RNAi-mediated suppression of the abscisic acid catabolism gene OsABA8ox1 increases abscisic acid content and tolerance to saline–alkaline stress in rice (Oryza sativa L.)
Xiaolong Liu,Xianzhi Xi,Chongk Zhng,Lixing Wi,Xiaowi Li,Yangyang Jin,Guohui Zhang,Chang-Ji Jiang, Zhngwi Lian
a Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun 130102, Jilin, China
b Shandong Rice Research Institute, Shandong Academy of Agricultural Sciences, Jinan 250100, Shandong, China
c College of Life Science and Resources and Environment, Yichun University, Yichun 336000, Jiangxi, China
d Da’an Sodic Land Experiment Station, Da’an 131317, Jilin, China
e Dongying Academy of Agricultural Sciences, Dongying 257091, Shandong, China
f College of Life Sciences, Engineering Research Center of the Chinese Ministry of Education for Bioreactor and Pharmaceutical Development, Jilin Agricultural University,Changchun 130118, Jilin, China
g Institute of Agrobiological Sciences, NARO, Kannondai 2-1-2, Tsukuba 305-8602, Japan
Keywords:Rice (Oryza sativa L.)Saline–alkaline stress Abscisic acid (ABA)OsABA8ox1-kd Endogenous ABA levels
ABSTRACT Saline–alkaline(SA)stress is characterized by high salinity and high alkalinity(high pH),which severely inhibit plant growth and cause huge losses in crop yields worldwide.Here we show that a moderate elevation of endogenous abscisic acid(ABA)levels by RNAi-mediated suppression of OsABA8ox1(OsABA8ox1-kd), a key ABA catabolic gene, significantly increased tolerance to SA stress in rice plants. We produced OsABA8ox1-kd lines in two different japonica cultivars,Dongdao 4 and Nipponbare.Compared with nontransgenic control plants (WT), the OsABA8ox1-kd seedlings accumulated 25.9%–55.7% higher levels of endogenous ABA and exhibited reduced plasmalemma injury, ROS accumulation and Na+/K+ ratio, and higher survival rates, under hydroponic alkaline conditions simulated by 10, 15, and 20 mmol L-1 of Na2CO3. In pot trials using SA field soils of different alkali levels (pH 7.59, 8.86, and 9.29), OsABA8ox1–kd plants showed markedly higher seedling survival rates and more vigorous plant growth, resulting in significantly higher yield components including panicle number(85.7%–128.6%),spikelets per panicle(36.9%–61.9%), branches (153.9%–236.7%), 1000–kernel weight (20.0%–28.6%), and percentage of filled spikelets(96.6%–1340.8%)at harvest time.Under severe SA soil conditions(pH=9.29,EC=834.4 μS cm-1),OsABA8ox1-kd lines showed an 194.5%–1090.8% increase in grain yield per plant relative to WT plants.These results suggest that suppression of OsABA8ox1 to increase endogenous ABA levels provides a new molecular approach for improving rice yield in SA paddies.
1. Introduction
Saline–alkaline (SA) stress has become a major limiting factor for world crop production owing to its high salinity and high alkalinity(high pH)[1,2].Plants growing in SA soil suffer not only from osmotic stress and high ion toxicity but also from high-pH stress[3–5]. SA stress exerts complex harmful effects on plants in the form of osmotic pressure,ion toxicity,and high alkalinity.Of these,alkalinity has been identified as the main factor inhibiting rice plant growth,suggesting that increasing alkalinity tolerance is critical for increasing rice growth and productivity in SA soils [6–8].High-pH stress can suppress plant growth by reducing survival rate, resulting in water desorption and cell death [6,9]. Our previous study [10] identified residual sodium carbonate (Na2CO3) in the 0- to 10-cm soil layer in SA soils during the reproductive stag as the most limiting factor in rice yield. Rice plants grown in SA soils suffered from damage induced by high salinity, osmotic stress, and high pH, and rice yield varied in the range 0.7–4.9 t ha-1[2,10]. Thus, SA stress is more damaging to plants than any single stress factor, and increasing rice yield in SA soils,especially severe SA soils, remains a challenge.
SA stress causes severe inhibition of plant growth and a decrease in rice yield in northeast China,where there is a large area of SA soil[11–13].Among the factors underlying SA stress,namely osmotic pressure, ion toxicity and high alkalinity, alkalinity has been identified [5,6,14,15] as the primary factor inhibiting rice growth and yield formation. Alkaline stress causes severe damage to root cells, as shown by an increase in membrane injury and malondialdehyde(MDA)content,as well as the upregulation of cell death-associated genes and downregulation of cell deathsuppressor genes [6,9,14]. Alkaline stress resulted in excessive accumulation of reactive oxygen species (ROS) in rice roots, and exogenous application of the ROS scavenging reagent procyanidins(a potent antioxidant)effectively rescued alkali-induced root damage, suggesting that ROS accumulation is a major damaging factor for rice seedlings exposed to alkaline stress [9].
