Promotional effect of silica on the combustion of nano-sized aluminum powder in carbon dioxide
Bozhong ZHU, Jinghui WANG, Qichng WANG, Yunln SUN,*,Weiqi CHEN, Jiqun WANG
a School of Petroleum Engineering, Changzhou University, Changzhou 213164, China
b School of Energy and Environment, Southeast University, Nanjing 243002, China
KEYWORDS Combustion;Mechanism;Nano-sized aluminum powder;Promotional effect;Silica
Abstract This paper presents how the combustion performance of nano-sized aluminum (nAl)powder in carbon dioxide are affected by silica. The ignition and combustion performance of nAl powder with silica addition were studied by a high-temperature tube furnace. An s-type thermocouple and a high-speed motion acquisition instrument were performed to evaluate the ignition temperature, maximum combustion temperature, maximum change of rate of temperature, and combustion propagation speed.The combustion efficiency and combustion products were measured and analyzed by a gas-volumetric method and an X-ray diffraction. The results show that silica added into nAl powder can enhance its maximum combustion temperature and maximum change of rate of temperature,while its ignition temperature increases slightly.The nAl powders with addition of 6.00 wt.%and 12.00 wt.%silica present high combustion propagation speeds,especially for the latter, it has high combustion efficiency. The effect mechanism of silica on the combustion of nAl powder in carbon dioxide was discussed.
1. Introduction
Metal combustion has received extensive attention and has been widely used in solid propellants owing to its high volumetric and gravimetric energy densities.The combustion of metal can significantly improve the specific impulse, and its combustion products can inhibit combustion oscillations.Aluminum (Al) content is abundant on the earth and has a high energy density (34.2 kJ/cm). It is an ideal fuel additive to increase the specific impulse of propellants. Moreover, Al powder can react with different oxidants such as air,carbon dioxide (CO), and steam (HO). In particular, COis the main component of the Martian atmosphere, and it can react with Al via the reaction (1)
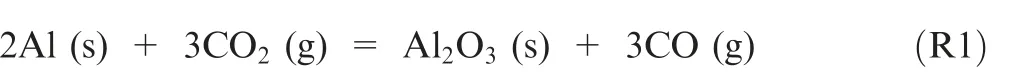
The reaction of Al with COcan release a lot of heat that is the source of power for Mars exploration, and can realize the‘In-situ Resource Utilization (ISRU)’. At present, many researches have been conducted to study the combustion performance of Al powder.There are two key issues in the combustion process of Al powder: (A) The alumina shell coated on the surface of Al powder can prevent the reaction of active Al with oxidizer and lead to the difficulty in ignition;(B)The alumina continuously formed during the burning process will interrupt the combustion of Al powder. Nano-sized aluminum (nAl) powder exhibits higher reactivity and better combustion behavior compared with micro-sized Al powder.For example, the oxidation rate of nAl powder is approximately two orders of magnitude higher than ordinary Al powder.Furthermore, its combustion efficiency is higher than that of micro-sized Al powder.However, nAl powder still exists the above problems.Additives such as sodium borohydride (KBH), ammonium perchlorate (AP), and sodium chloride(NaCl)can promote the combustion of nAl powder.In addition,the addition of thermites into nAl powder can also promote its combustion by a thermite reaction.Plantier et al.studied the combustion performance of nAl with ferric oxide(FeO) addition, and found that the combustion wave speed of nAl powder was improved significantly.
Thermite reaction refers to that Al reacts with oxides such as FeOand CuO, which can release a large amount of heat.In previous studies,thermites are primary metal oxides.Nonmetallic elements as thermites is rarely reported. Silicon(Si)is metal-like element and its metallicity is weaker than that of Al. Therefore, nAl powder can react with silica (SiO) and release heat. The reaction is as follows:
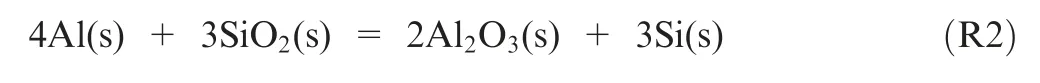
Si is distributed widely in the form of SiOin the earth crust,and Mars crust deposits an amount of opal(hydrated silica).Hence, SiOmay be an ideal additive for nAl combustion. However, the effect of SiOon the combustion of nAl powder is not still clarified.
