ZmWRKY104 positively regulates salt tolerance by modulating ZmSOD4 expression in maize
Jingwei Yn, Jing Li, Heping Zhng, Y Liu, Aying Zhng,b,*
a College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, Jiangsu, China
b State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University, Nanjing 210095, Jiangsu, China
Keywords:Zea mays ZmWRKY104 ZmSOD4 Salt tolerance
ABSTRACT Salinity impairs plant growth,limiting agricultural development.It is desirable to identify genes responding to salt stress and their mechanism of action.We identified a function of the Zea mays WRKY transcription factor, ZmWRKY104, in salt stress response. ZmWRKY104 was localized in the nucleus and showed transcriptional activation activity. Phenotypic and physiological analysis showed that overexpression of ZmWRKY104 in maize increased the tolerance of maize to salt stress and alleviated salt-induced increases in O2- accumulation, malondialdehyde (MDA) content, and percent of electrolyte leakage. Further investigation showed that ZmWRKY104 increased SOD activity by regulating ZmSOD4 expression. Yeast onehybrid, electrophoretic mobility shift test, and chromatin immunoprecipitation–quantitative PCR assay showed that ZmWRKY104 bound directly to the promoter of ZmSOD4 by recognizing the W-box motif in vivo and in vitro. Phenotypic, physiological, and biochemical analysis showed that ZmSOD4 increased salt tolerance by alleviating salt-induced increases in O2-accumulation,MDA content,and percent of electrolyte leakage under salt stress. Taken together, our results indicate that ZmWRKY104 positively regulates ZmSOD4 expression to modulate salt-induced O2- accumulation, MDA content, and percent of electrolyte leakage, thus affecting salt stress response in maize.
1. Introduction
Plants are frequently exposed during their growth and development to environmental stresses such as salt and drought stress[1].Plants have evolved various strategies to adapt to salt stress. In general,they sense and transmit stimulus signals from the cell surface to the cell interior,thus modulating the adaptive responses of genes to salt stress at physiological and biochemical level [2].Many transcription factors, including MYB, NAC, and WRKY, have been identified [3–6] as regulating plant response to salt stress.
WRKY is one of the largest families of plant transcription factors and has been identified in many species including Arabidopsis,Oryza sativa, Zea mays [7]. The characteristic feature of the WRKY superfamily is the presence of a highly conserved WRKY domain containing the almost invariant WRKYGQK peptide at the N terminus followed zinc-finger motifs [8]. WRKYs directly modulate the expression of target genes by binding to the W-box motif of promoters [8–10]. WRKY proteins regulate a wide range of development processes including stem elongation, pollen development,leaf senescence,and seed development [8,11–13]. A growing body of evidence [7,14–16] strongly indicates that WRKYs act through various interconnected networks to regulate multiple simultaneous responses to abiotic stresses. For instance, AtWRKY46 regulates responses to several abiotic and hormonal stresses in Arabidopsis[9,17,18] and AtWRKY15 modulates salt and osmotic stress responses [19]. Ectopic expression of ZmWRKY17 in Arabidopsis resulted in higher drought sensitivity [5]. Overexpression of ZmWRKY106 in Arabidopsis increased drought and heat tolerance[20].The maize genome encodes 136 WRKYs[20,21].Among them,several(ZmWRKY17,ZmWRKY33,ZmWRKY58,and ZmWRKY114)have been identified [5,22–24] as functioning in salt response by ectopic expression of their genes in Arabidopsis or rice. Whether other maize WRKYs are involved in salt response is unknown.
Salt stress to plants can cause the rapid production of reactive oxygen species (ROS), including superoxide anion (O2-), hydrogen peroxide (H2O2) and singlet oxygen (1O2). Excess ROS caused by salt stress is highly toxic to plants [2,25]. Thus, one of the important plant adaptations to salt stress is enhancing antioxidant defense systems by increasing activities of antioxidant enzymes,including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) [26]. SOD, catalyzing the conversion of O2-to H2O2, is the first line of defense against oxidative damage, so that increasing SOD activity can protect plants against abiotic stress.Overexpression of a Jatropha curcas SOD (JcSOD) in Arabidopsis increased salt tolerance [27], as did overexpression of a rice SOD (OsSOD) in rice [28] and a Potentilla atrosanguinea SOD(PaSOD)in potato[29].Salt stress induces some SOD gene expression,suggesting that SOD is regulated at the transcriptional level [27,30,31].However, the mechanism is unknown.In the present study,we investigated the function of a maize WRKY transcription factor, ZmWRKY104, in salt response in maize.
2. Materials and methods
2.1. Plant materials and growth conditions
Maize(Zea mays L.)inbred line B73 and tobacco(Nicotiana benthamiana L.)were used in this study.Seeds were sown on pots containing soil mixture(soil:vermiculite,1:1,v/v)or grown in Kimura B nutrient solution(Coolaber, Beijing,China)in a growth chamber(25 °C, 200 μmol m-2s-1, 14 h light/10 h dark, 60% humidity).Eight-day-old seedlings of wild type grown in nutrient solution were treated with 200 mmol L-1NaCl for various times, and samples were collected and immediately frozen in liquid nitrogen.
2.2. Isolation of total RNA and real-time PCR analysis
Total RNA was isolated from maize leaves as described in [32].Real-time PCR was performed using EvaGreen 2× qPCR MasterMix-No Dye (Applied Biological Materials, Zhenjiang,Jiangsu, China) according to the manufacturer’s instructions.Expression levels for ZmWRKY104 and ZmSOD4 were measured using the 2–ΔΔCTmethod with ZmActin2 as described previously[33].
