Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
Chopu Zhng, Yongjin Sun, Dinwen Wng, Wenqing Sun, Yuye Yu, Zhongli Hu, Siin Yu,*
a National Key Laboratory of Crop Genetic Improvement, College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, Hubei, China
b State Key Laboratory of Hybrid Rice, College of Life Science, Wuhan University, Wuhan 430072, Hubei, China
Keywords:Rice Heterotic loci Grain yield Overdominance
ABSTRACT Heterosis contributes greatly to crop production,but the genetic basis of heterosis is not fully understood.To identify heterotic loci(HLs)for grain yield,12 yield traits were evaluated in four rice(Oryza sativa L.)mapping populations:one parental population of chromosome segment substitution lines derived from a cross between the japonica cultivar Nipponbare and indica cultivar 9311 and three connected test populations in either a homozygous 9311 genetic background or a heterozygous background. A total of 390 HLs were detected for the measured traits in two environments. The genetic bases of heterosis differed between the backcross and testcross populations. At least 10 HLs were confirmed in F1 hybrids between 9311 and near-isogenic lines, each of which carried a heterotic locus of interest in the same 9311 background. All 10 showed overdominant or dominant effects on grain yield and yield components. Among them, three were verified as being associated with yield heterosis and colocalized in the same regions as those containing previously reported heterosis-associated genes. Five HLs were identified to be promising candidate loci that could be used to achieve more than 15%yield heterosis in several commercial rice hybrids. These findings suggest the potential of indica or japonica introgression for increasing yield in hybrid rice breeding programs.
1. Introduction
Heterosis is a phenomenon in which hybrids present yield,quality and abiotic and biotic stress adaptation that are superior to those of their parents[1].Heterosis has been observed in various plant species [2–5] and contributes to increased yield or quality improvement of crops [6,7]. Because exploiting heterosis in crop breeding is one of the most efficient ways to increase grain yield,its genetic basis has been studied in many crops [6–10] and appears to be polygenic, with main hypotheses of overdominance and dominance at a single locus.
Rice is the main staple food in Asia, which accounts for 90% of worldwide rice consumption [11]. The development of elite rice hybrids and exploitation of heterosis represent breakthroughs in rice breeding in the past fifty years [12]. Recent progress in genomics has facilitated the identification of heterotic loci or genes involved in rice yield traits [13]. As the most popular hybrid cultivars, Shanyou 63 (SY63) and Liang-you-pei 9 (LYP9), have been adopted as model systems for dissecting the genetic basis of heterosis in three-line and two-line hybrids[14–20]. For example,by the use of recombinant inbred lines and a backcross population derived from the cross (LYP9) of the elite sterile line Peiai 64S(PA64S) and a widely used restorer line (9311), several major quantitative trait loci(QTL)underlying yield heterosis were identified [14,15]. In a series of F2:3and ‘‘immortalized F2” populations that were derived from a cross between Zhenshan 97 and Minghui 63, several heterosis-associated loci with digenic interaction effects contributed to grain yield and seedling traits [16–19].Numerous other mapping populations have been developed for genetic dissection of heterosis [21,22]. Using F2segregating populations derived from various hybrid rice crosses, Huang et al. [23]found that several yield-associated genes were involved in heterosis advantages in yield-component traits.In a recent genome-wide association study [24,25], several QTL accounting for yield-trait heterosis for which the accumulation of superior alleles contributed to yield heterosis were found.Thus far,heterotic loci representing at least 29 candidate genes associated with heterosis of yield traits have been identified using biparental mapping populations and natural germplasm [14,23–26]. However, only a few heterosis-associated genes have been cloned, because the effects of a given locus are easily affected by a complex genetic background. The molecular mechanisms underlying heterosis remain poorly understood. Exploitation of these heterosis-associated QTL and genes has been limited by the genetic interactions that occur predominantly in breeding populations and commercial hybrid cultivars [27]. Whether the replacement of a parental allele with an optional allele at given heterosis-associated loci could increase the yield potential of commercial rice hybrids awaits investigation.
Chromosome segment substitution lines (CSSLs) and their derived secondary populations carry similar genetic backgrounds,facilitating the precise verification of the genetic effects of loci of interest [2,28]. Thus, CSSLs and their derived populations can be used to detect genetic factors of heterosis at the single-locus level[8,15].In the present study,to detect heterotic loci for yield-related traits, a CSSL population and CSSL derived backcross and testcross populations were used.To further verify major heterotic loci identified repeatedly in multiple populations and environments, several hybrid combinations were generated by crosses between elite sterile lines and near-isogenic lines that contain a heterotic locus of interest. As a result, a number of HLs were identified and the allele replacement at some HLs could improve the yield potential of elite commercial rice hybrids. These findings will be useful for improving grain yield in hybrid rice breeding programs.