Abscisic acid(ABA)is a phytohormone that acts as a regulatory messenger enabling plants to cope with various environmental stresses [16,17]. The priming effect is one of the mechanisms of ABA action allowing plants to resist various stress conditions[14,15,18]. Plants pretreated with a range of chemical compounds such as ABA show increased tolerance to subsequent external stresses,in a process referred to as priming[5,19,20].Pretreatment of rice seeds or seedlings with ABA increased seedling survival,plant growth,and grain yield under salt stress and saline soil conditions [21–23]. We previously reported [14] that pretreatment of rice seedlings by ABA priming increased tolerance to alkaline stress,as shown by increased seedling survival rate,relative water content,and root growth under alkaline stress conditions.A threeyear field trial showed that rice seedlings pretreated with ABA for 24 h before transplantation to SA paddy fields showed significantly reduced alkali-induced seedling death and leaf withering and increased grain yield [15]. Mechanistic analysis [5] revealed that ABA pretreatment resulted in activation of the antioxidant defense system and a reduction in ROS accumulation under alkaline conditions, which consequently mitigated alkali-induced root damage and seedling death. ABA pretreatment also superinduced stress tolerance-associated genes in response to alkaline stress conditions. These results suggested that ABA primes rice seedlings for increased tolerance to alkaline stress by upregulating downstream antioxidant defense systems and stress tolerance-associated genes[5]. These findings imply that ABA priming provides a new and effective approach for increasing tolerance to alkaline stress and rice production in SA paddy fields.
Studies [5,15,23] including our own have suggested the potential of ABA priming for agricultural use to improve crop yield in stress environments. However, ABA application under field conditions has long been hindered by its high cost and light sensitivity[15,24,25]. Molecular biological modification of ABA metabolism to strengthen the ABA signaling pathway could be a valuable approach to increasing rice yield in SA paddy fields [26,27].
Because ABA levels in plants are controlled by biosynthesis,metabolism, transport, and other processes, strengthening ABA biosynthesis or suppressing the ABA metabolism pathway will be effective for modulating ABA levels in plants to activate stress responses [28]. NCED is the key enzyme in ABA biosynthesis[16,29,30], and ectopic expression of rice OsNCED4 in Arabidopsis increased ABA levels and drought tolerance [31]. Knockout of OsNCED5 by CRISPR/Cas9 significantly decreased salt tolerance in rice [32]. ABA catabolism was activated when stress signals were diminished [24]. The hydroxylation of three methyl groups (C-7′,C-8′, and C-9′) is the main regulatory pathway of ABA catabolism.Among these, hydroxylation of C-8′is known as the key catalytic pathway[33,34],and OsABA8ox is the dominant regulatory enzyme of ABA catabolism [35,36]. In rice, ABA 8′-hydroxylase is encoded by three homologous genes, OsABA8ox1, OsABA8ox2, and OsABA8ox3, and RNA interference applied to OsABA8ox3 significantly increased ABA levels, antioxidative capability, and stressassociated gene expression, resulting in drought tolerance [26].However, to date, no such study has been conducted with a focus on alkaline tolerance in rice.
In this study, we employed RNAi-mediated suppression of the OsABA8ox1 to investigate the effect of endogenous ABA levels on the SA tolerance of rice.
2. Materials and methods
2.1. Cultivars
Two japonica rice cultivars were used: ‘Dongdao 4’ (D4) and‘Nipponbare’ (NB). The D4, an elite cultivar in the SA land area in northeast China, was bred by crossing ‘Akitakomachi’ with‘Nongda-10’ at Da’an Sodic Land Experiment Station, Jilin, China[37]; and NB, a well characterized Japanese standard cultivar,was bred by crossing‘Shanyan’with‘Xingfeng’in Japan(China Rice Data Center, http://www.ricedata.cn/).
2.2. DNA construction and plant transformation
To construct a plasmid for OsABA8ox1 RNAi (OsABA8ox1-kd), a part of the 3′-UTR (nucleotides 1694–1867) of OsABA8ox1 cDNA(Os02g0703600, AK067007) was amplified by PCR using genomic DNA as template and cloned into the pANDA vector [38,39]. Rice(cvs.D4 and NB)was transformed by an Agrobacterium tumefaciens(strain EHA105)-mediated technique, as described by Toki et al.[40].
Several primary tranformants (T0 plants) from each cultivar were selfed to produce T1-generation seeds. Among the T1 progenies, hygromycin B-resistant plants (30 μg mL-1hygromycin B,0.8% agar) with segregation ratio of 3:1 (Hyg-R vs. Hyg-S, which potentially resulted from a single transgene insertion) were selected and selfed to yield T2 seeds homozygous for the transgene. Four independent homozygous lines of T2 OsABA8ox1-kd from each cultivar were obtained and tested for OsABA8ox1 expression (Fig. S1). Two OsABA8ox1-kd lines from each cultivar (D4#2 and D4#4 from D4, NB#4 and NB#7 from NB) showing apparent reduction in OsABA8ox1 expression(Fig.S1)were selected and used for all further experiments.
2.3. Plant growth conditions
Seeds were surface-sterilized with 75% (v/v) alcohol for 5 min and rinsed with deionized water five times. After immersion in water for 2 days, the seeds were sprinkled onto wet filter paper in a Petri dish and germinated for 24 h at 28°C in a dark incubator.Eighteen uniformly germinated seeds were transplanted onto a multi-well plate floating in a 320 mL-1cup containing deionized water, grown for 7 days, and then grown for 7 days in halfstrength Kimura B nutrient solution [41] in a controlled growth chamber under the following conditions: 25 °C day/20 °C night,12-h photoperiod, and 350 μmol photons m-2s-1light intensity.