2.3. Maize transformation and regeneration
The full-length of ZmWRKY104 was amplified by PCR using specific primers(Table S1)and introduced into the plant express vector pCUN-NHF driven by the ubiquitin promoter.The maize inbred line B73 was used as the plant recipient.Agrobacterium-mediated maize shoot-tip transformation was used [34]. Positive transformants were selected by spraying with 25 μg mL-1DL-phosphinothricin(Sigma-Aldrich, USA), and further confirmed by PCR. Resistant T2 seedlings with 3:1 segregation of resistance were transferred to soil to obtain homozygous T3 seeds from individual lines.
2.4. Subcellular localization of ZmWRKY104
Maize protoplasts were isolated as described previously [35].The coding sequence of ZmWRKY104 was cloned into a pXZP008-YFP vector driven by the ubiquitin promoter. Plasmids (100 μg)were transfected into 1 mL maize protoplasts using a PEG–calcium-mediated method [35]. Transfected protoplasts were stained with 1 μg μL-14′,6′-diamino-phenylindole(DAPI,Solarbio,Shanghai, China). After staining for 16 h in the dark at 25 °C, fluorescence signals were examined under a Leica laser-scanning confocal microscope with the following parameters: DAPI, excitation at 360 nm and emission at 466 nm; YFP, excitation at 513 nm and emission at 527 nm (Leica, TCP SP8, Germany) [6].
2.5. Cell fraction analysis
According to the manufacturer’s instructions, Plant Plasma Membrane Protein Extraction Kit (BestBio, Shanghai, China) and Plant Nuclear Protein Extraction Kit (BestBio) were used to isolate the plasma membrane, nucleus and cytoplasm fractions. Histone H3.1,detected by monoclonal anti-histone H3.1 antibody(Abmart,Shanghai, China), acted as a nucleus protein marker.
2.6. Western blot analysis
Proteins extracted from maize leaves were separated by 12%SDS-PAGE,then transferred to a PVDF membrane(Merck Millipore,USA).The membrane was blocked with 5%[w/v]skim milk powder in PBST buffer for 2 h at 25°C.The membrane was incubated with a 1000-fold dilution of polyclonal anti-ZmWRKY104 antibody(Abmart), a 5000-fold dilution of anti-histone H3.1 antibody(Abmart),anti-Flag antibody(Abmart),or anti-actin antibody(Biodragon, Beijing, China) in PBST buffer for 4 h. The membrane washed with PBST buffer was incubated with a 2000-fold dilution of secondary antibody (goat anti-mouse or goat anti-rabbit IgG coupled with horseradish peroxidase, Abmart) in PBST buffer for 1 h. A camera (Tanon 5200 Multi, China) was used to capture the chemifluorescent signal.
2.7. Phenotype and oxidative damage analysis
For the growth phenotype, eight-day-old seedlings grown on Kimura B nutrient solution were treated with or without 200 mmol L-1NaCl in Kimura B nutrient solution for an additional 6 days.After recovery in nutrient solution for 6 days, surviving seedlings were counted and photos were taken.
For analysis of oxidative damage,eight-day-old maize seedlings were subjected to the nutrient solution containing with or without 200 mmol L-1NaCl for 2 days, after which the maize leaves were harvested. The content of MDA and the percentage of electrolyte leakage were measured as described previously [32].
For the analysis of total chlorophyll content, eight-day-old maize seedlings were subjected to the nutrient solution containing with or without 200 mmol L-1NaCl for 4 days, after which total chlorophyll contents were determined as described [36].
2.8. Determination of O2- level
For staining with O2-, nitroblue tetrazolium (NBT) staining was used to stain the second maize leaf as described previously [37].Briefly,eight-day-old maize seedlings were subjected to the nutrient solution containing with or without 200 mmol L-1NaCl for 2 days, after which the second leaves were incubated in 0.5 mg mL-1NBT solution (Solarbio) for 12 h in dark at 25 °C.The decolored leaves washed with 95%ethanol were photographed and recorded.
For quantification of O2-, eight-day-old maize seedlings were subjected to nutrient solution with or without 200 mmol L-1NaCl for 2 days, after which the second leaves were harvested. O2-content was measured with a Hydrogen Peroxide Assay Kit(Beyotime,Shanghai, China) according to the manufacturer’s instructions.
2.9. Measurement of SOD activity
Eight-day-old maize seedlings were subjected to the nutrient solution containing with or without 200 mmol L-1NaCl for 2 days,after which the second leaves were harvested.Second leaves were homogenized in 1 mL of 50 mmol L-1potassium phosphate buffer(pH 7.0)containing 1 mmol L-1EDTA and 1%polyvinylpyrrolidone 40. The homogenate was centrifuged at 12,000×g for 30 min at 4 °C, and the supernatant was immediately used to measure SOD activity [32].
2.10. Luciferase assays
The coding sequence of ZmWRKY104 was cloned into vector 1305-YFP. The promoters of ZmSOD1, ZmSOD2, ZmSOD3, ZmSOD4,and ZmSOD4A were separately cloned into the p1381-LUC vector.Agrobacterium tumefaciens strain GV3101 carrying the above vectors and 1305-REN vector were infiltrated into 4-week-old tobacco leaves in various combinations as indicated. After infiltration for 4 days,LUC/REN ratio was calculated following the manufacturer’s instructions for the Dual Luciferase Reporter Gene Assay Kit(Vazyme, Nanjing, China).
2.11. Yeast one-hybrid (Y1H) assay
The fragments of ZmSOD4 promoter were inserted into the vector pAbAi(Table S1),then transformed into strain Y1HGold to generate the bait strain. The full-length coding sequence of ZmWRKY104 was cloned into the pGADT7 AD vector using the specific primers listed in Table S1. The construct pGADT7 ADZmWRKY104 or empty vector pGADT7 AD was transformed into the Y1H bait strain containing the fragment of ZmSOD4 promoter and cultured on a synthetic dropout/-Leu/-Ura plate also containing 100 ng mL-1aureobasidin A (AbA) (Clontech, USA).