2. Materials and methods
2.1. Plant materials
Four mapping populations were used.The first population,consisting of 122 CSSLs,was developed from a cross between two rice cultivars whose genome has been sequenced,the japonica cultivar Nipponbare(NIP)as donor and the elite indica restorer line 9311 as recurrent parent, using a backcross scheme in conjunction with marker-assisted selection [29,30]. The backcross population (BC)was generated by backcrossing each CSSL with 9311.Two testcross populations (named TC1 and TC2) were generated by testcrossing each CSSL with two elite sterile lines: Yuetai A (YTA)and Guangzhan 63S (GZ63S).
2.2. Experimental design and phenotypic evaluation
Three experiments were conducted to study the genetic basis of yield heterosis in rice.First,CSSL and BC populations were used to identify midparent heterotic loci (HLMP) in the homozygous background of 9311. The parental lines (CSSLs and 9311) were used as controls. Two TC populations were used to detect overstandard heterotic loci (HLOS) for heterosis advantage over the check cultivars in a similar heterozygous background of either YTA/9311 or GZ63S/9311. Second, to confirm the midparent heterosis (MPH) effects of HLMP, 10 CSSLs and near-isogenic lines(NILs) that carried the introduced NIP segment of the heterotic locus of interest were selected and crossed with 9311 to generate hybrid combinations to validate heterotic effects on grain yield and yield-related traits. Third, to determine the over-standard heterosis (OS) effects of heterotic regions, five NILs harboring the target HLs were each crossed with six elite sterile lines, PA64S, GZ63S,Y58S, Xinan S (AS), Luohong 3A (3A), and YTA, to generate corresponding hybrid combinations. Among these combinations, five generated from the crosses between the NILs and four sterile lines(PA64S, Y58S, AS, and 3A) were described in a previous study for predicting hybrid performance [31]. The hybrids that were generated from the crosses of 9311 and the corresponding six sterile lines were used as controls to assess over-standard heterosis.
To estimate heterotic effects, plants of the CSSL, backcross, and testcross populations were grown at the experimental station of Huazhong Agricultural University in Wuhan (30.48°N, 114.2°E) in two environments (2007 and 2011),using a randomized complete block design for the CSSL and BC populations in 2007(E1)and 2011(E2),and for TC1 in E1 and TC2 in E2.The hybrids with various allelic combinations at ten HLMPof interest along with the corresponding parental lines(CSSLs and 9311)were planted in 2014(E3),with two replications.Each line was planted in two rows,with 10 plants per row at a spacing of 16.7 × 26.6 cm. The field was managed according to local standard practices.
Twelve quantitative traits were measured: grain number (GN),heading date (HD), number of primary branches (PB), plant height(PH), panicle length (PL), panicles per plant (PP), panicle weight(PW), number of secondary branches (SB), spikelet number (SN),seed setting percentage (SS), thousand-grain weight (TGW), and grain yield per plant (YD). These 12 traits were measured in E1,and nine traits (all but HD, PH, and SB) were measured in E2. For the hybrid combinations, 10 traits (all but HD and SB) were measured in E3.
2.3. DNA extraction and hybrid genotyping
Genomic DNA was extracted from young leaves using the CTAB method [32] with minor modifications. Genotyping of 122 CSSLs using the RICE 6 K array [30] generated a total of 3383 highquality single-nucleotide polymorphisms.A bin map with 387 bins was constructed for the CSSL population[30].The genotypes of BC and TC were deduced from each corresponding CSSL. Polymerase chain reaction was performed following a previously described procedure [33]. Insertion/deletion and simple sequence repeat markers were separated by 4% polyacrylamide gel electrophoresis and visualized by silver staining.
2.4. Genetic effect estimation
The additive effect a was estimated as a = (CSSL – 9311)/2. The dominance effect d was estimated as d=F1–(CSSL+9311)/2.MPH was estimated as MPH = (F1– MP)/MP × 100%, MP =(CSSL + 9311)/2, where F1is the phenotypic value of the BC. OS was estimated as OS=(F1–CK)/CK×100%,where F1is the phenotypic value of the TC.The estimated additive and dominance effects were used to calculate|d/a|and thereby classify HLMPas having an additive effect(A)(|d/a|<0.2),partial dominance(PD)(0.2 ≤|d/a|<0.8), complete dominance (CD) (0.8 ≤|d/a| <1.2), or overdominance (OD) (|d/a| ≥1.2), as described previously [34].
