Far-red light: A regulator of plant morphology and photosynthetic capacity
Tingting Tan, Shenglan Li, Yuanfang Fan, Zhonglin Wang, Muhammad Ali Raza,Iram Shafiq, Beibei Wang, Xiaoling Wu, Taiwen Yong, Xiaochun Wang, Yushan Wu,Feng Yang,*, Wenyu Yang
a College of Agronomy, Sichuan Agricultural University, Chengdu 611130, Sichuan, China
b Sichuan Engineering Research Center for Crop Strip Intercropping System, Chengdu 611130, Sichuan, China
c Key Laboratory of Crop Ecophysiology and Farming System in Southwest, Ministry of Agriculture, Chengdu 611130, Sichuan, China
Keywords:Far-red light Photosynthetic capacity Photosystem Photosynthetic electron transport
ABSTRACT Plant photosynthetic capacity directly determines crop yield. Light quality regulates photosynthetic capacity. This review discusses plant responses to far-red light from the phenotypic to the molecular level, focusing specifically on the improvement of photosynthetic capacity by adjustment of photosynthetic electron transport and the path of light energy. Far-red light can also regulate leaf angle and increase plant height and leaf area, via expression of associated genes, to capture more light energy.Thus, far-red light regulates plant morphology and photosynthetic capacity. Identifying the mechanism of this regulation may lead to increased crop yields.
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 301
2. Effect of far-red light on plant morphology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 301
2.1. Plant height. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 301
2.2. Leaf morphology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 302
2.3. Stomata . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 302
2.4. Plant biomass . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 302
3. Effect of far-red light on leaf structure and chloroplast . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 302
3.1. Leaf structure. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 302
3.2. Chloroplast. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 302
4. Effect of far-red light on plant photosynthetic characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 303
4.1. Photosynthetic pigments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 303
4.2. Chlorophyll fluorescence. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 304
5. Effect of far-red light on the photosystem and photosynthetic electron transport in plant leaves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 304
5.1. Photosystem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 304
5.2. Photosynthetic electron transport . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 305
6. The effect of far-red light on carbon assimilation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 305
7. Effect of far-red light on photosynthate products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 305
8. Conclusion and perspectives. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 306
Declaration of competing interest . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 306
Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 306
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 306
1. Introduction
Photosynthesis is a process by which plants use light energy to convert carbon dioxide (CO2) and water (H2O) into organic matter and release oxygen (O2) [1]. Light quality strongly influences photosynthesis[2].Because light with a wavelength of 400–700 nm is the most photosynthetically effective [3], most studies of photosynthesis have been performed in this wavelength range [4,5].However, far-red light (700–800 nm) mediates plant growth and developmental processes, especially in shaded environments [6–9].Only a few studies on the influence of far-red light on the morphological and photosynthetic parameters of plants have been reported. A shaded environment (such as strip intercropping) is well known [10] to reduce the red-to-far-red ratio of light (R/Fr)in the plant canopy, ultimately changing plant morphology, physiology, and biochemistry. Increasing far-red light increases plants’internodal length, petiole length, plant height, and gibberellin(GA) content [11–13], and adding far-red light increases the canopy gross photosynthetic rate in C3and C4plants[14].To date,most studies have focused on the effect of far-red light on plant morphological characteristics, photosynthetic pigment content,chlorophyll fluorescence, and other indicators in field cultivation and facility agriculture [15,16]. Only a few studies [17,18] have investigated the effect of far-red light on the photosystem and photosynthetic electron transport.Identifying the physiological mechanisms by which far-red light influences the photosynthetic capacity of plants could lead to increases in crop yield.This review summarizes research progress on the effect of far-red light on plant morphology and photosynthetic capacity, including height, leaf morphology, chloroplast ultrastructure, photosystem, and photosynthetic electron transport.