2.12. Electrophoretic mobility shift assay
The full-length ZmWRKY104 was cloned into the pET30a vector using specific primers(Table S1),then transformed into Escherichia coli BL21 (DE3) (Navogen, China). His-ZmWRKY104 recombinant proteins were purified and incubated with the biotin-11-UTPlabeled DNA fragment (ZmSOD4 promoter oligonucleotides) for 30 min in electrophoretic mobility shift assay(EMSA)binding buffer.Chemifluorescent signals were recorded a camera(Tanon 5200 Multi).A 10-fold excess of unlabeled oligonucleotides acted as the competitive probe.
2.13. Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR)assay
ChIP was performed as reported previously [38]. Ubi:ZmWRKY104-Flag transgenic plants (OE-ZmWRKY104#1), anti-Flag antibodies produced in mouse (Abmart), and Imprint Chromatin Immunoprecipitation Kit (Sigma-Aldrich, St. Louis, MO, USA) were used for ChIP experiments following the manufacturer’s instructions. The enrichment of DNA fragments was quantified by qPCR using specific primers (Table S1). A fragment of the ZmActin2 coding region was used as a reference gene.
2.14. Transcriptional activity test
The full-length ZmWRKY104 was cloned into the pGBKT7 vector using specific primers(Table S1).Then this construct or empty vector was transformed into the Y2H yeast strain and cultured for three days on a synthetic dropout/-Trp/-His/-Ade plate also containing 100 mg mL-1X-α-gal (Clontech).
2.15.Protoplast preparation and transfection with fusion construct or dsRNA
For in vitro synthesis of dsRNA, DNA templates were produced by PCR using primers containing the T7 promoter sequence (5′-T TAATACGACTCACTATAGGGAGG-3′) on both 5′and 3′ends(Table S1). DsRNA of ZmSOD4 was synthesized in vitro using the RiboMAX Large Scale RNA Production System-T7 (Promega, USA)according to the manufacturer’s instructions. Protoplast isolation and transfection with Ubi-ZmWRKY104-YFP fusion construct or dsRNA of ZmSOD4 were based on the protocol for maize mesophyll protoplasts [32].
3. Results
3.1. Subcellular localization and transcriptional activity of ZmWRKY104
To investigate the subcellular localization of ZmWRKY104,transient expression of ZmWRKY104 fused with yellow fluorescence proteins (YFP) driven by ubiquitin promoter in maize protoplasts was characterized. As shown in Fig. 1A, ZmWRKY104-YFP fusion protein was observed in the nucleus, co-localized with DAPI. To further confirm the localization of ZmWRKY104,the plasma membrane,cytosol and nucleus fractions were separately isolated from maize leaves. The nucleus fraction was confirmed by the nuclear marker, histone H3.1. In agreement with the localization of ZmWRKY104 in nuclei of maize protoplasts, ZmWRKY104 protein was detected only in the nucleus fractions (Fig. 1B).
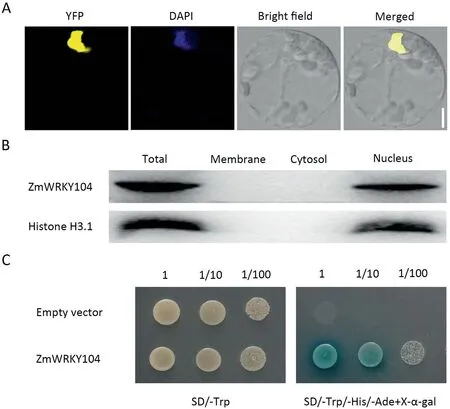
Fig. 1. Subcellular localization and transcriptional activity of ZmWRKY104. (A) The Ubi:YFP-ZmWRKY104 were transiently expressed in maize protoplasts. 4′,6′-diaminophenylindole (DAPI, blue) was applied to stain the nucleus. Scale bar, 5 μm. (B) Immunoblotting assay showing ZmWRKY104 localization. Plasma membrane, cytosol and nucleus were separately isolated,and ZmWRKY104 was detected by polyclonal anti-ZmWRKY104 antibody.Histone H3.1 acts as a nucleus protein marker.(C)Transcriptional activity of ZmWRKY104 in yeast.Y2HGold strain transformed with ZmWRKY104 or empty vector was grown on SD/-Trp and-Trp/-His/-Ade supplemented with 100 mg mL-1 X-α-gal for three days.Number at top represents the dilutions of an optical density of 600 nm.Experiments in(A–C)were performed at least three times with similar results.
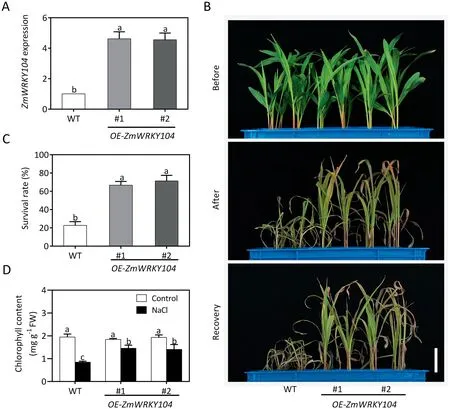
Fig. 2. Overexpressing ZmWRKY104 in maize increases salt tolerance. (A) Expression of ZmWRKY104 in wild type and ZmWRKY104-overexpressing plants. (B) Phenotype of ZmWRKY104 overexpressors and wild type under salt stress.Eight-day-old seedlings were treated with 200 mmol L-1 NaCl for 6 days and then recovered for 6 days.Scale bar,5 cm.(C)Corresponding survival rates in(B).The experiment in(B)was repeated at least three times and at least 50 plants for each genotype were assessed for survival rate in each experiment.(D)Chlorophyll contents in maize leaves of ZmWRKY104 overexpressors and wild type under NaCl treatment.Eight-day-old seedlings were treated with or without 200 mmol L-1 NaCl for 4 days,after which chlorophyll contents was measured.Error bars in(A,C and D)indicate SD(n=3).Different letters in(A,C and D)indicate significant difference at P <0.05 according to Duncan’s multiple range test.