2.5. QTL and HL detection
Linear ridge regression [35]was used for QTL analysis with the bin map constructed for the CSSLs. This method was also performed for HL analysis using MPH and OS values estimated from the interconnected CSSLs, BCs, and TCs. A significance level of P <0.05 was set as the threshold for declaring the presence of a QTL [35]. The phenotypic variance explained by each QTL and HL was determined using the relaimpo package (lmg function) of R[36](www.r-project.org/).QTL and HL nomenclature followed that of McCouch [37]. Circos software [38] was used for QTL and HL heatmap presentation. Boxplots were generated using the ggplot2 package of R [39].
3. Results
3.1. Heterosis of CSSL, BC, and TC populations
Transgressive segregation in both directions and continuous distribution of all the measured traits were observed in the CSSLs,BCs, and TCs (Table S1). The majority of the CSSLs presented yield and yield-associated phenotypes similar to those of parental line 9311, but several lines showed phenotypic values significantly higher or lower than those of 9311 (Fig. 1; Table S1). Compared with their corresponding hybrid controls, many hybrids (those of BCs and TCs) generated by crossing 9311 or sterile lines also presented significantly higher or lower MPH and OS values for yield and yield-associated traits(Fig.2a–c;Table S1).These results indicated that heterosis showed a typical quantitative trait inheritance pattern controlled by multiple genes. In comparison with the BC population, TC1 and TC2 showed even wider variation in phenotypic values and heterosis for the measured traits (Table S1).
3.2. Detection of QTL in CSSLs
The QTL detected in CSSLs are summarized in Table S2.A total of 265 QTL controlling 12 yield-associated traits were identified in the CSSLs in the two environments (E1 and E2). Among them, 59 QTL were detected in both environments (Table S2). Nine to 23 QTL were detected for each of the assayed traits and explained 21.9%–83.3% of the phenotypic variance (Table S2). In particular,17 QTL were detected for HD, and 16 QTL were identified for PH.For panicle traits, 28, 26, 19 and 24 QTL were detected for PB, PL,PP and PW,respectively.Among these loci,nine QTL regions affecting multiple panicle traits were detected.For spikelet traits,28,17 and 25 QTL were identified for GN,SB and SN,respectively.Among them, two QTL regions showed positive effects on three spikelet traits: qGN1.4/qSB1.3/qSN1.5 colocalized in the same region(42.04–42.63 Mb) on chromosome 1, and qGN6.1/qSB6/qSN6 mapped to the same region (0.39–0.60 Mb) on chromosome 6(Table S2).
3.3. Detection of HLMP for yield-associated traits
Midparent heterosis of the yield-associated traits was evaluated by comparison of the BC lines with the parental lines (CSSLs and 9311) across the two environments. Respectively 130 and 110 HLMPwere identified for all 12 traits in E1 and for 9 traits in E2(Fig. 2; Tables S3–S4). Three genetic components (additive, dominant, and overdominant effects) at each HLMPwere estimated(Tables S3–S4).Most(greater than 85%)of HLMPshowed overdominant or dominant effects. These findings indicate that dominant and overdominant effects play important roles in the heterosis of yield-associated traits.Among them,48 loci were detected repeatedly in both E1 and E2,and 13 HLMPfor HD and 11 HLMPfor PH.For panicle-related traits,41 HLMPwere detected for PB,PL,PP and PW in E1,and 55 HLMPwere detected in E2.Among them,34 loci were detected in both E1 and E2,and 24 loci presented heterozygous NIP alleles that increased the MPH values.Nine HL regions were found to control multiple panicle traits. For spikelet traits, 30 HLMPwere detected for GN, SB and SN in E1, and 23 HLMPwere detected for GN and SN in E2. Eight and 17 HLMPwere detected for YD in E1 and E2, respectively (Tables S3–S4).