In a pot experiment, germinated rice seeds were sown in a pot with normal soil (non-SA). Approximately 30-day-old seedlings were transplanted to pots containing 3 kg of soil with three SA levels: moderate (pH 7.59), medium (pH 8.86), and severe (pH 9.29). Ten plants were transplanted to each pot. During seedling establishment, plants were thinned to six uniform seedlings per pot. All pots were placed in a controlled growth chamber under the following conditions:25°C day/20°C night,12-h photoperiod,and 350 μmol photons m-2s-1light intensity,with the pots moved intermittently to ensure uniform light intensity.
2.4. Alkaline-stress treatment
In a hydroponic experiment, Na2CO3at 10 mmol L-1(pH = 10.64, EC = 2.35 mS cm-1), 15 mmol L-1(pH = 10.87, EC =2.72 mS cm-1), and 20 mmol L-1(pH = 11.17,EC = 3.43 mS cm-1)were used to simulate alkaline stress[5,9,14].The EC and pH of the solutions were measured with a DDS-12 conductivity meter (Lida Inc.,Shanghai,China)and a PHS-25 pH meter(Baiyuan Inc.,Beijing,China), respectively. The solutions were changed every 2 days to maintain the stress conditions throughout the experiment. The seedlings were sampled for various measurements after 3, 5, and 7 days of alkaline stress.
2.5. Measurement of survival rate, seedling growth and sodium potassium
The survival rate of rice seedlings was determined after 3,5,and 7 days of Na2CO3treatment.Individual seedlings were classified as dead if all the leaves were dry and brown [5,9,14].
Ten rice seedlings were randomly selected in each treatment group to measure seedling growth status during alkaline stress.These seedlings were scanned with an Epson Expression 10000XL(Epson America Inc., Long Beach, CA, USA) at 0, 3, 5, and 7 days of alkaline stress. The resulting images were digitized with WinRHIZO (Regent Instruments Canada Inc., Ville de Québec, QC,Canada), and mean shoot length (SL), total root length (TRL), total root surface area(RSA),root diameter(RD),total root volume(RV),and root number (RN) were recorded. The fold change in each growth index was calculated to evaluate the influence of alkaline stress on rice seedlings.
These 10 rice seedlings after growth measurement were divided into shoots and roots, and their fresh weights were measured.These samples were dried in a forced-air oven at 105°C for 2 h,followed by drying at 70 °C to constant mass. The roots were rinsed four times with deionized water and then dried.
After dry mass was recorded,the samples were cut with scissors into 5–10 mm pieces and digested completely with an HNO3and HClO4(v:v 2:1) mixture, which was diluted to 50 mL. The Na+and K+concentrations in the shoots and roots were determined by flame emission spectrometry (FP6410, Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China).
2.6. Measurement of withered-leaf proportion, plant growth, root growth, grain yield and yield components in the pot experiment
Seedling survival rate was determined at the seedling establishment and mature stages.Withered-leaf proportions were recorded at the heading and mature stages. Rice roots were rinsed and indexes of growth were measured at the mature stage. A leaf was classed as withered if the whole leaf was dry and brown.The withered-leaf proportion of a seedling was recorded as the proportion of withered leaves.Plant shoot length,chlorophyll content, and biomass were recorded throughout growth.
At the mature stage, all surviving plants were harvested to determine the following parameters: plant height, panicle weight,stem weight, main root length, root number, yield components including numbers and weight of filled and empty spikelets, panicle number per hill (PN), spikelets per panicle (SP), 1000-kernel weight (TKW), percentage of filled spikelets (PFS), and harvest index(HI).All panicles and spikelets from an individual plant were measured and averaged. Measurement of grain yield and yield components followed Yoshida [42]. The TGW and aboveground biomass were adjusted to 0.14 g g-1moisture content on a dry weight basis. HI was calculated as grain yield divided by aboveground biomass.
2.7. Measurement of chlorophyll content
Chlorophyll content was measured as described by Wellburn and Lichtenthaler [43], with some modifications. Leaf samples(0.1 g) were extracted using a 5-mL mixture of ethanol (2.5 mL)and acetone (2.5 mL). The absorbance of the supernatant was determined at 645 and 663 nm with a spectrophotometer (UV-2700, Shimadzu, Kyoto, Japan). Total chlorophyll content was calculated as (20.29 × A645+ 8.05 × A663) V/(1000 × W).
2.8. Measurement of membrane injury (MI) and MDA content
Membrane injury (MI) was measured by electrolyte leakage[44]. Rice seedlings were randomly selected from each treatment group, washed with deionized water to remove surface-adhering electrolytes, and divided into shoots and roots. Samples (2 g fresh weight) of the roots were submerged in 15 mL of deionized water in 50 mL conical tubes and held at 20°C for 1 h.The electrical conduction of the effusion was then measured (R1) with the conductivity meter. The tissue samples were killed by heating tubes in a boiling bath for 40 min and cooled to 20°C,and the electrical conduction of the effusion was measured again(R2).MI was evaluated as MI (%) = R1/R2 × 100%.
The MDA content was determined by the thiobarbituric acid reaction following Heath and Packer [45]. A fresh root sample(0.1 g)was homogenized in 1 mL of 50 mmol L-1phosphate buffer(pH 7.8) using a bench-top ball mill (Scientz-48, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, Zhejiang, China) at 50 Hz for 30 s, and centrifuged at 12,000×g for 15 min. Then, 400 μL of supernatant was mixed with 1 mL of 0.5% thiobarbituric acid and the mixture was placed in a boiling water bath for 20 min. The mixture was then cooled and centrifuged, and the absorbance of the supernatant was measured at 532, 600, and 450 nm using a spectrophotometer. MDA content was calculated as 6.45 × (A532-- A600) - 0.56 × A450.