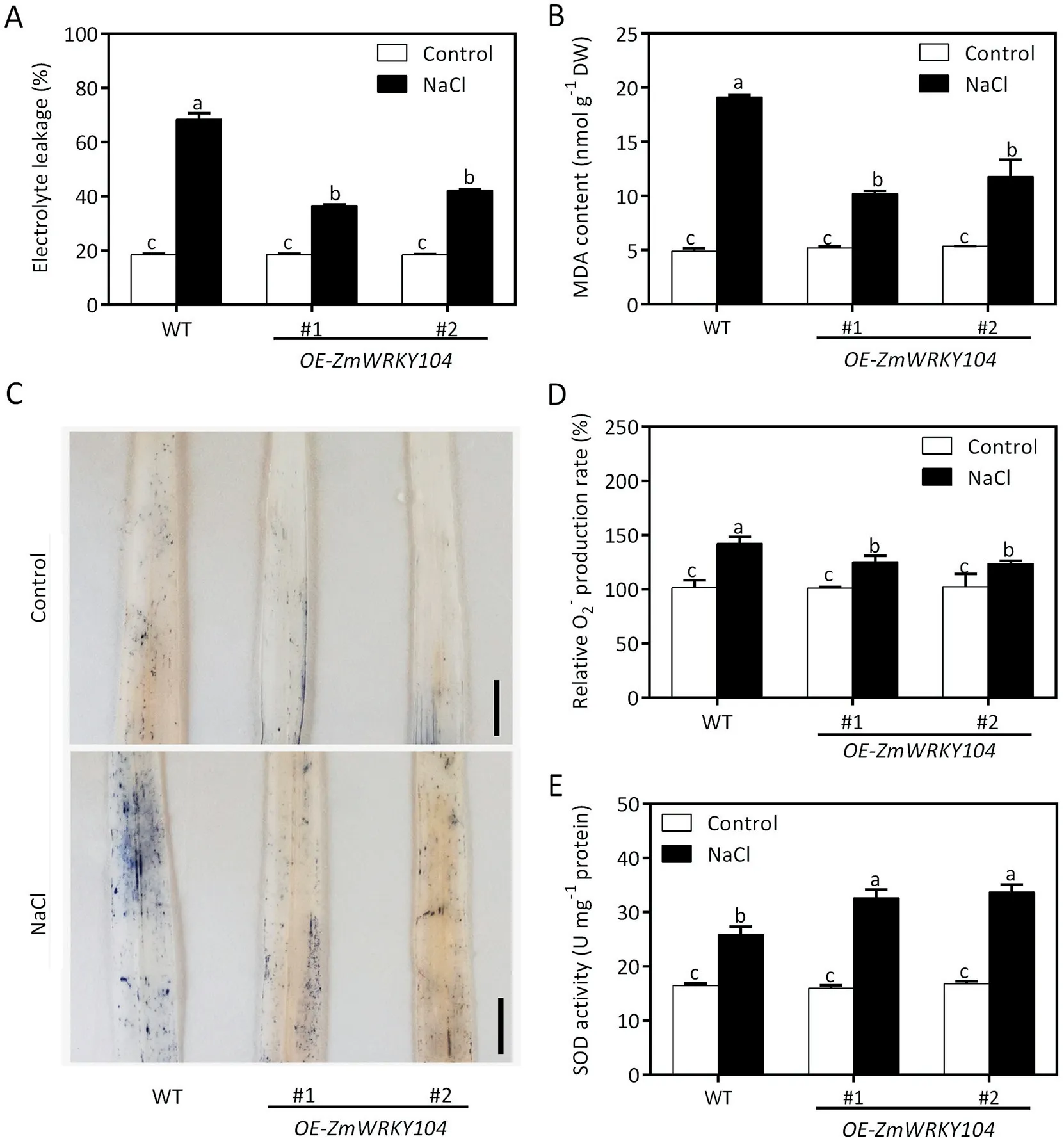
Fig. 3. Effect of ZmWRKY104 on lipid peroxidation, O2- accumulation and SOD activity under salt stress. (A) Electrolyte leakage rates in maize leaves of ZmWRKY104 overexpressors and wild type under NaCl treatment. (B) Malondialdehyde (MDA) contents in maize leaves of ZmWRKY104 overexpressors and wild type under NaCl treatment.(C)O2-accumulation in leaves of ZmWRKY104 overexpressors and wild type under NaCl treatment detected by nitroblue tetrazolium staining.Scale bars,1 cm.(D)O2-contents in leaves of ZmWRKY104 overexpressors and wild type under NaCl treatment.(E)SOD activities in leaves of ZmWRKY104 overexpressors and wild type under NaCl treatment.Eight-day-old seedlings of ZmWRKY104 overexpressors and wild type in(A–E)were treated with or without 200 mmol L-1 NaCl for 2 days,after which the above physiological indexes were measured.Error bars in(A,B,D and E)indicate SD(n=3).Different letters in(A,B,D and E)indicate significant difference at P <0.05 according to Duncan’s multiple range test.