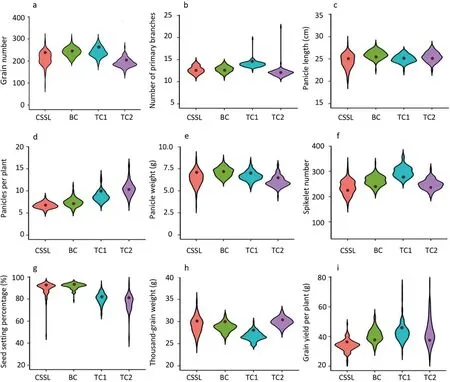
Fig. 1. Phenotypic variation in grain yield and its associated traits among chromosome segment substitution lines (CSSLs), backcross population (BC), and testcross populations(TCs).TC1,testcross population 1(Yuetai A×CSSL);TC2,testcross population 2(Guangzhan 63S×CSSL).The dark dot near the center indicates phenotype values of the corresponding parental line or commercial hybrid.

Fig.2. Heterotic loci detected for midparent heterosis(MPH)and over-standard heterosis(OS)in BC and TC populations.(a–c)Distribution of MPH and OS for grain yield and yield-associated traits.(d)Venn diagram of the number of QTL and heterotic loci(HL)detected in CSSLs,BCs,and TCs.(e–g)Genome-wide distribution of midparent heterotic loci (HLMP) and over-standard heterotic loci (HLOS) for (e) grain yield per plant (YD), (f) panicle length (PL), and (g) spikelet number (SN). Rice chromosomes with bins are indicated in the outer circle.The circles from inside outward show HLMP (A),HLOS detected in TC1(B)and HLOS in TC2(C). The x-axis represents the physical location along each numbered chromosome.The y-axis represents the negative log10P-values from the linear ridge regression model.Dashed lines indicate declaration thresholds.Red dots in (e–g) indicate major heterosis-related genes associated with grain yield and yield-associated traits in previous studies.
Heterotic loci for multiple traits that colocalized to the same or overlapping regions were designated heterotic hotspots or regions.Nine heterotic regions were found to affect four or more traits simultaneously(Table S5).Among them,seven regions increased MPH for many yield traits. In particular, HL1.1 (2.79–4.62 Mb) and HL1.2(40.15–40.90 Mb)on chromosome 1,HL2(33.68–35.63 Mb)on chromosome 2,HL4(30.72–31.49 Mb)on chromosome 4,HL6.2(24.09–24.52 Mb)on chromosome 6,HL8.1(1.70–3.50 Mb)on chromosome 8, and HL11 (2.79–3.03 Mb) on chromosome 11 were detected as having positive effects on MPH. However, two regions, HL6.1 (0–0.59 Mb) located on chromosome 6 and HL10.2 (13.94–14.0 Mb)on chromosome 10, reduced the MPH of the measured traits(Table S5).These major heterotic regions were further investigated to dissect genetic effects on heterosis.
3.4. Detection of HLOS for yield-associated traits
The TC populations had a heterogeneous background similar to that of commercial hybrids (either Feng-liang-you generated from GZ63S × 9311 or LYP9 generated from PA64S × 9311). The HLOSdetected in TC1 and TC2 are summarized in Tables S6 and S7,respectively. A total of 152 HLOSwere identified for 12 traits in TC1, and 116 HLOSwere identified for nine traits in TC2. A total of 47.3%of HLOSpresented heterozygous NIP alleles(YTA/NIP)that increased the OS in TC1(Table S6),and 46.5%of the loci presented heterozygous alleles (GZ63S/NIP) that increased the OS in TC2(Table S7). A total of 50 loci were repeatedly identified in both TC populations, and 28 loci carried heterozygous NIP alleles that increased the OS values in two heterozygous backgrounds. Thirteen HLOSfor HD and 13 HLOSfor PH were detected in TC1.Among these loci, YPH1.3 in Bin31 (38.10–39.07 Mb) of chromosome 1 showed the largest effect,explaining 31.9%of the phenotypic variance of PH.For panicle traits,43 HLOSwere detected for PB,PL,PP,and PW in TC1,and 52 HLOSwere detected in TC2.Among them,28 loci were identified in both TC populations, and 19 loci carried heterozygous NIP alleles that increased the OS values. For spikelet traits, 47 HLOSwere found to affect GN, SB, and SN in TC1, and 32 HLOSwere found to affect GN and SN in TC2.Among them,18 HLOSwere detected in both TC1 and TC2,and nine carried heterozygous NIP alleles that increased the OS values.Nine and eight HLOSfor YD were detected in TC1 and TC2, respectively.