2.9. Measurement of ROS levels
The O2-contents were measured as described by Elstner and Heupel [46] by monitoring nitrite formation from hydroxylamine in the presence of O2-, with some modifications as described by Jiang and Zhang[47].Absorbance values at 530 nm were calibrated to calculate the contents of O2-from the chemical reaction of O2-and hydroxylamine.
H2O2contents were measured as described by monitoring the A415of the titanium-peroxide complex [48]. Absorbance values were calibrated to a standard curve generated with known concentrations of H2O2.The analytical reagents used to measure the H2O2and O2-contents were included in the determination kit (Comin Biotechnology Co., Ltd., Suzhou, Jiangsu, China) [5,9].
2.10. Measurement of ABA content
Abscisic acid (ABA) was determined immunologically using enzyme-linked immunosorbent assay (ELISA) with monoclonal ABA antibodies as described by Weiler [49]. The validity of this immunoassay of ABA content has been verified in maize tissues and a range of other plant species and soil solutions [50–52]. The plant ABA ELISA kit was used according to the manufacturers instructions (Shanghai Lengton Bioscience Co., Ltd., Shanghai,China).
2.11. RNA isolation and quantitative real-time PCR (qRT-PCR)
Rice leaves or roots were sampled in liquid nitrogen and ground using a benchtop ball mill at 50 Hz for 30 s. Total RNA was extracted with TRIzol reagent(TaKaRa Bio,Tokyo,Japan)and firststrand cDNA was synthesized using M-MLV reverse transcriptase(Thermo, Carlsbad, CA, USA) according to the manufacturer’s protocols. Quantitative real-time PCR (qRT-PCR) was performed to determine the transcriptional expression of genes, including two ABA-response genes, SalT and OsWsi18, two stress toleranceassociated genes,OsPEX11 and OsJRL,and 20 ROS-scavenging genes(R1-R20) [5,53]. Gene-specific primers were designed with Primer 5.0 software (Primer-E Ltd., Plymouth, UK) (Tables S1 and S2).
The housekeeping gene β-actin (GenBank ID: X15865.1) was used as an internal standard. PCR was conducted in a 20 μL reaction mixture containing 1.6 μL of cDNA template (50 ng), 0.4 μL of 10 mmol L-1specific forward primer, 0.4 μL of 10 mmol L-1specific reverse primer, 10 μL of 2× SYBR Premix Ex Taq (TaKaRa),and 7.6 μL of double-distilled H2O in a PCRmax machine(Illumina,ECORT48,UK).The procedure was performed as follows:1 cycle for 30 s at 95°C,40 cycles for 5 s at 95°C,and 20 s at 60°C,and 1 cycle for 60 s at 95 °C, 30 s at 55 °C, and 30 s at 95 °C for melting curve analysis.Relative expression was calculated by the 2-△△CTmethod[54].
2.12. Experimental design and statistical analyses
All of the experiments were conducted in a controlled growth chamber with three biological replicates. Statistical analyses were performed using the statistical software SPSS 21.0 (IBM Corp.,Armonk, NY, USA). Based on one-way analysis of variance(ANOVA), Duncan’s multiple range test (DMRT) was used to identify differences in means among treatments.
3. Results
3.1. OsABA8ox1-kd increased endogenous ABA levels
Two homozygous lines of OsABA8ox1-kd (D4#2 and D4#4 from D4,NB#4 and NB#7 from NB)showed 20%–27%reduction in basal levels of OsABA8ox1 expression under unstressed control(CK)conditions (Fig. S1). The D4#2 showed a steady reduction in OsABA8ox1 expression, although with slightly lower statistical significance (P = 0.058), and increase in ABA levels under alkaline stress conditions(Fig.1C,D).Although NB#2 showed a 36%reduction in OsABA8ox1 expression, it was not used owing to its poor plant growth and seed set (Fig. S1).
Expression analysis of OsABA8ox1 showed that it is upregulated in response to alkaline stress in wild-type (WT) plants, especially at high concentrations of Na2CO3(≥15 mmol L-1) (Fig. 1A, B).Moreover, a significant further reduction in OsABA8ox1 expression was observed in OsABA8ox1-kd plants under alkaline stress conditions compared with that under CK conditions.
The RNA-mediated suppression of OsABA8ox1 (Fig. 1A, B) did not significantly alter the basal low levels of ABA under CK conditions (Fig. 1C, D), and ABA levels were increased in both WT and OsABA8ox1-kd lines in response to alkaline stress. However,OsABA8ox1-kd lines showed significantly higher ABA contents in both D4 (by 29.1%–55.7%) and NB (by 25.9%–47.9%) backgrounds(Fig. 1C, D) compared with WT plants. These results were in good agreement with the expression patterns of OsABA8ox1 (Fig. 1A, B).
Similarly, in pot trials, the ABA content of OsABA8ox1-kd lines was not significantly different from that of WT plants under soil conditions of moderate (pH 7.59) and medium (pH 8.86) alkali levels (Fig. 1E, F). However, it increased to a significantly higher levels than those in WT plants in severe SA soil (pH 9.29) conditions; by respectively 2.8% and 32.7% in D4#2 and D4#4(Fig. 1E), and by 10.5% and 33.6% in NB#4 and NB#7 (Fig. 1F).