A phylogenetic tree was used to evaluate the evolutionary relationships between ZmWRKY104 and the other WRKY proteins.ZmWRKY104 displayed a close relationship with BdWRKY62 from Brachypodium distachyon, SbWRKY62 from Sorghum bicolor, and OsWRKY62 from Oryza sativa (Fig. S1A). Sequence alignment indicated that ZmWRKY104 showed high identity (41.5%–74.4%)with its close WRKY relatives. ZmWRKY104 contained a single WRKY domain followed by a C2H2-type zinc-finger motif(Fig. S1B), indicating that ZmWRKY104 is a member of the group II WRKY family [8].
To determine whether ZmWRKY104 has transcriptional activation activity, the full-length ZmWRKY104 was cloned into the pGBKT7 vector, which carries a GAL4 DNA-binding domain.As shown in Fig. 1C, only the yeast strain transformed with ZmWRKY104 grew well and also appeared blue on synthetic dropout/-Trp/-His/-Ade plate supplemented with X-α-gal.Thus, ZmWRKY104 showed transcriptional activation activity in yeast.
3.2. ZmWRKY104 positively regulated salt tolerance in maize
To investigate the biological function of ZmWRKY104 in maize,ZmWRKY104 overexpressors were generated and two independent lines (OE-ZmWRKY104#1 and OE-ZmWRKY104#2) were confirmed by qRT-PCR(Fig.2A).Eight-day-old seedlings of ZmWRKY104 overexpressors and wild type were treated with or without 200 mmol L-1NaCl for 6 days. In the absence of NaCl, no morphological difference was observed between ZmWRKY104 overexpressors and wild-type plants. In the presence of NaCl, ZmWRKY104-overexpressing plants showed less chlorosis and growth inhibition than wild-type plants (Fig. 2B). After recovery for 6 d, the survival rate of ZmWRKY104 overexpressors was higher than that of the wild type (Fig. 2C). As shown in Fig. 2D, the chlorophyll content in the wild type was significantly lower than those in ZmWRKY104 overexpressors. These results suggest that ZmWRKY104 positively regulates salt tolerance in maize.

Fig.4. ZmWRKY104 positively regulates expression of ZmSOD4 in maize.(A)The effect of ZmWRKY104 on luciferase activity driven by various ZmSODs promoter in tobacco leaves.The panel shows a schematic representation of the double-reporter and effector plasmids.LUC and REN represent firefly luciferase and renilla luciferase.The LUC/REN ratios,which represent ZmSODs promoter activities,were calculated.(B)Salt stress up-regulates ZmWRKY104 expression in wild type.Eight-day-old seedlings of the wild type were treated with 200 mmol L-1 NaCl for indicated times, after which ZmWRKY104 expression was measured by qRT-PCR. (C) Expression of ZmSOD4 in ZmWRKY104 overexpressors and wild type under NaCl treatment.Eight-day-old seedlings of ZmWRKY104 overexpressors and wild type were treated with or without 200 mmol L-1 NaCl for 12 h, after which ZmSOD4 expression was measured by qRT-PCR. Different letters in (A–C) indicate significant difference at P <0.05 by Duncan’s multiple range test.

Fig. 5. ZmWRKY104 binds directly to the promoter of ZmSOD4 in vitro. (A) ZmWRKY104 binds to the ZmSOD4 promoter in yeast. Y1HGold strain co-transformed ZmSOD4-promoter linked to Aureobasidin 1-C (AbAr) and ZmWRKY104-AD or empty AD vector alone (AD) was grown on the SD/-Leu/-Ura with or without 100 ng/mL AbA for three days. Number at top represents dilutions of optical density of 600 nm. (B) Electrophoretic mobility shift assay shows that ZmWRKY104 binds to ZmSOD4 promoter. The purified protein (His-ZmWRKY104) was incubated with biotin-labeled probe (Biotin probe). 10-fold unlabeled probe (Cold probe) acted as the competitive probe. The experiments in (A and B) were performed at least three times with similar results.
3.3.ZmWRKY104 alleviates oxidative damage in response to salt stress
Salt stress typically causes oxidative damage in plants. Accordingly, MDA and ion leakage which could reflect the degree of oxidative damage were measured [39]. Without NaCl treatment,there were no significant differences in MDA content and electrolyte leakage between ZmWRKY104 overexpressors and wild-type plants. Under NaCl treatment, both MDA content and electrolyte leakage were significantly lower in ZmWRKY104 overexpressors than in the wild type (Fig. 3A, B). Thus, ZmWRKY104 alleviates oxidative damage in response to salt stress.
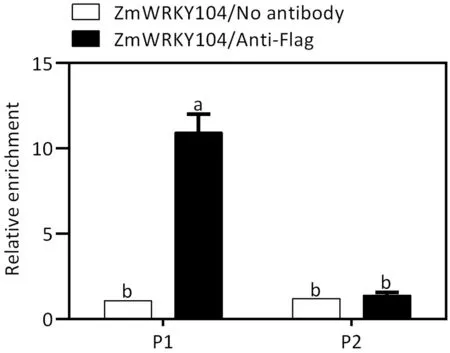
Fig.6. ZmWRKY104 binds to the promoter of ZmSOD4 in vivo.The upstream region shows the W-box, which is a putative ZmWRKY104 binding site in the region 2000 bp upstream of the start site in ZmSOD4 promoter. Gray lines indicate the GGTCAA motif. P1 and P2 represent the fragments amplified in the ChIP assay.Chromatin from eight-day-old maize leaves of ZmWRKY104 overexpressor (OEZmWRKY104#1) was immunoprecipitated with anti-Flag antibody produced in mouse (anti-Flag, Abmart). The measured values in control (no antibody) were set to 1 after normalization against ZmActin2 for quantitative PCR. Values are mean ± SD (n = 3). Different letters indicate significant difference at P <0.05 by Duncan’s multiple range test.