Therefore, in this study, the combustion performance of nAl with SiOaddition in COwas experimentally investigated through a high-temperature tube furnace. A number of key issues were clarified, including the ignition temperature, maximum combustion temperature, maximum change of rate of temperature,and combustion propagation speed.The combustion products were detected through an X-Ray Diffraction(XRD). Also, the combustion efficiency was measured by the reaction of the combustion products with the sodium hydroxide (NaOH) solution to generate hydrogen, namely a gasvolumetric method.The effect mechanism of SiOon the combustion of nAl powder in COwas revealed. These results should help to understand the catalytic actions of SiOon the combustion of Al powder in CO.
2. Experimental
2.1. Materials and samples
Nano-sized Al powder whose average diameter is 150 nm was produced by Henan Nanometer Material Limited Company,which active Al content measured by a gas-volumetric method is 85.0%.SiO(analytical grade 99.7%) was supplied by Sinopharm Group Chemical Reagent Company.
Three mixtures of SiOand nAl powder were prepared by using SiO-to-nAl ratios (ω) of 6.00, 12.00, and 18.00 wt%.Firstly,a certain amount of nAl powder and SiOwas weighed according to the aforesaid percentage,respectively.Then,they mixed homogeneously with cyclohexane by a sonic oscillator in an ice bath.Finally, the cyclohexane was evaporated by a vacuum drying box,and the mixture was collected by containers with argon gas as protective gas.The nAl powder without addition of SiOwas prepared by the same procedures,which is used as reference.Thus,the four samples have similar physical properties, whose components are shown in Table 1.
2.2. Ignition and the maximum combustion temperatures
The experimental setup of ignition and combustion is shown in Fig. 1, which consists of a gas supply system, a hightemperature reaction system, and a data acquisition system.Before the experiments, the furnace temperature was set to a target temperature (700 °C) using a thermoregulator. Then,the CO(purity, 99.999%) with a flow rate of 30 mL/min was introduced the quartz tube (inner diameter of 3.0 cm,external diameter of 3.6 cm,and length of 95.0 cm)in the tube furnace for 5 min to expel the inside air. After the air in the quartz tube was exhausted,the 0.05 g sample with a fixed volume of 2.0 cm(length)×0.2 cm(thickness)×0.7 cm(width)in a cuboid strand molds was pushed into the tube furnace by a push rod. The temperature evolution of the ignition and combustion process was recorded in real time by S-type thermocouple (10Rh-Pt/Pt, thermocouple head with a diameter of 1.0 mm)embedded in the middle of samples connecting a data collecting instrument (Agilent, 34972A, USA), which acquisition frequency is 25 Hz. To guarantee the precision of the experimental results, two repeatability tests were performed for each sample under the same experimental condition.
For the used spherical thermocouple,the temperature error can be analyzed through comparing the thickness of the thermal wave with the size of the thermocouple head.If the size of thermocouple head is smaller than the thickness of the heat wave, this method for temperature measurement can be used.
For a steady-state burning stacked mixture, the temperature (θ) profile can be expressed as Eq. (1).

where ris the burning rate of mixture,which is obtained by the following experiments;αis the mixture thermal diffusivity, cm/s; and y indicates the thickness of sample.
The thermal diffusivity of the mixture of nAl and SiOis given by Eq. (2).