2. Effect of far-red light on plant morphology
2.1. Plant height
The light-harvesting ability and carbon assimilation of plants are linked closely to their architectural features. For example,variations in far-red light change plant height, especially under shaded conditions [19]. Plants possess photoreceptors that monitor red and far-red light. These photoreceptors are known as phytochromes, which are composed of chromophores and apoproteins [20]. Phytochromes can be classified into phytochrome A (phyA), a far-red light photoreceptor, and phytochrome B (phyB), a red-light photoreceptor [21]. Earlier study [22] have shown that phyB also influences the manner in which plants respond to far-red light. A decrease in the R/Fr ratio of the plant canopy slightly changes the chromophore structure. Some phytochromes change from a biologically active far-red-light-absorbing state (Pfr) to a biologically inactive redlight-absorbing form (Pr) in an environment with a low R/Fr ratio [23,24]. Pfr and Pr are present simultaneously in plants.When far-red light increases in the plant canopy, the negative regulator of plant photomorphogenesis SPA1 (suppressor of phyA-105) interacts with COP1 (constitutive photomorphogenesis 1) to form E3 ubiquitin ligase [25,26]. This ligase inhibits plant photomorphogenesis and promotes hypocotyl elongation by degrading phosphorylated phyA and the positive regulator HY5 (long hypocotyl 5) [22,27–30]. The low R/Fr environment also reduces the activity of phyB. However, this environment regulates the expression of PIF7 (phytochrome interacting factor 7), which interacts with phyB in Arabidopsis leaves, leading to the increased binding of PIF7 to downstream target genes. The expression of flavin monooxygenase (YUC), the rate-limiting enzyme in the auxin synthesis pathway, is up-regulated,increasing the content of auxin (indole-3-acetic acid, IAA). IAA is transported from leaves to stems, elongating the internodes and increasing the plant height (Fig. 1) [31–33]. Expression of kaurenoic acid oxidase genes in the biosynthetic pathway of GA is up-regulated in a low-R/Fr environment [34], increasing GA content, accelerating cell division and cell elongation in the stem, and ultimately increasing plant height [35–37]. In summary, a low-R/Fr environment can regulate the endogenous hormone levels of plants via expression of related genes to regulate plant morphogenesis and capture more light energy[38–42].

Fig. 1. Regulation of plant height by far-red light.
2.2. Leaf morphology
Photosynthesis is one of the main physiological functions of leaves.The effect of far-red light on leaves strongly influences plant photosynthetic capacitys [43,44]. An abundance of far-red light in the canopy environment increases leaf length and reduces leaf width, making leaves narrower than those under normal light[45,46]. A high R/Fr ratio inhibits leaf expansion, resulting in a smaller leaf area. By contrast, a low R/Fr ratio increased leaf area by 1.31-fold compared with a control by increasing the extensibility of the cell wall in the plant cells (Fig. 2). Thus, leaf area influences radiation use efficiency. Far-red light is beneficial to plant radiation use efficiency [46–50].
Like leaf area, leaf angle directly affects light interception. The petiole can control the orientation of the leaf relative to the incident light by changing the angle of its adaxial side.A low R/Fr ratio may change petiole length and leaf angle by promoting the elongation of the cells on this adaxial side,thereby increasing the area of the leaf intercepting light [12,51–53]. The increased abundance of far-red light also optimizes light interception by reducing the overlap area of leaves [54,55].
2.3. Stomata
Stomata on the leaf epidermis are the main channels for water and gas exchange[56].The stomata of different plants respond distinctly to far-red light.High far-red light increased the numbers of stomata in chrysanthemum and Rotala hippuris leaves but reduced the number in tobacco leaves and the stomatal conductance of cucumber leaves [45,57–59]. Far-red light also reduced stomatal density in leaves of Arabidopsis thaliana, cucumber, and wheat[57,60,61].Red and blue light promote stomatal opening.However,far-red light does not necessarily promote stomatal opening. In response to blue light, the C3and C4guard cells of plants synthesize ATP by photophosphorylation, activating H+-ATPase on the plasma membrane and driving K+flux into the guard cells. The accumulation of K+increases the water potential of guard cells,facilitating the entrance of water into them and inducing stomatal opening [62–64]. In contrast, far-red light can reverse stomatal opening caused by blue light [65]. However, the influence of farred light on the regulation of stomata is controversial. Karlson[66] believed that far-red light does not affect stomatal opening.Talbott et al. [65] found that far-red light at 700 nm promoted stomatal opening but that far-red light at 720 nm reversed this process.