3.4. ZmWRKY104 regulates O2- accumulation by modulating SOD activity in response to salt stress
Excess ROS accumulation caused by salt stress results in oxidative damage. To determine whether there was a relationship between ROS and ZmWRKY104, O2-accumulation was assessed by NBT staining. As shown in Fig. 3C, bluer precipitation in wild type leaves signified a greater level of O2-accumulation than in ZmWRKY104 overexpressors exposed to salt stress. In agreement with the results of NBT staining, the O2-content was much higher in the wild type than in ZmWRKY104 overexpressors under NaCl treatment (Fig. 3D). SOD, catalyzing the conversion of O2-to H2O2,is the first line of defense against oxidative damage.Accordingly, the SOD activity in wild type and ZmWRKY104 overexpressors in response to salt stress was measured. As expected, SOD activity was higher in ZmWRKY104 overexpressors than in wildtype plants under NaCl treatment (Fig. 3E). These results suggest that ZmWRKY104 reduces O2-accumulation by increasing SOD activity in response to salt stress.
3.5.ZmWRKY104 positively regulates ZmSOD4 expression in response to salt stress
Given that SOD activity was regulated by ZmWRKY104 in response to salt stress(Fig.3E)and the maize genome harbors five SOD genes (ZmSOD1, ZmSOD2, ZmSOD3, ZmSOD4, and ZmSOD4A),the effect of ZmWRKY104 on the activity of ZmSODs promoter was investigated in tobacco leaves using a dual luciferase assay system. The ZmSODs promoter was amplified and fused to firefly luciferase protein (LUC) at the N-terminus. The effector plasmids contain either ZmWRKY104-YFP or YFP expression cassette. The LUC/REN ratio represents the ability of ZmWRKY104 to transcriptionally activate its putative downstream genes. As shown in Fig. 4A, ZmWRKY104 increased only the activity of LUC driven by the ZmSOD4 promoter and did not affect the activity of LUC driven by the other ZmSODs promoter. This result suggests that ZmWRKY104 might positively regulate ZmSOD4 expression.
To investigate whether ZmWRKY104 regulates the ZmSOD4 expression in salt response,the effect of salt stress on ZmWRKY104 expression was investigated. NaCl treatment significantly upregulated ZmWRKY104 expression (Fig. 4B). Next, the expression of ZmSOD4 in ZmWRKY104 overexpressors and wild type exposed to salt stress was measured. In the presence of NaCl, ZmSOD4 expression was higher in ZmWRKY104 overexpressors than in the wild type (Fig. 4C). Thus, ZmWRKY104 positively regulates ZmSOD4 expression in response to salt stress.
3.6.ZmWRKY104 directly binds to the ZmSOD4 promoter in vitro and in vivo
Given that ZmWRRKY104 positively regulated the expression of ZmSOD4 in response to salt stress (Fig. 4), we wondered whether ZmWRKY104 could directly regulate ZmSOD4 expression. WRKY proteins can regulate the expression of their target genes by recognizing W-box motif [8,10]. We identified one W-box motif in the ZmSOD4 promoter (–164 to –169). To determine whether ZmWRKY104 protein could bind to the promoter of ZmWRKY104,we first used a Y1H assay. We tested the interaction between ZmWRKY104 and a fragment of the ZmSOD4 promoter(–1 to –280) by growing on a medium lacking Leu and Ura and supplemented with 100 ng mL-1AbA. As shown in Fig. 5A,ZmWRKY104 bound to the ZmSOD4 promoter in yeast. Next, we performed EMSA. ZmWRKY104 directly bound to probes labeled with biotin, and this interaction was almost abolished by addition of a 10-fold excess of unlabeled competitive probes (Fig. 5B).
To confirm the interaction between ZmWRKY104 and ZmSOD4 promoter in vivo, a chromatin immunoprecipitation (ChIP) assay was performed. The ZmWRKY104-Flag proteins were successfully detected in leaves of OE-ZmWRKY104#1 plants (Fig. S2). As shown in Fig. 6, ZmWRKY104 strongly bound to the P1 but not the P2 region of the promoter, which does not harbor a W-box motif.Thus, ZmWRKY104 binds directly to the ZmSOD4 promoter in vitro and in vivo.
3.7. ZmSOD4 increases salt tolerance in maize
To further confirm the role of ZmSOD4 in response to salt stress,ZmSOD4 overexpressors were generated and two independent lines (OE-ZmSOD4#4 and OE-ZmSOD4#5) were confirmed by qRTPCR (Fig. S3). Eight-day-old seedlings of ZmSOD4 overexpressors and wild type were treated with or without 200 mmol L-1NaCl for 6 days. In the absence of NaCl, no morphological difference was observed between ZmSOD4 overexpressors and wild-type plants. In the presence of NaCl, ZmSOD4 overexpressors showed less chlorosis and growth inhibition than wild-type plants(Fig.7A).After recovery for 6 days,the survival rate of ZmWRKY104 overexpressors was higher than that of the wild type (Fig. 7B).Under NaCl treatment,the chlorophyll contents in ZmSOD4 overexpressors were significantly lower than those in the wild type(Fig. 7C). MDA content, electrolyte leakage and O2-accumulation were significantly lower in ZmSOD4 overexpressors than in the wild type (Fig. 7D–F). Thus, ZmSOD4 increases salt tolerance by alleviating oxidative damage.
