor binding and expression, incretin stimulants, and to name a few. However, as yet, the evidence available on the efficacy of such bioactive peptides is rare and more research is required to validate these potential benefits using suitable animal models.Moreover, most reported glucoregulatory peptides lack mechanistic studies, which warrant further studies in the future. It should be noted that delineating the mechanism(s) of action is challenging due to the complex nature of peptide structures and the multi-functional properties of bioactive peptides. Bioactive peptides are usually liable to proteolytic cleavage in the gastrointestinal tract during absorption and uptake. Peptides susceptible to digestion may require protection when administered orally using strategies like encapsulation.Several physicochemical parameters like charge, molecular weight,lipophilicity, and solubility affect the bioavailability of bioactive peptides. Moreover, the mode of administration (fed or fasted phase,with or without water), the dosage form (suspension, powder, micelle,emulsion, etc.), and inter-individual variability due to age, sex, disease conditions, ethnicity are other factors greatly affect the bioavailability of peptides in clinical trials [164]. Therefore, pharmacokinetic data is required to determine the effective dosage and frequency of administration.
In conclusion, while the results obtained from in vitro and in vivo studies of bioactive peptides are encouraging for their potential uses against complications of MetS, studies on humans are scarce.Therefore, randomized clinical trials are necessary to confirm the safety and effectiveness of these peptides.
Con flicts of interest
The authors declare no con flict of interest.
Acknowledgments
The laboratory of Jianping Wu is supported by Grants from Natural Sciences and Engineering Research Council (NSERC) of Canada,Alberta Agriculture and Forestry, and Egg Farmers of Canada.
- 食品科学与人类健康(英文)的其它文章
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas
- Protective effects of tilapia (Oreochromis niloticus) skin gelatin hydrolysates on osteoporosis rats induced by retinoic acid