Our picture of the influence of far-red light on stomata remains inconclusive.Far-red light affected intracellular Ca2+concentration by reducing the phytochrome Pfr content,regulating the entry and exit of K+and thereby controlling stomatal opening [67]. But in another study[68],a low-R/Fr environment changed stomatal density by changing the ratio of the biologically active form of phytochrome, phyB, and the expression of stomatal development genes.
2.4. Plant biomass
In one study [69], red, blue, and green light showed no significant effect on dry or fresh weight of lettuce, but supplementation with far-red light significantly increased both. Ai et al. [70]reported that the dry and fresh biomass of tomatoes increased significantly by 28.46% and 33.26% under a low-R/Fr environment.The biomass of soybean and lettuce increased significantly under high far-red light conditions [50,71]. Thus, supplementing with far-red light (low R/Fr ratio) can increase plant biomass. Reducing the R/Fr ratio also promotes the distribution of plant dry matter to the stem [72,73].
3. Effect of far-red light on leaf structure and chloroplast
3.1. Leaf structure
The leaf is the main organ of photosynthesis in higher plants.Inside the leaf, the mesophyll tissue regulates light transmission for photosynthesis. It can form palisade and spongy tissues to reduce the difference between light reception on the dorsiventral surface of the leaf. Plant leaves can change the mesophyll tissue structure to adjust the capture of light energy and exchange gases to acclimatize to different environments [74]. Many studies[6,46,75–77] have shown that the addition of far-red light increases net photosynthetic rate and leaf area but reduces leaf thickness. The explanation of this phenomenon may be that low R/Fr conditions reduce the thickness of palisade tissue by reducing its cell size and cell number,thereby reducing the thickness of the leaf (Fig. 3) [78,79].

Fig. 2. Effect of far-red light on plant leaf area. Low-R/Fr ratio treatment significantly increased leaf area under the same light intensity.
3.2. Chloroplast

Fig.3. Effects of far-red light on leaf anatomical structure and leaf thickness.(A)Low light treatment;(B)low light plus far-red light treatment;(C)compared with low light,the addition of far-red light reduced the thickness of palisade tissue, spongy tissue, and the leaf.
Photosynthesis occurs in the chloroplast. The distribution and internal structure of the chloroplast strongly influence the capture and transmission of light energy. Far-red light is essential for chloroplast development. Its absence impairs chloroplast structure,resulting in irregularly arranged grana thylakoids[80].Under a combination of blue,green,red,and far-red light,the long side of the chloroplast tends to be vertical to the incident direction of the far-red light.This phenomenon indicates that chloroplast distribution is closely related to far-red light [81]. Far-red instead of red light received at the end of the day increased chloroplast length but reduced chloroplast width in tobacco leaves,leading to the formation of an oblong-shaped chloroplast [82]. A low R/Fr ratio increased the number of chloroplasts in pepper leaves,the number of chloroplast grana in maize and tobacco leaves,and grana stacking in soybean leaves compared with a control. However, the stacks of thylakoids in maize and tobacco leaves decreased in a low-R/Fr environment [79,82–84]. Thus, the effect of far-red light on plant leaf chloroplast structure is still uncertain.Some researchers [85] believe that high far-red light increases the density of granum and the grana stacking, while others [82] believe that the number of granum thylakoid layers is reduced, resulting in a decrease in grana stacking under high far-red light environments.Studies of the response of chloroplasts to far-red light have focused mainly on the structure of chloroplast DNA, proton motive force,and photosystem II (PSII) content in the thylakoid membrane[86–88]. Only a few studies have investigated how far-red light regulates the number and structure of chloroplasts. In Arabidopsis,FHY3 (far-red elongated hypocotyls3)/ CPD45 (chloroplast division45) controls chloroplast division by regulating the expression of ARC5 and FHY1, thereby affecting the number of chloroplasts[89]. A lack of chlorophyll b (Chl-b) reduces the level of LHC II(light-harvesting complex II) protein,leading in turn to a decrease in grana stacking[90].Whether far-red light affects the structure of the chloroplast by regulating chlorophyll content awaits further research. In addition to reshaping plant morphology, far-red light affects plant photosynthetic characteristics (Table 1).