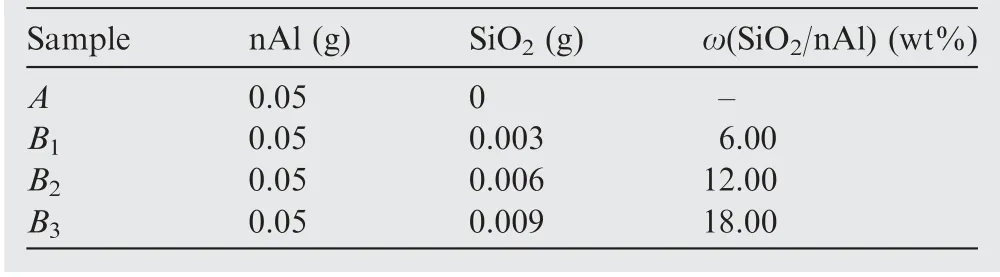
Table 1 Composition of samples.
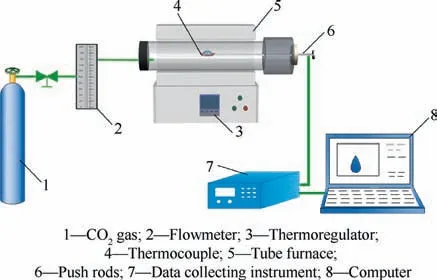
Fig. 1 Schematic diagram for the experimental system.
where λis the thermal conductivity of mixture,W/m·K;ρis the density of mixture, kg/m; Crepresents the specific heat of mixture, J/kg·K.
Take the sample A for example due to the largest thickness of the thermal wave in these samples. All the parameters used for the calculating are shown in Table 2.Hence,α=0.176-cm/s and θ = 0.0033 (<0.01).
As the calculated temperature distribution equivalent is less than 0.01, the thickness of the thermal wave can be expressed as Eq. (3).
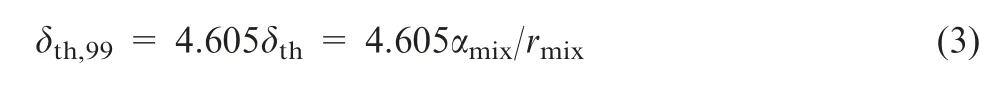
Hence, the thickness of thermal wave is 2.3 mm. while the diameter of the used spherical thermocouple head is 1.0 mm,which can theoretically guarantees the reliability of temperature measurement.
The combustion products were collected to measure the combustion efficiency by a gas-volumetric methodand analyze the components using an X-ray diffraction (XRD,D8Advance, Bruker).
2.3. Combustion propagation speed
The effect of SiOon the combustion propagation of nAl powder was studied in a channel shown in Fig.2.The channel with overall dimensions of 5.0 cm (length) × 0.3 cm (height) × 0.3 cm(width)is made of stainless steel.An embedded nichrome(NiCr) heating wire at one side of the channel was used to ignite the sample and the operating voltage of the NiCr heating wire was 10 V. Firstly, the sample was filled into the channel.Then, the channel with the sample was placed into the quartz tube that filled with CO.When the sample was ignited in CO,the combustion propagating process of nAl with COwas recorded by a high-speed camera (Phantom V311, VRI,USA), whose acquisition frequency is 1000 fps. The data of combustion propagation speed were acquired by calculating the average of three tests.

Table 2 Used parameters for calculating the thickness of thermal wave.
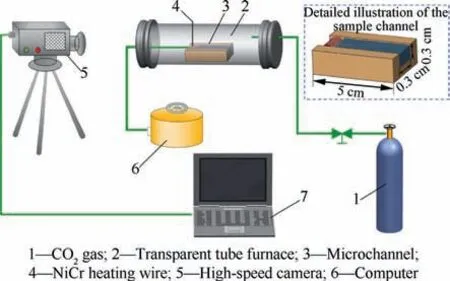
Fig. 2 Schematic diagram of experimental device for combustion propagating.