3.5. Common regions of QTL and HLs affecting yield traits
A comparison between the QTL and HLMPidentified for yield traits of the CSSLs and BCs revealed that 88 loci colocalized in the same or overlapping regions(Fig. 2d). This colocalization indicates that these loci were strongly expressed in either the homozygous or heterozygous 9311 background. However, 152 HLMPwere detected in BCs independently of those loci in CSSLs, suggesting that HLs showed mainly dominant or overdominant effects.Moreover,57 HLMPwere found to be colocalized in the same or overlapping regions harboring HLOS, suggesting that the same gene may control both MPH and OS.Of these 57 loci,16 carried heterozygous NIP alleles that increased both MPH and OS values(Tables S3–S7).In addition, 29 loci were detected simultaneously in the CSSL, BC and TC populations (Fig. 2d). Of these loci, nine presented increased phenotypic values and heterotic performance. Thirty of 268 HLOSwere repeatedly identified in both TC1 and TC2 (Tables S6–S7). These results indicate that complex genetic backgrounds influence heterosis.
3.6. Validation of heterotic effects of HLMP
To further validate the heterotic effects of HLs, 10 CSSLs (or NILsNIP),each carrying only one introduced NIP segment containing an HLMPof interest,were crossed with 9311 to produce F1hybrids with specific allelic combinations (Table 1). Within the hybrids,five heterotic regions (HL1.1, HL1.2, HL4, HL6.2,and HL11) were found to increase MPH and one (HL6.1) to reduce MPH for many yield traits simultaneously (Figs. 3–4; Table 1). For example,NILHL1.1, which carried a single introduced NIP segment encompassing HL1.1, was crossed with 9311 to produce F1hybrids(Fig. 3a). Compared with the parental lines (NILHL1.1or 9311), the hybrids heterozygous at HL1.1 showed significantly increased YD,PP,and PL(Fig.3b–d).Heterozygosity at HL1.1 was associated with high MPH for YD(17.6%)and PP(25.0%)(Table 1).The MPH of HL11 was measured in terms of its effect on grain yield and four yield traits (Fig. 3; Table 1). Hybrids heterozygous at HL11 showed high MPH for YD (19.0%) and GN (26.1%) and moderate MPH for SN(9.9%), PL (8.9%) and PW (8.0%) (Table 1). NILHL4, which carries a single introduced NIP segment harboring HL4, was crossed with 9311 to produce an F2segregating population (n = 144) (Fig. 3i).Compared with NILHL4, the heterozygotes at HL4 showed significantly higher YD, GN, and PW (Fig. 3j–l) and moderate MPH for YD (11.7%), GN (5.3%) and PW (9.3%) (Table 1). Except for these six heterotic loci,the remaining four HLMPof the hybrids were also associated with high or moderate MPH for grain yield and yield traits (Table 1). Taken together,these results confirmed that these 10 HLs influenced yield heterosis and that almost all increased rice grain yield.
3.7. Heterotic regions associated with OS for yield components
Five HLs (HL1.1, HL1.2, HL4, HL6.1, and HL6.2) for yield-trait heterosis were identified in the same or overlapping regions as those harboring both HLMPfor MPH and HLOSfor OS (Tables 1, S6 and S7). To further investigate the heterotic effects at these regions, five NILsi, each containing a target HL(i) with NIP alleles,were crossed with six elite sterile lines to generate 30 hybrid combinations (Fig. 4). Almost all these hybrids presented yield and yield component values that were higher than those of hybrids between the corresponding sterile lines and 9311 (Fig. 4). For YD,HL1.1 showed greater than 15% OS in three hybrid combinations,with the highest OS for YD (38.1%) detected in GZ63S/NILHL1.1.HL1.2 showed high OS for YD in the combinations of GZ63S/NILi(20.9%)and PA64S/NILi(33.8%).HL4 showed high OS for YD in combinations of PA64S/NILi(37.6%)and Y58S/NILi(22.5%)and showed the highest OS for GN (39.6%) and PW (40.1%) in three combinations: PA64S/NILi, GZ63S/NILiand Y58S/NILi. HL6.1 and HL6.2 showed high OS (23.1%) and moderate OS (9.9%) in PA64S/NILi(Fig. 4).