3.2. OsABA8ox1-kd mitigated alkali-caused growth inhibition
Suppression of OsABA8ox1 rescued rice seedlings from withering and death,as shown by the higher survival rates in OsABA8ox1-kd lines under alkaline stress conditions(Figs.1G,H,S2).There was no apparent difference in survival rate between OsABA8ox1-kd lines and non-transgenic wild type (WT) plants under < 10 mmol L-1Na2CO3conditions; however,under ≥15 mmol L-1Na2CO3conditions, the OsABA8ox1-kd lines showed significantly(5.1%–80.0%)higher survival rates in Dongdao 4 (Fig. 1G) and 5.4%–61.9% in Nipponbare plants (Fig. 1H).OsABA8ox1-kd mitigated the alkaline suppression of seedling growth, as shown by higher fold changes in growth indexes of the OsABA8ox1-kd lines (Table S3).
3.3. OsABA8ox1-kd mitigated root damage under alkaline stress conditions
Alkaline treatment caused rapid root damage in both OsABA8ox1-kd lines and WT plants, but OsABA8ox1-kd mitigated root damage, as shown by lower alkali-induced root MI (Fig. 2A,B) and MDA overaccumulation (Fig. 2C, D). Root MI and MDA decreased by 1.8%–36.5% and 1.2%–38.1%, respectively, in the OsABA8ox1-kd lines (Fig. 2A–D). Expression levels of OsKOD1, an inducer of programmed cell death (PCD) [55], were significantly upregulated (Fig. 2E, F), and OsBI1, a cell death suppressor that modulates PCD in response to abiotic and biotic stresses [56],was downregulated (Fig. 2G, H), by alkaline stress. This alkaliinduced expression of OsKOD1 was significantly suppressed by 31.4%–63.9% (Fig. 2E, F), while the alkali-induced downregulation of OsBI1 was significantly mitigated by 22.0%–122.8%, in the OsABA8ox1-kd lines compared with the corresponding values in WT plants under 15 and 20 mmol L-1Na2CO3stress conditions(Fig. 2G, H). Under ≥ 15 mmol L-1Na2CO3conditions, the OsABA8ox1-kd lines showed higher fold changes in root growth indexes compared with the WT plants(Table S3),a finding consistent with the degree of root damage (Fig. 2).
3.4. OsABA8ox1-kd reduced alkali-induced accumulation of ROS and upregulated antioxidant and stress tolerance-related genes
The accumulation of ROS was observed in both WT plants and OsABA8ox1-kd lines (Fig. 3). However, compared with WT plants,OsABA8ox1-kd lines of both D4 and NB showed significantly lower accumulation of O2–and H2O2(Fig.3),showing decreases of 16.5%–46.7% and 2.4%–25.8% for O2–(Fig. 3A, B) and 16.6%–25.7% and 7.4%–24.7% for H2O2(Fig. 3C, D) in D4 and NB, respectively, under alkaline conditions.
Consistently, among the 20 ROS-scavenging genes (R1-20)examined, 12 genes, R1, R4, R5, R9, R10, R13, and R15-R20, were significantly upregulated in OsABA8ox1-kd lines of both D4(Fig.S3 A,C,E and G)and NB(Fig.S3 B,D,F and H),under alkaline stress conditions.
Four stress-associated genes, including two ABA-response genes, SalT and OsWsi18, as well as two stress toleranceassociated genes, OsPEX11 and OsJRL, were significantly upregulated by 30.3%–282.7% and 37.2%–328.5% in OsABA8ox1-kd lines of both D4 (Fig. S4 A, C, E and G) and NB (Fig. S4 B, D, F and H),respectively, under alkaline stress conditions. SalT is a plant mannose binding condensate-associated gene and a gene induced in plant response to salt stress induced by ABA,drought,and SA stress[57]. OsWsi18 is referred to as stress-inducible protein 18 in rice and is induced by salt and water stress.The 5′-upstream sequence of OsWsi18 contains an ABA response element [58]. Our previous studies [5,15,59] showed that the two ABA-response genes were induced by alkaline stress and ABA priming. OsPEX11 is a biosynthesis factor of peroxidase, and overexpression of OsPEX11 increased salt tolerance by increasing antioxidant scavenging capacity in rice [60]. OsJRL is a mannose binding lectin gene and acts in cellular defense and signal transduction in rice in response to stress factors [61].

Fig.1. Expression of OsABA8ox1,ABA content,and survival rate of OsABA8ox1-kd lines under alkaline stress conditions.Two-week-old rice seedlings of WT and OsABA8ox1-kd plants(D4:left column;NB:right column)were grown under unstressed control(CK)or three alkaline stress conditions(Na2CO3;10,15,20 mmol L-1)for 3,5,and 7 days.(A,B) Expression levels of OsABA8ox1 at 3 d; shown as fold changes relative to that under CK conditions. (C, D) ABA content in the leaves and (G, H) survival rates of seedlings under alkaline stress conditions were measured on indicated days.(E,F)In a pot experiment,rice seedlings were transplanted into saline–alkaline soils with three different SA levels:moderate(T1,pH 7.59),medium(T2,pH 8.86),and severe(T3,pH 9.29).ABA contents in the leaves were measured on the indicated days.Values are means±SD,n=3.Different letters on columns represent significant (P <0.05) difference between rice lines based on Duncan’s test.