3. Results and discussion
3.1. Effect of SiO2 on ignition and combustion behaviors
Ignition and maximum combustion temperatures are two important parameters for nAl powder combustion in CO.Fig. 3 shows one set of ignition and combustion temperature evolution curves for the sample A.To further understand their behaviors, the change of rate of temperature, dT/dt, is also manifested in Fig. 3. When the sample is placed into the high-temperature reactor, its temperature increases. The time of the sample temperature reaching 100 °C is defined as t=0 s.A sharp increase in the temperature-time curve presents, which is also the peak of dT/dt, illustrating that the nAl powder is ignited, and the corresponding temperature is defined as the ignition temperature,T.The peak temperature in the temperature-time curve is defined as the maximum combustion temperature,T.According to the three special temperatures mentioned above, the combustion process can be separated three stages: a slow heating zone (Stage I), a rapid reaction zone (Stage II), and a cooling zone (Stage III).
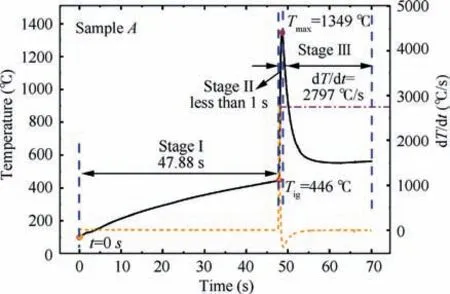
Fig. 3 Ignition and combustion temperature evolution of the sample A in CO2.
In the Sage I,the oxidation rate of nAl powder is relatively slow owing to the low temperature at the beginning. With the heat accumulating, the sample A is ignited at 446 °C after approximately 47.88 s.The burning of the sample A enters into the Stage II, and the temperature increases dramatically. Its combustion temperature reaches the maximum in 0.72 s,which is 1349 °C. Furthermore, the maximum change of rate of the temperature reaches 2797 °C/s, indicating that the reaction of nAl powder with COis harsh and releases a lot of heat.With the consumption of nAl powder,the reaction enters into the Stage III. The oxidation reaction becomes slow and the reaction temperature decreases. Moreover, with the continuous production of AlO, the activated Al is completely wrapped in AlOshell that can lead to the combustion interruption. Therefore, the sample temperature decreases rapidly.
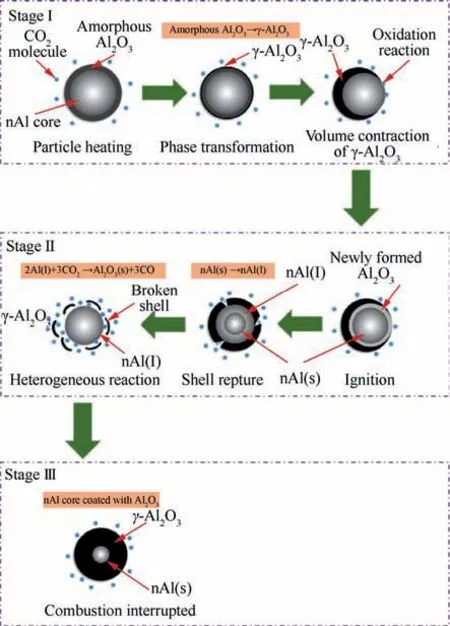
Fig. 4 Combustion process depiction of nAl particles in CO2.