Table 1Effects of far-red light on plant morphology and photosynthesis.
4.Effect of far-red light on plant photosynthetic characteristics
4.1. Photosynthetic pigments
Chlorophyll functions in the absorption,transmission,and conversion of light energy,and the content and composition of chlorophyll directly affect leaf photosynthetic capacity. The chlorophyll content of tomato, maize, and tobacco leaves decreased in a low-R/Fr environment [45,75,99]. However, the contents in soybean and chrysanthemum leaves were negatively correlated with R/Fr ratio [100,101]. The ratio of Chl a to Chl b affects the photosynthetic activity of chloroplasts. High far-red light reduces this ratio in most plants [51,88,92,93,101]. A decrease in Chl a/Chl b increased the reducing ability of 2,6-dichlorophenolindophenol,increasing the photophosphorylation activity of chloroplasts[102].
Carotenoids can transfer energy to chlorophyll for photosynthesis and protect the chlorophyll from photooxidation [103]. Like chlorophyll content,carotenoid content varies among plants whenfar-red light increases.The carotenoid contents of chrysanthemum,strawberry, and Dipterocarpaceae leaves increased [100,104,105]but those in soybean and tomato leaves decreased[106,107].Given the influence of far-red light on carotenoid content, studies[108,109] have shown that high far-red light can lead to overexpression of the mRNA and protein levels of phytoene synthase,enriching carotenoids in tissues.Pfr can prevent the loss of carotenoids, whereas far-red light reduces the relative content of Pfr[110].The decrease in Pfr reduced the number of lipid globules that prevent carotenoid degradation, resulting in a decrease in carotenoid content.
The photosynthetic pigment content represents the photosynthetic ability of plants. The responses of plant photosynthetic pigments to light intensity are not consistent among species [111].Like the effect of far-red light on these pigments, the effects of intensity and quality of light on the content of these pigments vary among plant species.
4.2. Chlorophyll fluorescence
When leaves absorb light,solar energy is converted by the plant into chemical energy, heat, and chlorophyll fluorescence. The pigment molecules are excited by red light,and the electrons in these molecules are transferred to the lowest excited state. Given the instability of the excited state, the state quickly changes to the ground state, and the energy is either consumed in the form of light or released as heat. Light emitted from the lowest excited state back to the ground state is called fluorescence[112]. Chlorophyll fluorescence is produced by energy-level changes in pigment molecules.This characteristic reflects the absorption,transmission,dissipation,and distribution of solar energy called a probe of photosynthesis [113,114]. The quantum yield of PSII (ΦPSII) is also known as the actual photosynthetic capacity of PSII. However,the nonphotochemical quenching coefficient (NPQ) reflects the ability of plants to turn excess light energy into heat.
After the long-term addition of far-red light to white light,ΦPSIIincreases and NPQ decreases (Fig. 4). This phenomenon indicates that high far-red light reduces the heat dissipation of PSII and increases the light energy available for photosynthesis in the leaves. The increase in ΦPSIIand decrease in NPQ occur as far-red light increases photosystem I (PSI) activity, leading to faster reoxidation of plastoquinone and promoting the reopening of the PSII reaction center [77,95].