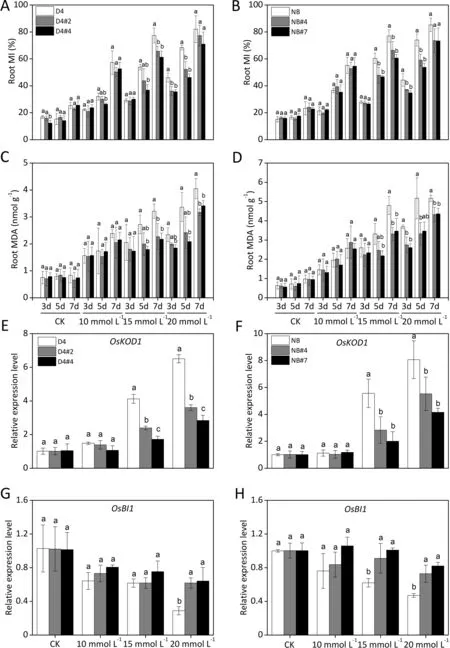
Fig.2. Root damage in OsABA8ox1-kd lines under alkaline stress conditions.Two-week-old rice seedlings of WT and OsABA8ox1-kd plants(D4:left column;NB:right column)were grown under unstressed control (CK) or three alkaline stress conditions (Na2CO3; 10, 15, 20 mmol L-1) for 3, 5, and 7 days. (A, B) Membrane injury and (C, D)malondialdehyde(MDA)content of roots were measured on the indicated treatment days.(E–H)Expression levels of the cell death-associated genes,OsKOD1(E,F)and OsBI1(G, H) in roots were measured at 3 d; shown as fold changes relative to those under CK conditions. Values are means ± SD, n = 3. Different letters on columns represent significant (P <0.05) difference between rice lines based on Duncan’s test.

Fig. 3. ROS accumulation in OsABA8ox1-kd lines under alkaline stress conditions. Two-week-old rice seedlings of WT and OsABA8ox1-kd plants (D4: left column; NB: right column)were grown under unstressed control(CK)or three alkaline stress conditions(Na2CO3;10,15,20 mmol L-1)conditions for 3,5,and 7 days.(A,B)The accumulation of O2– and (C, D) H2O2 in the roots was measured on the indicated treatment days. Values are means ± SD, n = 3. Different letters on columns represent significant (P <0.05)difference between rice lines based on Duncan’s test.
3.5. OsABA8ox1-kd increased plant growth under severe SA soil conditions
In pot experiments,there was no appreciable difference in plant growth between OsABA8ox1-kd lines and WT under moderate (pH 7.59) and medium (pH 8.86) SA soil conditions (Figs. 4 and 5).However, under severe SA soil conditions (pH 9.29, EC = 834.4 μS cm-1), OsABA8ox1-kd lines showed significantly greater growth than WT plants (Fig. 4A, B). The survival rate of OsABA8ox1-kd plants was 4.2%–38.5% (D4) and 4.2%–63.6% (NB) higher (Fig. 4C,D), and the withered-leaf proportions were 16.2%–47.8% (D4) and 23.6%–48.9%(NB)lower(Fig.4E,F),than those values in WT plants.
At the end of the plant growth period, OsABA8ox1-kd lines showed greater growth than WT plants, in the form of a 20%increase in shoot length (Fig. 5A, B), 20%–121% increase in chlorophyll content(Fig.5C,D),1–3-fold biomass accumulation(Fig.5E–H), 21%–85% increase in root length (Fig. 6C, D) and 85%–133%increase in root numbers (Fig. 6E, F), under severe SA soil stress conditions.
3.6. OsABA8ox1-kd increased grain yield under severe SA soil conditions
As shown in Fig.7,no difference in yield was observed between OsABA8ox1-kd lines and WT plants under moderate (pH 7.59) and medium(pH 8.86)SA soil conditions,a finding consistent with the plant growth rate. However, under severe SA soil conditions (pH 9.29, EC = 834.4 μS cm-1), OsABA8ox1-kd lines showed 1.9–10.9-fold more grain per plant than WT plants (Fig. 7H).
Under severe SA soil conditions,in OsABA8ox1-kd lines showed SP (Fig. 7A), SBP (Fig. 7B), FSP (Fig. 7E), TGW (Fig. 7F) and HI(Fig. 7G), increased by 27.8%–104.5%, 54.0%–236.7%, 96.6%–1340.8%, 20.0%–28.6%, and 16.5%–65.0% respectively, compared with WT control plants (Fig. 7). No significant difference in these indexes was observed between WT and OsABA8ox1-kd plants under moderate and medium SA soil conditions (Fig. 7).