The physical and chemical behaviors in the combustion process of nAl particles with COare schematically shown in Fig. 4. This process is similar to that reported in previous study.However, there are some differences in the phase change process of AlO. nAl particles have a layer of amorphous AlOcoated on their surface due to passivation treatment. At the Stage I, the amorphous AlOhinders the reaction of the active Al with COat low temperature.Increasing the temperature, the amorphous AlOcoated on the surface of nAl is gradually transformed into γ-AlOand the density of AlOincreases (ρ= 2.45-3.38 g/cmvs ρA= 3.65-3.67 g/cm) because the oxygen atoms and Al atoms accumulate more closely,which results from the volume shrinkage of AlO,so AlOis not fully coated on the surface of activated Al. Fresh Al starts to react with CO. While at the Stage I, the oxidation rate of nAl particles is relatively slow due to the low temperature. However, nAl particles are eventually ignited because heat continues to accumulate. Subsequently,the combustion reaction of nAl particles enters into the Stage II. At the Stage II, with the occurrence of nAl combustion, large amounts of heat are released and the temperature quickly increases to the melting point (660 °C) of nAl,so it rapidly enters the liquid phase. The formed γ-AlOon the Al surface is fragmented due to the large internal and external pressure difference generated during the nAl phase transition,and the active component of nAl is completely exposed to CO. Then the heterogeneous reactions between the exposed nAl and COproceed rapidly, and the combustion temperature reaches the maximum value in less than 1 s. After that,the combustion process enters into the Stage III. Although a large amount of AlOis formed in the stage II, the burning of Al is not interrupted. The remaining Al continues to react with CO. When the alumina shell completely encases the active Al, the combustion of nAl particles is interrupted. The combustion process is over.
Three sets of results of combustion temperature evolution curves of nAl powder with different addition amounts of SiOare shown in Fig.5.The specific values of related parameters in combustion process are listed in Table 3. It is evident that the SiOat different levels has a strong influence on the ignition and combustion temperatures of nAl powder in CO.
Obviously, the maximum combustion temperature in the combustion process of nAl powder with SiOaddition is greatly improved. As shown in Fig. 5 and Table 3, the Tof sample A without addition of SiOis only 1349 °C, while the Tof the samples B, B, and Bare 1580, 1717,1624 °C, respectively. Compared with the sample A, the Tof the samples B, B, and Bare increased by 17.1%,27.3%, and 20.3%, respectively, indicating that nAl powder with the addition of SiOcan significantly increase the heat release,which may be caused by the thermite reaction between nAl powder and SiOvia the R2.
The maximum change of rate of temperature is also significantly increased due to the large amount of heat released from the thermite reaction of nAl powder and SiO. The maximum change of rate of the temperature for the sample A is only 2797 °C/s, while the maximum changes of rate of temperature of the samples B, B, and Bare 3390, 3845, and 3561 °C/s,respectively. Compared with the maximum change of rate of the temperature for the sample A, they are increased by 21.2%,37.5%,and 27.3%,respectively.However,the addition of SiOcannot effectively reduce the ignition temperature of nAl powder and even increase its ignition temperature. This phenomenon may result from the higher apparent activation energy of nAl powder with SiO. In addition, the lower thermal conductivity of SiOrelative to nAl powder also affects the ignition temperature.
In conclusion, SiOcan significantly enhance the heat release of nAl combustion, which causes an effective increase of the maximum combustion temperature and the maximum change of rate of temperature.However,the ignition temperature of nAl with the addition of SiOincreases slightly.
3.2. Effect of SiO2 on the combustion propagation speed
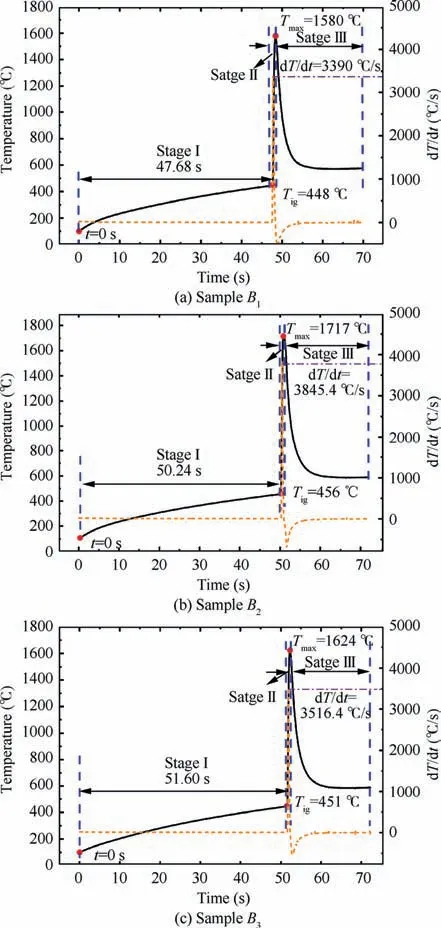
Fig.5 Temperature evolution of burning for different samples in CO2.