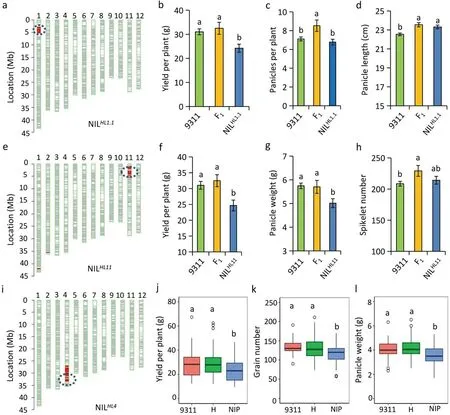
Fig. 3. Validation of major heterotic loci effects using the hybrid combinations with near-isogenic lines (NILs) and 9311. (a, e, i) Graphical genotype showing the NILNIP carrying the introduced Nipponbare segment harboring HL1.1,HL11,and HL4 in a 9311 background.(b–d)Phenotypic values of HL1.1 for grain yield per plant(YD),panicles per plant(PP),and panicle length(PL)of the hybrids and parental lines(NILHL1.1 and 9311).(f–h)Phenotypes of HL11 for YD,panicle weight(PW),and spikelet number(SN)of the hybrids and parental lines(NILHL11 and 9311).Error bars represent means±standard error.(j–l)Phenotypes of HL4 for YD,grain number(GN)and PW in a NILHL4-derived F2 population (n = 144). 9311 (n = 29), H (n = 74) and NIP (n =41) denote respectively 9311, heterozygous, and NIP genotypes at HL4. Different letters above the error bars denote significant difference according to the Duncan test at P <0.05.
4. Discussion
A large number of HLs for 12 yield traits were identified in the NIP/9311 CSSLs and their derived backcross and testcross populations. By comparing the QTL in CSSLs in this study with those in previous studies, we found that at least 50 QTL were colocalized in the same or overlapping regions that harbor functional genes reported to be associated with grain yield or yield traits(Table S2). For example, qPH1.3 associated with plant height in CSSLs is located at the same location as the semidwarf gene sd1[40]. Seven QTL (qGN4.4/qPB4.4/qPL4/qPW4.4/qSB4.2/qSN4.3/qYD4.3) were localized in the overlapping region (30.72–31.49 Mb) that contains the narrow-leaf gene NAL1 [41]. Six loci (qHD7.5/qGN7.2/qPB7/qPL7.3/qSB7/qSN7.2) were mapped in the region (28.13–29.70 Mb) overlapping that of the yield-associated gene Ghd7.1[42].
Unlike the QTL with additive genetic effects identified in CSSLs,the HLs for MPH and OS present mainly dominance effects at any given locus. Several HLs for grain yield and yield traits were detected (Tables S3–S7). A total of 33.0% of the HLMPfor MPH or HLOSfor OS were found in the same or overlapping regions as those of the QTL for the measured traits (Fig. S1; Table S5), suggesting that these colocalized loci influenced trait performance by both additive and dominant effects. However, only 12.6% of HLMPand HLOSwere identified as being in common for MPH and OS,indicating different genetic bases of heterosis in the backcross and testcross populations (Fig. 2d). The BC population had the homozygous background of 9311, while the TC populations had a heterozygous background (YTA × 9311 or GZ63S × 9311). More HLOSfor wider variation in OS suggested that the TC populations within the heterozygous backgrounds (either YTA/9311 or GZ63S/9311) had a more complex genetic basis of heterosis for the measured yield traits (Table S1; Fig. 2). Among the detected HLs, 42 HLMPand 45 HLOSmapped to the same or overlapping regions as those harboring previously reported heterosisassociated genes associated with yield traits (Tables S3–S7)[23,26]. In particular, 10 heterosis-associated genes: Gn1a, sd1,OsMADS22, Ehd1, Ehd2, Ehd4, NAL1, Ghd7, Ghd7.1, and IPA1, were identified as possible candidate genes influencing both MPH and OS. Thus, integration of BC and TC populations is an effective strategy for elucidating the genetic basis of heterosis for complex traits such as yield. Taken together, these results indicate that the heterosis-associated genes identified could be useful for improving yield potential in either homozygous or heterozygous backgrounds.