4. Discussion
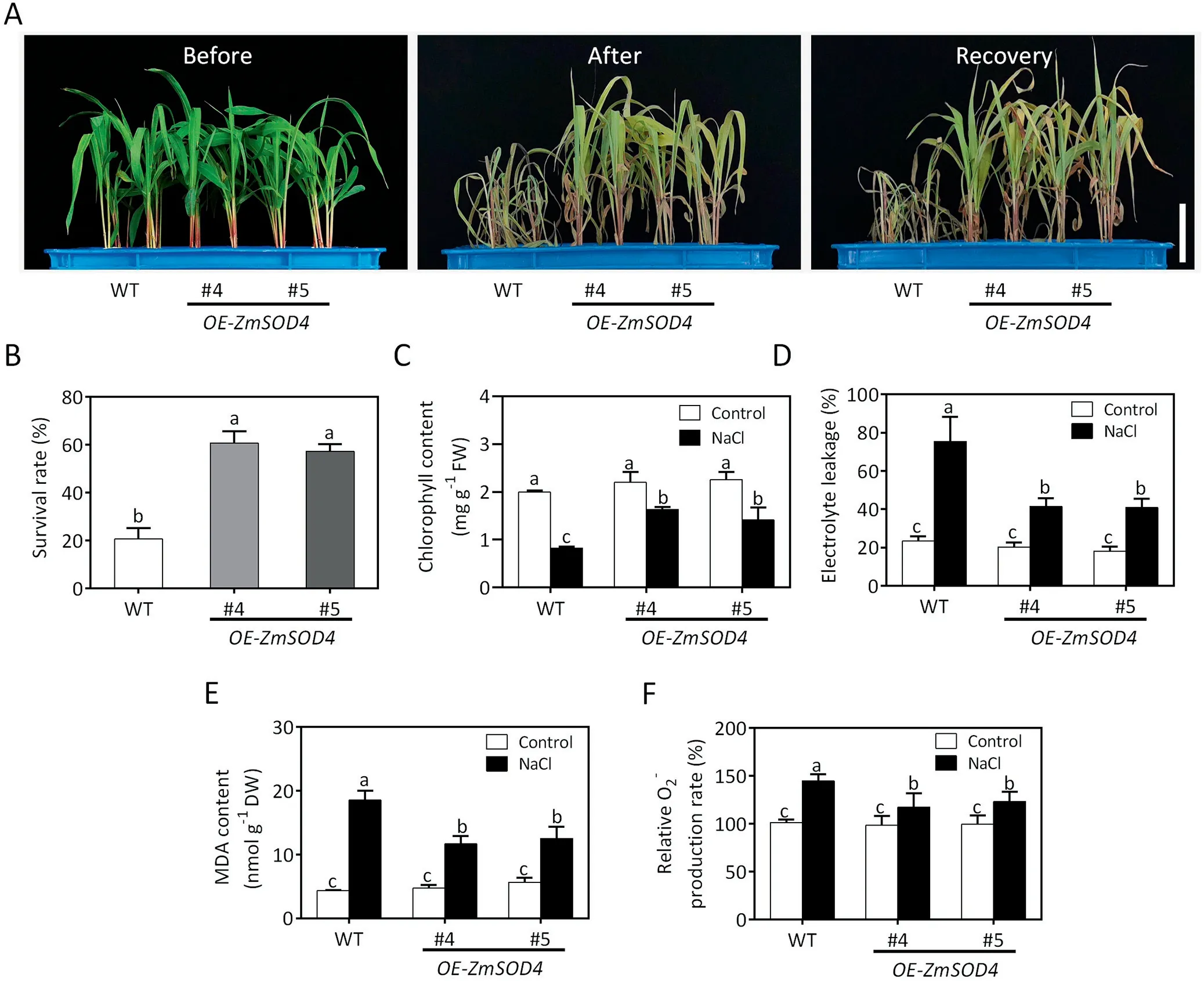
Fig.7. Overexpression of ZmSOD4 in maize increases tolerance to salt stress.(A)Phenotype of ZmSOD4 overexpressors and wild type under salt stress.Eight-day-old seedlings were treated with 200 mmol L-1 NaCl for 6 days and then recovered for 6 days.Scale bar,5 cm.(B)Corresponding survival rates in(A).The experiment in(A)was repeated at least three times and at least 50 plants for each genotype were assessed for survival rate in each experiment.(C)Chlorophyll contents in leaves of ZmSOD4 overexpressors and wild type under NaCl treatment. Eight-day-old seedlings were treated with or without 200 mmol L-1 NaCl for 4 days, after which chlorophyll contents were measured. (D)Electrolyte leakage rates in leaves of ZmSOD4 overexpressors and wild type under NaCl treatment.(E)Malondialdehyde(MDA)contents in leaves of ZmSOD4 overexpressors and wild type under NaCl treatment. (F) O2- contents in leaves of ZmSOD4 overexpressors and wild type under NaCl treatment. Eight-day-old seedlings of ZmSOD4 overexpressors and wild type in(D–F)were treated with or without 200 mmol L-1 NaCl for 2 days,after which the above physiological indexes were measured.Error bars in(B–F) indicate SD (n = 3). Different letters in (B–F) indicate significant difference at P <0.05 by Duncan’s multiple range test.

Fig.8. A model of ZmWRKY104 activity in maize salt response.Salt stress induces the expression of ZmWRKY104,which causes an increase in ZmSOD4 expression and SOD activity by directly recognizing the ZmSOD4 promoter, leading to the decrease of O2- accumulation and promoting the salt stress response.