The above experimental results show that the SiOaddition effectively facilitates the combustion and heat release of nAl powder in CO.The combustion propagation speed is another important parameter for evaluating the reaction of nAl powder with CO,so we investigated the effect of SiOon the combustion propagation speed of nAl/COin a microchannel.For consistency,each set of sample is tiled within the microchanneland filled up the channel to keep the packing density of each sample constant because the combustion propagation speed of mixtures is sensitive to the packing density of powder.Fig. 6 shows the combustion propagation behaviors of each set of sample. Time zero is set as the time when the sample is ignited. As the nAl powder is ignited, the flame propagates from left to right within the microchannel. To describe the flame propagation characteristics clearly, the flame propagation images of the samples A and Bwithin 0.50 s are selected,while for the samples Band B,the flame propagation images are selected within 0.60 s. And the time interval between each picture is 0.05 s.
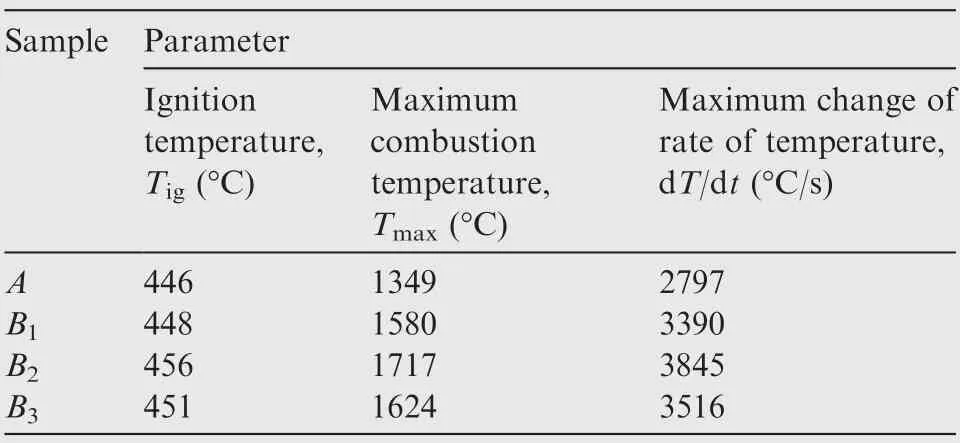
Table 3 Combustion parameters of different samples in CO2.
In the initial stage of ignition (about 0-0.05 s), the area of the flame is nearly equal for all samples.As the flame continues to spread in the mircochannel, the area of the flame starts to increase. In particular, the area of the flame frontal plane apparently expands. However, the flame area of the sample A is almost equal to the surface area of the microchannel covered by its combustion propagation.In contrast,the flame area of other samples is significantly larger. This indicates that the combustion for the nAl powder with SiOaddition is more vigorous. In addition, the speed of combustion propagation is also an important parameter to evaluate the combustion intensity. The average combustion propagation speed of each sample is shown in Table 4.