Studies [115–117] have investigated chlorophyll fluorescence technology in a stable light environment. Real-time fluorescence technology has been applied to investigate changes in the photosystem in a dynamic light environment [118,119]. The shortterm addition of far-red light increased the ΦPSIIand reduced the NPQ of the leaf [94], a result consistent with that in a stable light environment(Fig.4).After addition of far-red light to red or white light, the quantum yield of fluorescence immediately drops and reaches a steady-state minimum within 10–15 s [77]. A decrease in the fluorescence yield may be due to the increase in photochemical efficiency or heat dissipation according to the path of light energy captured by plants. Heat dissipation depends on the xanthophyll cycle involving a series of enzymatic reactions that usually take a few minutes. The photochemical efficiency regulated by the redox signal can change on a time scale of milliseconds.Thus, the decrease in fluorescence yield is due to the increase in PSII photochemical efficiency [77,120–122].

Fig. 4. Influence of far-red light on the fate of light energy.
5.Effect of far-red light on the photosystem and photosynthetic electron transport in plant leaves
5.1. Photosystem
The light reaction in the thylakoid membrane converts light energy into chemical energy in NADPH and ATP, as the first step of photosynthesis. The photosynthetic electron transport chain with PSI and PSII as the primary members functions in the light reaction [123]. Light-harvesting complex I (LHC I) and LHC II are associated with PSI and PSII, respectively, and transfer captured light energy to antenna pigments in the reaction center [124].Far-red light preferentially excites PSI and increases its activity in C3and C4plants [125]. However, owing to the unbalanced distribution of light energy between PSI and PSII, plants regulate the distribution of light energy by state transition, photosystem stoichiometry, or the light-harvesting complex [92,94,126].
First, far-red light increases the relative content of the chlorophyll protein complex in the PSII reaction center by upregulating the gene expression of Lhcb1, Lhcb2, and Lhcb4[101,127]. Second, far-red light excites PSI preferentially, causing the oxidation of plastoquinones and then inhibiting LHC II kinase activity.LHC II is dephosphorylated and combined with PSII to promote light energy distribution to PSII [128–130]. Finally, far-red light can reshape the photosynthetic structure [131]. PSI is distributed on the non-stacked stromal thylakoid membrane,whereas PSII is distributed on the stacked grana thylakoid membrane[84,127]. High far-red light increases the stoichiometric ratio of PSII/PSI because of thylakoid stacking changing [88,93]. Far-red light increases the number of grana formed by thylakoid stacks,leading to a high PSII/PSI ratio[132].However,the responses of different plant leaf chloroplasts to a high far-red light environment are inconsistent.High far-red light may adjust the structure of thylakoids by affecting chlorophyll, thereby increasing the ratio PSII/PSI [125].
5.2. Photosynthetic electron transport
Cyclic electron transport is one of the modes of photosynthetic electron transport.An abundance of far-red light accelerates cyclic electron transport through the PSI [133]. After addition of far-red light, PSI is preferentially excited, and the chlorophyll dimer P700 in PSI is oxidized to P700+. It then transfers electrons to the electron acceptor ferredoxin. The limitation of electrons in P700+promotes the transfer of electrons from plastocyanin to PSI,thereby accelerating the re-oxidation of plastoquinone. Thus, the cyclic electron transport around PSI is accelerated (Fig. 5) [96–98,134].