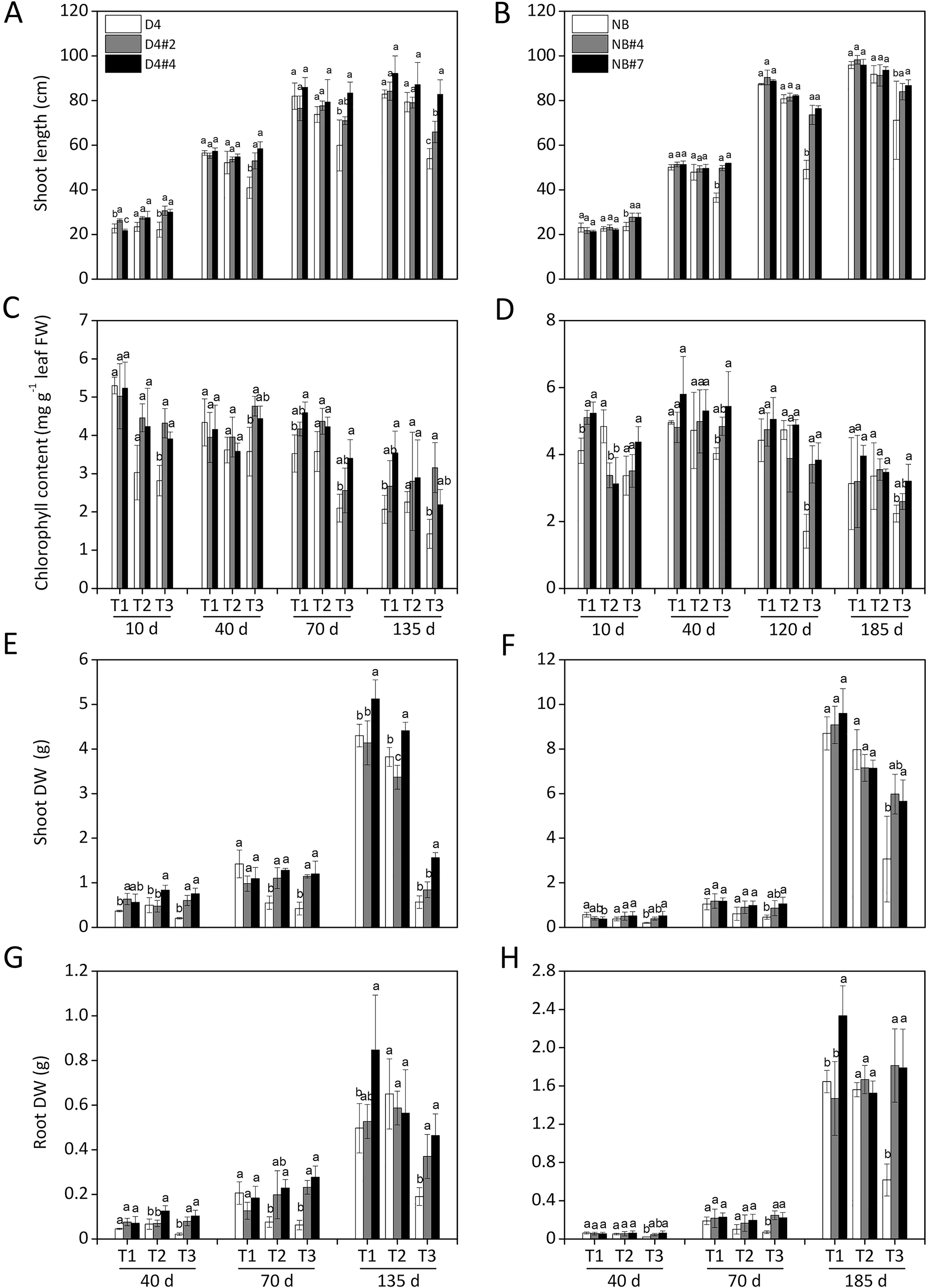
Fig. 5. Plant growth of OsABA8ox1-kd lines in saline-alkaline soils throughout growth. Rice seedlings of WT and OsABA8ox1-kd plants (D4: left column; NB: right column)were transplanted to saline–alkaline (SA) soils with three SA levels: moderate (T1, pH 7.59), medium (T2, pH 8.86), and severe (T3, pH 9.29). (A, B) Shoot length, (C, D)chlorophyll content, (E, F) shoot dry weight, and (G, H) root dry weight were measured throughout growth. Values are means ± SD, n = 3. Different letters on columns represent significant (P <0.05) difference between rice lines based on Duncan’s test.

Fig. 6. Root growth of OsABA8ox1-kd lines in saline-alkaline soils at mature stage. Rice seedlings of WT and OsABA8ox1-kd plants (D4: A, C and E; NB: B, D and F) were transplanted to saline-alkaline(SA)soils with three SA levels:moderate(T1,pH 7.59),medium(T2,pH 8.86),and severe(T3,pH 9.29).(A,B)Image of root growth,(C,D)root length,and(E,F)root numbers were measured at mature stage.Values are means±SD,n=3.Different letters on columns represent significant(P <0.05)difference between rice lines based on Duncan’s test.