Obviously, SiOcan effectively increase the combustion propagation speed of nAl powder due to the huge heat release from the thermite reaction between nAl powder and SiO.The combustion propagation speed of the sample A is 3.36 cm/s,while the corresponding values of the samples B, B, and Bare 4.50,4.25,and 3.45 cm/s,respectively,which are increased by 33.9%,26.5%,and 2.7%compared with the sample A.We can see from Table 4 that the combustion propagation speed increases first and then decreases as the addition amount of SiOincreases. When the addition amount of SiOis 6.00 wt.%, the combustion propagation speed is the maximum. This may be because the reaction of nAl with SiOis easily slagging (the location marked with yellow line shown in Fig. 6), which can hinder the combustion propagation.Fig.6 shows that for the sample B,the slagging phenomenon exists but it is unobvious.The huge heat release of the thermite reaction between nAl powder and SiOcan effectively promote the combustion flame spread. However, as the addition amount of SiOincreases, the slagging seriously hinders the combustion propagation. Since the heat release of the sample Bis relatively larger than that of the sample B,the reduction of the combustion propagation speed for the sample Bis relatively small. Although SiOcan improve the combustion propagation speed of nAl powder, it is not advisable to add a large amount of SiObecause the slagging is more serious with the increase of addition amount of SiOin the combustion process, which can result in the decrease of combustion propagation speed.
3.3. Effect of SiO2 on the combustion efficiency
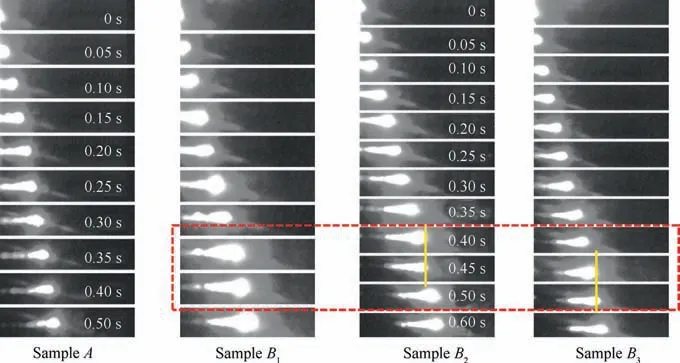
Fig. 6 Combustion propagation behaviors of different samples.
The AlOshells cover on the nAl surface and prevent COfrom reacting with the fresh Al, and thus the combustion of nAl powder is interrupted.Therefore,the samples cannot completely react with CO.The combustion efficiency is an important indicator of combustion evaluation. The residual active nAl contents in the combustion products of different samples were determined through a gas-volumetric method and the average results of three tests are shown in Fig.7.The combustion efficiencies of the samples Band Bare lower than that of the sample A because there is a serious agglomeration in the combustion process of nAl powder (shown in Fig. 6). While the combustion efficiency of the sample Bis higher than that of the sample A due to the high combustion temperature.
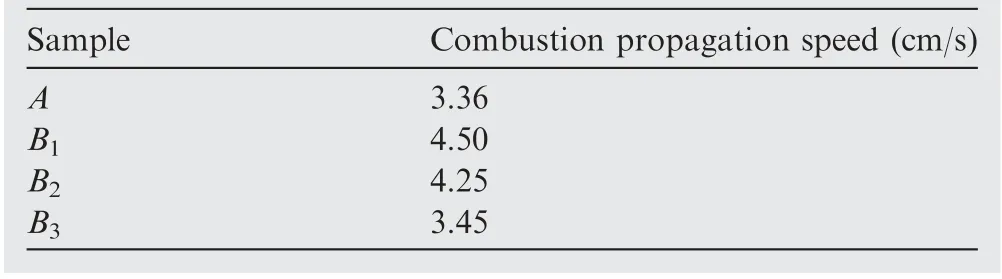
Table 4 Combustion propagation speeds of different samples.
3.4. Effect mechanism of SiO2 on the combustion
To reveal the effect mechanism of SiOon the combustion of nAl powder in CO, the combustion products were detected through an XRD. The results are shown in Fig. 8.
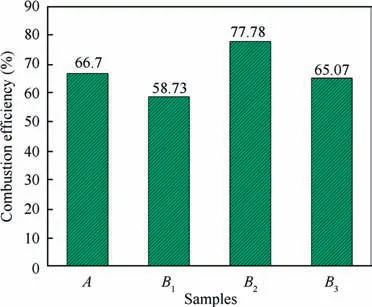
Fig. 7 Combustion efficiencies of different samples.