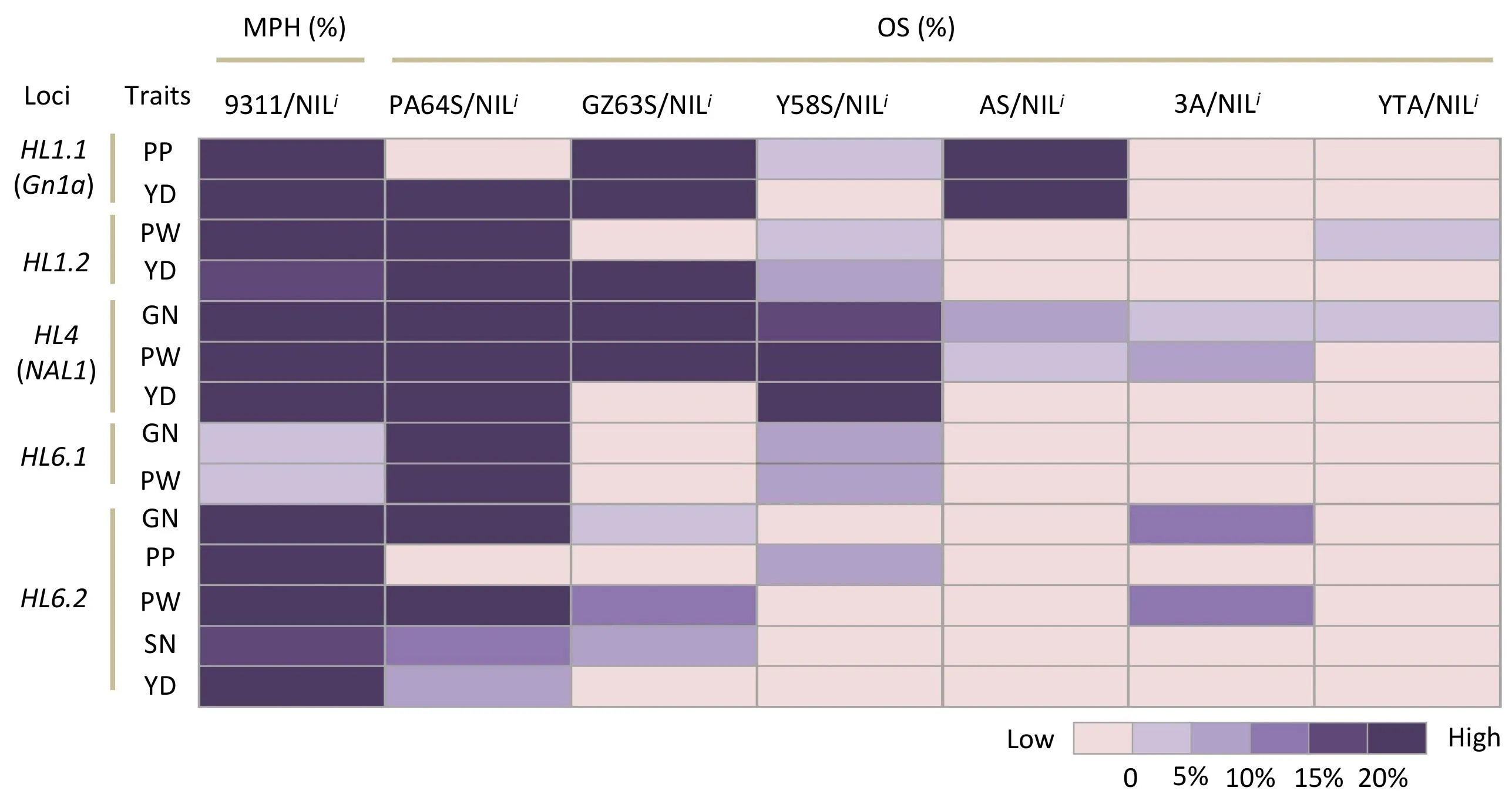
Fig.4. Midparent heterosis(MPH)and over-standard heterosis(OS)for grain yield and yield components for five major heterotic loci.NILi harbors the Nipponbare alleles at a corresponding heterotic locus within the 9311 background. PA64S/NILi, GZ63S/NILi, Y58S/NILi, AS/NILi, 3A/NILi, and YTA/NILi denote six hybrid combinations derived from crosses between NILi and six elite sterile lines.PA64S,Peiai 64S;GZ63S,Guangzhan 63S;Y58S;AS,Xinan S;3A,Luohong 3A;YTA,Yuetai A.The colors in the heatmap denote the heterosis levels of the grain yield per plant (YD) and yield components: panicles per plant (PP), panicle weight (PW), grain number (GN).