WRKY family is one of the largest transcription factor families in higher plants, implying that they have distinct functions. Indeed,WRKY proteins have been found to play pivotal roles in the regulation of plant growth and development process including secondary cell wall formation, germination, trichome outgrowth, seed development and leaf senescence [40–44]. In addition, WRKY proteins regulate plant hormone signaling and plant response to abiotic stresses and biotic stresses [5,23,45,46]. However, there are few prior reports about the function of WRKY in maize. To our knowledge, four maize WRKYs (ZmWRKY17, ZmWRKY33, ZmWRKY58 and ZmWRKY114)have been investigated in salt response by ectopic expression of these genes in Arabidopsis or rice [5,22–24]. The roles of additional maize WRKY proteins in regulating salt response await discovery. We demonstrated that ZmWRKY104 is a positive regulator in modulating maize salt response based on the following results. First, overexpressing ZmWRKY104 in maize increased salt stress tolerance(Fig.2).Second,ZmWRKY104 alleviated oxidative damage in response to salt stress (Fig. 3).
WRKY function in response to abiotic stresses via activation of the cellular antioxidant defense system has been reported in Arabidopsis [18,47]. Our study showed that ZmWRKY104 positively regulated SOD enzyme activity (Fig. 3E). The maize genome encodes five SOD genes (ZmSOD1, ZmSOD2, ZmSOD3, ZmSOD4 and ZmSOD4A). Our results showed that ZmWRKY104 regulated only ZmSOD4 expression via direct binding to the promoter of ZmSOD4 in response to salt stress (Figs. 4–6). Transient expression of ZmWRKY104 in maize protoplasts markedly increased SOD activity, but this effect was partly blocked by transient silencing of ZmSOD4 (Fig. S4). Thus, ZmWRKY104 acts upstream of ZmSOD4 to regulate SOD activity. The observation that SOD activity in protoplasts with both transiently expressed ZmWRKY104 and silenced ZmSOD4 was much higher than in protoplasts with transiently silenced ZmSOD4 suggests that there are other targets of ZmWRKY104 in regulating SOD activity. The genetic relationship between ZmWRKY104 and ZmSOD4 in salt response invites further investigation using transgenic plants overexpressing ZmWRKY104 in a zmsod4 mutant or knockout of ZmWRKY104 in a ZmSOD4 overexpressor.
In agreement with the putative role of WRKY proteins as transcriptional factors, ZmWRKY104 was indeed localized in the nucleus, suggesting that ZmWRKY104 might function in the nucleus(Fig.1).WRKY proteins can act as positive or negative regulators. For example, AtWRKY46 is a transcriptional activator,whereas AtWRKY40 is a transcriptional repressor.AtWRKY6 could act as either activator or repressor [18,48,49]. In this study, we showed that ZmWRKY104 was a transcriptional activator (Fig. 1C and Fig. 4). The effect of ZmWRKY104 on ZmSOD4 expression showed that SOD could be regulated at the transcriptional level.The expression of two Arabidopsis SOD genes (CSD1 and CSD2)was modulated by miR398-directed mRNA cleavage in osmotic stress response [50]. A very recent study [51] showed that a rice SOD protein,ALM1,stabilized OsTrxz protein via directly interacting with OsTrxz. These studies indicate that SOD proteins also could be modified at the post-transcriptional and posttranslational levels.Studies of such modification of maize SOD proteins would shed light on the mechanism of SOD response to salt stress.
Excess reactive oxygen species (ROS) caused by salt stress severely harms the plants, and plants have evolved a variety of adaptive mechanisms to deal with it. One of the important plant adaptations to salt stress is increasing the capacity of nonenzymatic antioxidants such as ascorbic acid (AsA). AsA regulated by the ABA INSENSITIVE 4 (ABI4)-VITAMIN C DEFECTIVE 2 (VTC2)module scavenged accumulated ROS,increasing salt tolerance[52–54]. Another strategy is increasing the activities of antioxidant enzymes such as SOD.Superexpression of SOD genes in Arabidopsis,rice or potato increased tolerance to salt stress [27–29]. In agreement with these results, constitutive expression of ZmSOD4 in maize increased salt tolerance (Fig. 7). SOD-gene knockout Arabidopsis (CSD1 or CSD2) plants showed suppressed growth [55].Overexpression of a rice SOD (OsSOD) in rice increased plant fresh weight [28], suggesting that SOD affected the plant growth and development. We observed no morphological differences between ZmSOD4 overexpressors and wild type plants. A similar phenomenon was observed in previous studies. Overexpression of a Jatropha curcas SOD (JcSOD) in Arabidopsis or a Potentilla atrosanguinea SOD (PaSOD) in potato did not affect plant growth in the normal condition [27,29]. Thus, it is reasonable to speculate that SOD genes function differently among species.
We present a model by which ZmWRKY104, directly recognizing the ZmSOD4 promoter,positively regulates ZmSOD4 expression to modulate salt-induced O2-accumulation,MDA content,and electrolyte leakage, thus mediating salt stress response in maize(Fig. 8).
Accession number
Sequence data from this study can be found under the accession numbers: ZmWRKY104 (Zm00001d020495), ZmActin2(Zm00001d013873), ZmSOD1 (Zm00001d036135), ZmSOD2(Zm00001d022505), ZmSOD3 (Zm00001d037859), ZmSOD4(Zm00001d029170), ZmSOD4A (Zm00001d047479).
CRediT authorship contribution statement
Jingwei Yan:Methodology, Writing - original draft, Funding acquisition,Project administration,Data curation,Formal analysis.Jing Li:Data curation, Formal analysis, Methodology, Writing -original draft.Heping Zhang:Methodology.Ya Liu:Methodology.Aying Zhang:Writing-review&editing,Funding acquisition,Project administration, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (32001445 and 31871534), the Natural Science Foundation of Jiangsu Province(BK20200557),and the China Postdoctoral Science Foundation (2019M651846).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.05.010.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet