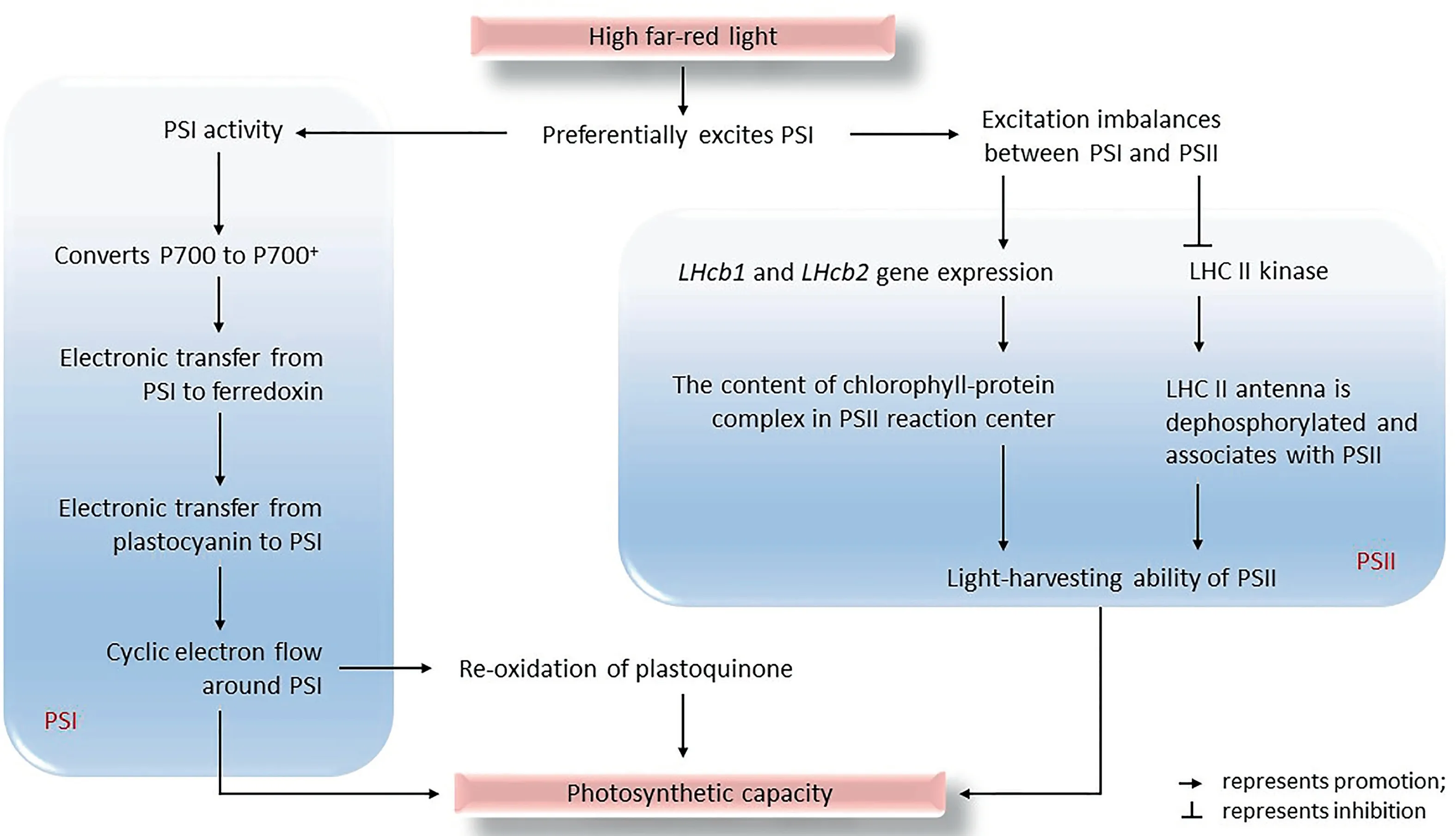
Fig. 5. Effects of far-red light on the photosystem and photosynthetic electron transfer.
D1 is one of the polypeptides that PSII binds to the electron transfer body, and PsbA is the first identified D1 polypeptideencoding gene.High far-red light promotes the expression of PsbA,increasing the content of D1 protein and the ability of PSII to bind electrons [63].
6. The effect of far-red light on carbon assimilation
Carbon assimilation is the fixation of CO2and formation of sugars using ATP and NADPH [135]. This process is divided into three stages: carboxylation, reduction, and regeneration. Carboxylation is the rate-limiting reaction of carbon assimilation [136].Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) catalyzes the carboxylation reaction of ribulose 1,5-bisphosphate(RuBP) in the Calvin cycle [137]. An influence of red or blue light on Rubisco has been reported [138,139], but only a few studies have investigated the effect of far-red light on Rubisco or carbon assimilation. Spinach leaves grown under low R/Fr showed higher Rubisco enzyme activity than those grown under high R/Fr under the same light intensity. Far-red light has been inferred [140] to promote carbon assimilation and increase the efficiency of CO2conversion into carbohydrates. However, study [141] has shown that light intensity, not light quality, regulates changes in Rubisco levels.
7. Effect of far-red light on photosynthate products
Types of photosynthate products vary among plants. Starch is the main photosynthate produced in most higher plants. The primary photosynthetic product of wheat is sucrose, in contrast to glucose and fructose in onions [142]. Sucrose is the main form of transportation of photosynthate products,and starch is the storage form of photosynthate products for most plants [143]. In comparison with normal light, increased far-red light increased sucrose and starch contents in soybean [50] and chrysanthemum leaves[144].However,strawberry and peach leaves showed high sucrose and reduced starch content [145]. The effect of far-red light on plant photosynthesis may be species-dependent. The pathways for the effect of red and blue light on photosynthate production have been elucidated [146–149]. However, the mechanism of the influence of far-red light on photosynthate production and degradation remains unclear and rarely reported. FHY3 and FAR1 (farred-impaired response 1) can activate FHY1 (far-red elongated hypocotyl 1)and FHL(FHY1-like)to participate in the far-red light signal transduction pathway via transcription [150,151]. Far-red light stimulates FHY3 and FAR1 to transcriptionally activate ISA2(ISOAMYLASE 2),thereby promoting starch synthesis by regulating the activity of the starch debranching enzyme [152].
8. Conclusion and perspectives
Far-red light accelerates plant flowering, regulates plant nutrition, and shapes plant morphology (Fig. 6) [9,95,153,154]. When far-red light in the canopy increases, plants increase the interception of solar energy by increasing leaf area and adjusting leaf angle(changing the angle of the leaves relative to the incident light),the orientation of the chloroplast, and the distribution of canopy leaves. After sunlight is captured, far-red light increases the proportion of light energy used in the light reaction,reduces heat dissipation, and improves photochemical efficiency (Fig. 4). Far-red light promotes energy transfer from PSII to PSI and increases the cyclic electron transfer rate and photophosphorylation activity(Fig.5).Thus,high far-red light can increase light capture capacity,photosynthetic electron transfer rate,photophosphorylation activity, and plant photosynthetic capacity and biomass.
Research into the effects of far-red light on the photosynthetic capacity of plants has some shortcomings. The following study areas invite attention:(1)characterization of the difference in photosynthetic capacity between C3and C4plants, grasses and legumes, woody and herbaceous plants, and other types of plants in response to high far-red light; (2) use of transcriptomics, proteomics, metabolomics, and other technologies to further identify the mechanism of action of far-red light in improving plant photosynthesis; and (3) from the perspective of agricultural production,determination of how high far-red light can be used to increase crop yields via optimized configuration of crops.
CRediT authorship contribution statement
Tingting Tan:Writing - original draft.Shenglan Li:Formal analysis.Yuanfang Fan:Visualization.Zhonglin Wang:Visualization.Muhammad Ali Raza:Resources.Iram Shafiq:Resources.Beibei Wang:Visualization.Xiaoling Wu:Funding acquisition.Taiwen Yong:Resources.Xiaochun Wang:Funding acquisition.Yushan Wu:Formal analysis.Feng Yang:Writing-review&editing.Wenyu Yang:Supervision.
Declaration of competing interest
Authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (32071963), the International S & T Cooperation Projects of Sichuan Province (2020YFH0126), and the China Agriculture Research System (CARS-04-PS19).
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet
- RNAi-mediated suppression of the abscisic acid catabolism gene OsABA8ox1 increases abscisic acid content and tolerance to saline–alkaline stress in rice (Oryza sativa L.)