Notably,the indica cultivar 9311 is widely used as an elite parent for two-line rice hybrids [14]. For example, LYP9(PA64S × 9311), Feng-liang-you (GZ63S × 9311), and Y-liang-you(Y58S×9311)have been successfully used for commercial production in recent decades in China [15]. We detected 10 heterotic regions and verified that they were major heterotic loci for yield traits in BCs with the homozygous 9311 background (Table 1).Among these 10 regions, five (HL1.1, HL1.2, HL4, HL6.1, and HL6.2)were also identified for yield traits in TCs with heterozygous backgrounds(Tables S6–S7).These five heterotic loci were also found to increase the OS of yield component traits of several commercial hybrids:LYP9,Feng-liang-you,and Y-liang-you(Fig.4),suggesting that these heterotic loci could be widely used to improve yield potential in different hybrid backgrounds. In particular, HL1.1 increased MPH by 31.2% and OS by 33.1% for grain yield of four hybrid combinations and was located in the same region as Gn1a[43], which was reported [15] to be a heterosis-associated gene that controls multiple agronomic traits of rice. Comparison of Gn1a sequence between NIP and 9311 revealed that Gn1a9311carried a 6-bp deletion in the coding region,which causes amino acid substitution (www.elabcaas.cn/rice/), suggesting that heterotic advantage may depend on the allelic variation of the gene. HL4 showed high MPH for grain yield and yield traits in both the F2and the BC population, as well as high OS for YD, GN, and PW in several hybrid combinations. HL4 increased OS by more than 30.0%for the measured yield traits compared to those of the commercial hybrid LYP9.In agreement with this result,the HL4 region contains NAL1 [44], which was identified in previous studies[15,23] as a major heterotic candidate gene for yield traits.Sequence differences between NIP and 9311 were also found in the promoter and coding regions of NAL1 and led to allelic variation at the transcription and protein levels [45]. Seven identified HLs(HL1.2, HL6.1, HL6.2, HL11, BPB1.1, BPB2.1, and BSN8.3) may be novel heterosis-associated loci. Hybrids heterozygous at HL6.1 showed a partial dominance effect on grain yield and yield components,with a mean increase of 24.3% OS for YD, GN, and PW compared with those of LYP9 (Table 1). HL6.1 was mapped in the heterotic region, 2 Mb from Hd3a [46]. HL6.2 increased the MPH of grain yield and five yield traits (GN, SN, PB, PP, and PW) by an average of 23.8% (Table 1). In addition, one CSSL carried only one introduced NIP segment of a small size(approximately 790 kb)carrying HL6.2 in the homozygous 9311 background,and provides an excellent resource for further mapping and cloning of the causal genes for yield heterosis.Our findings suggest that these HLs identified as promising candidate loci are used directly for improving yield potential of some commercial hybrids.Upgrading elite parental lines (i.e., 9311 and PA64S) by replacement of their alleles at a given HL with alternative NIP alleles to generate a desirable allelic combination would be of practical value to improve the yield potential of commercial hybrid cultivars. Further investigation is needed to determine whether a much higher yield heterosis advantage could be achieved by pyramiding two or more HLs in either elite restorer lines or sterile lines for hybrid rice breeding.
5. Conclusions
Many QTL and HLs for yield and yield-associated traits were identified in CSSLs and their derived testing populations. A total of 29 HLMPand HLOSwere colocalized in the same or overlapping regions in interconnected CSSL,BC and TC populations.Integration of BC and TC populations provides an efficient strategy for heterosis exploitation. At least 10 HLs affected heterosis for grain yield.Five of them with promising NIP alleles were confirmed as being used to increase yield heterosis by more than 15% in several commercial rice hybrids that are widely grown in China.These findings concerning the identified HLs will not only be helpful for identifying causal genes and for characterization of the molecular mechanisms underlying yield heterosis but also provide an optional strategy for the replacement of heterotic alleles in elite parental lines to improve the yield potential of hybrid rice.
CRediT authorship contribution statement
Chaopu Zhang:Writing - original draft, Data curation, Investigation, Formal analysis.Yongjian Sun:Investigation, Resources.Dianwen Wang:Investigation, Resources.Wenqiang Sun:Data curation.Yuye Yu:Data curation.Zhongli Hu:Data curation.Sibin Yu:Project administration, Data curation, Writing - review &editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (2662018YJ025), National Natural Science Foundation of China (31971864), and National High Technology Research and Development Program of China(2014AA10A604),and the Major Project of Science and Technology of Hubei (2019ABA104, 2020ABA016).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.07.002.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet
- RNAi-mediated suppression of the abscisic acid catabolism gene OsABA8ox1 increases abscisic acid content and tolerance to saline–alkaline stress in rice (Oryza sativa L.)