Fig. 8 shows that the main combustion products of the sample A are Al, AlO, and AlOC. When SiOis added to the sample,the combustion products are also found to contain Si and AlSiC. Consequently, the following reactions may occur.
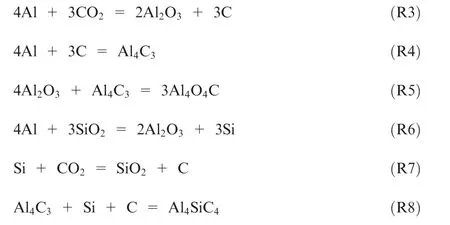
For the sample A,R3-R5 occur in the nAl combustion process. And the main exothermic reaction is R3. While adding SiO, it is obvious that the R2 is the key reaction to promote the heat release of nAl combustion.In addition,the formation of AlSiCthrough R7-R8 may be the primary reason for the decrease of combustion efficiency of the samples Band B.Actually, AlSiCis a kind of refractory material, which has strong antioxidant activity under high temperature and can be generated at above 1300°C.Hence,the existence of AlSiCwill prevent the combustion reaction between nAl powder and CO,and result in the decrease of combustion efficiency.However, when the combustion temperature is extremely high and the amount of heat released is enough,the effect of AlSiCon the combustion efficiency will be greatly reduced such as the sample B.
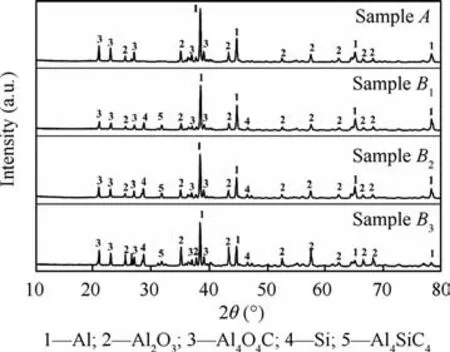
Fig. 8 XRD patterns of the combustion products of different samples.
4. Conclusions
In summary, current work investigated the effect of SiOon the combustion behavior of nAl powder in CO. The larger increase of the maximum combustion temperature of the nAl powder with SiOaddition is obtained. However, the ignition temperature cannot be effectively decreased, while it increases slightly.
The combustion efficiency of nAl powder with the addition of SiOdoes not always increase. When 6.00 wt.% and 18.00 wt% SiOare added into nAl powder, the combustion efficiencies of both samples decrease due to the serious slagging in the combustion process. While the combustion efficiency of nAl powder with addition of 12.00 wt% SiOincreases due to the relatively high combustion temperature. The heat release and the combustion propagation speed of nAl powder with addition of SiOare higher than those of nAl powder without addition of SiO. The maximum combustion propagation speed is reached when the addition amount of SiOis 6.00 wt%.
To sum up, SiOcan effectively facilitate the combustion process and heat release of nAl powder in CO. However,the addition amount of SiOshould be appropriate due to the slagging during the combustion process. In current work,the addition of 12.00 wt% SiOinto nAl powder can receive the best combustion benefit through comprehensive evaluation.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
s
This work was supported by the National Natural Science Foundation of China (Nos. 52176099, 51376007 and 51806001) and the Project of Jiangsu Provincial Six Talent Peak, China (No. JNHB-097).
 Chinese Journal of Aeronautics2022年4期
Chinese Journal of Aeronautics2022年4期
- Chinese Journal of Aeronautics的其它文章
- An automatic isotropic/anisotropic hybrid grid generation technique for viscous flow simulations based on an artificial neural network
- Optimization design of airfoils under atmospheric icing conditions for UAV
- Pressure distribution feature-oriented sampling for statistical analysis of supercritical airfoil aerodynamics
- Recent progress of machine learning in flow modeling and active flow control
- Design method of optimal control schedule for the adaptive cycle engine steady-state performance
- Using tandem blades to break loading limit of highly loaded axial compressors