4. Discussion
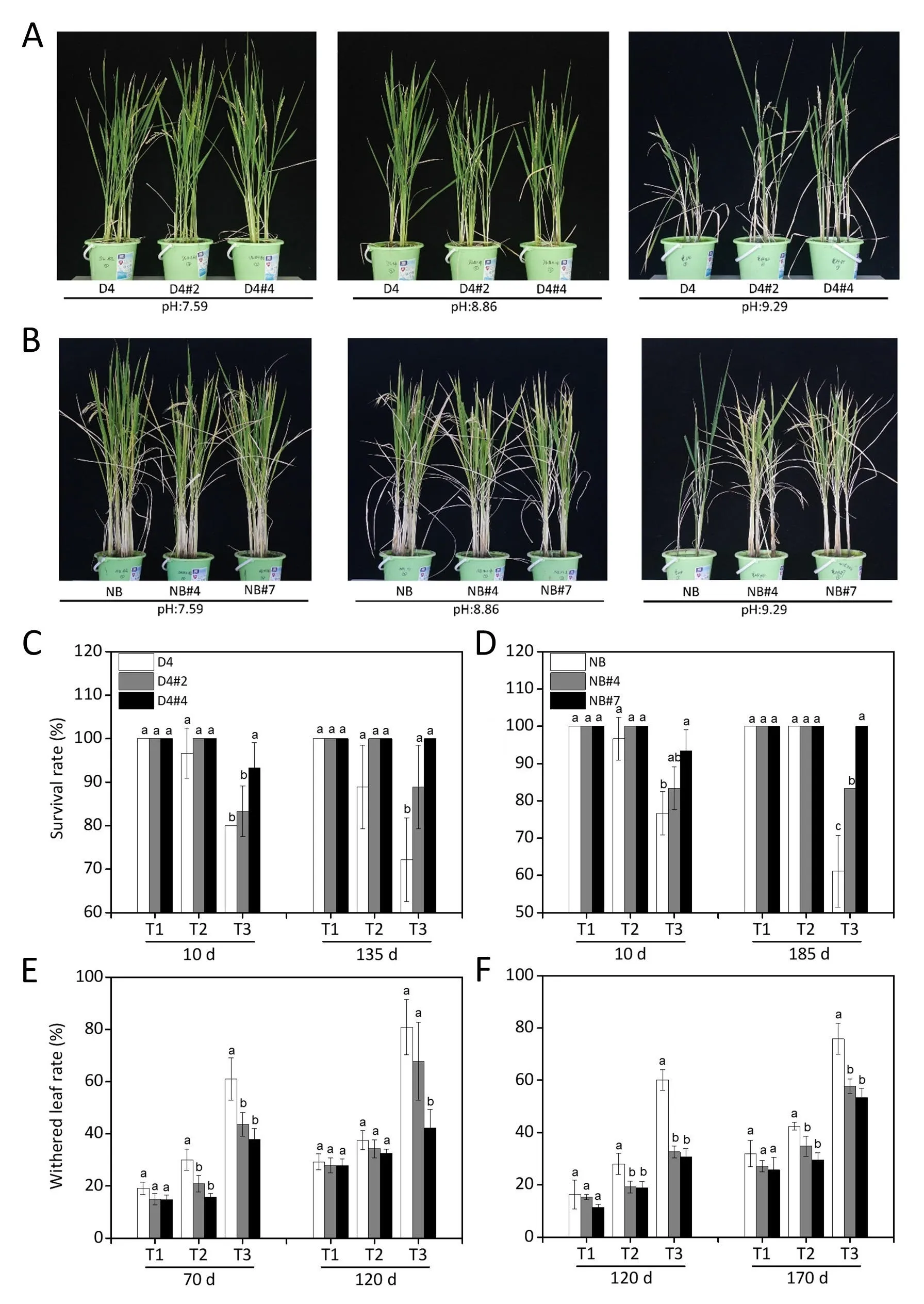
Fig.4. Survival rate and withered-leaf proportion in OsABA8ox1-kd lines in saline-alkaline soils.Rice seedlings of WT and OsABA8ox1-kd plants(D4:A,C and E;NB:B,D and F)were transplanted to saline–alkaline(SA)soils with three SA levels:moderate(T1,pH 7.59),medium(T2,pH 8.86)and severe(T3,pH 9.29)conditions.(A,B)Image of plant growth taken at heading stage. (C, D) Survival rates measured at the seedling establishment and mature stages. (E, F) Withered-leaf proportions measured at heading and mature stage. Values are means ± SD, n = 3. Different letters on columns represent significant (P <0.05) difference between rice lines based on Duncan’s test.
It has been shown that ABA-priming increases alkaline tolerance of rice seedlings in both laboratory experiments and field trials [5,14,15]. However, it may currently be unrealistic to promote the use of exogenous ABA in field scale,owing to its expensive cost and unstable nature under ambient conditions[25].In this context,molecular manipulation of ABA metabolism to strengthen the signaling pathway could be an alternative and effective approach to improve plant growth and grain yield under SA stress conditions.In this study, we found that the expression of OsABA8ox1 was upregulated in response to alkaline stress in wild-type(WT)plants(Fig. 1A, B), implying a negative feedback regulation of OsABA8ox1 expression in response to increasing ABA levels (Fig. 1C–F). RNAimediated suppression of OsABA8ox1 (OsABA8ox1-kd) effectively suppressed the induction of OsABA8ox1 and significantly increased ABA content (Fig. 1). Compared with WT plants, OsABA8ox1-kd lines showed low ROS overaccumulation (Figs. 3 and S3), Na+/K+ratio (Fig. S5) and alkali-induced plasmalemma injury (Fig. 2)under alkaline stress conditions. These results indicate that OsABA8ox participates in adaptation to alkaline stress in rice by regulating endogenous ABA levels. OsABA8ox1-kd lines also showed increased growth(Figs.4–6)and yield formation(Figs.7,S6)under severe SA soil conditions,providing a new pathway for the molecular design and breeding of SA-tolerant rice cultivars. We also observed a further reduction in OsABA8ox1 expression in OsABA8ox1-kd plants under alkaline stress conditions. The explanation for this observation is unknown, but one possibility is that the transcriptional activity of the maize Ubiquitin promoter that drives OsABA8ox1-RNAi is increased in response to alkaline stress.A similar observation was previously reported [62] when GUS expression driven by the maize ubiquitin promoter increased in response to thermal stress in transgenic rice plants.
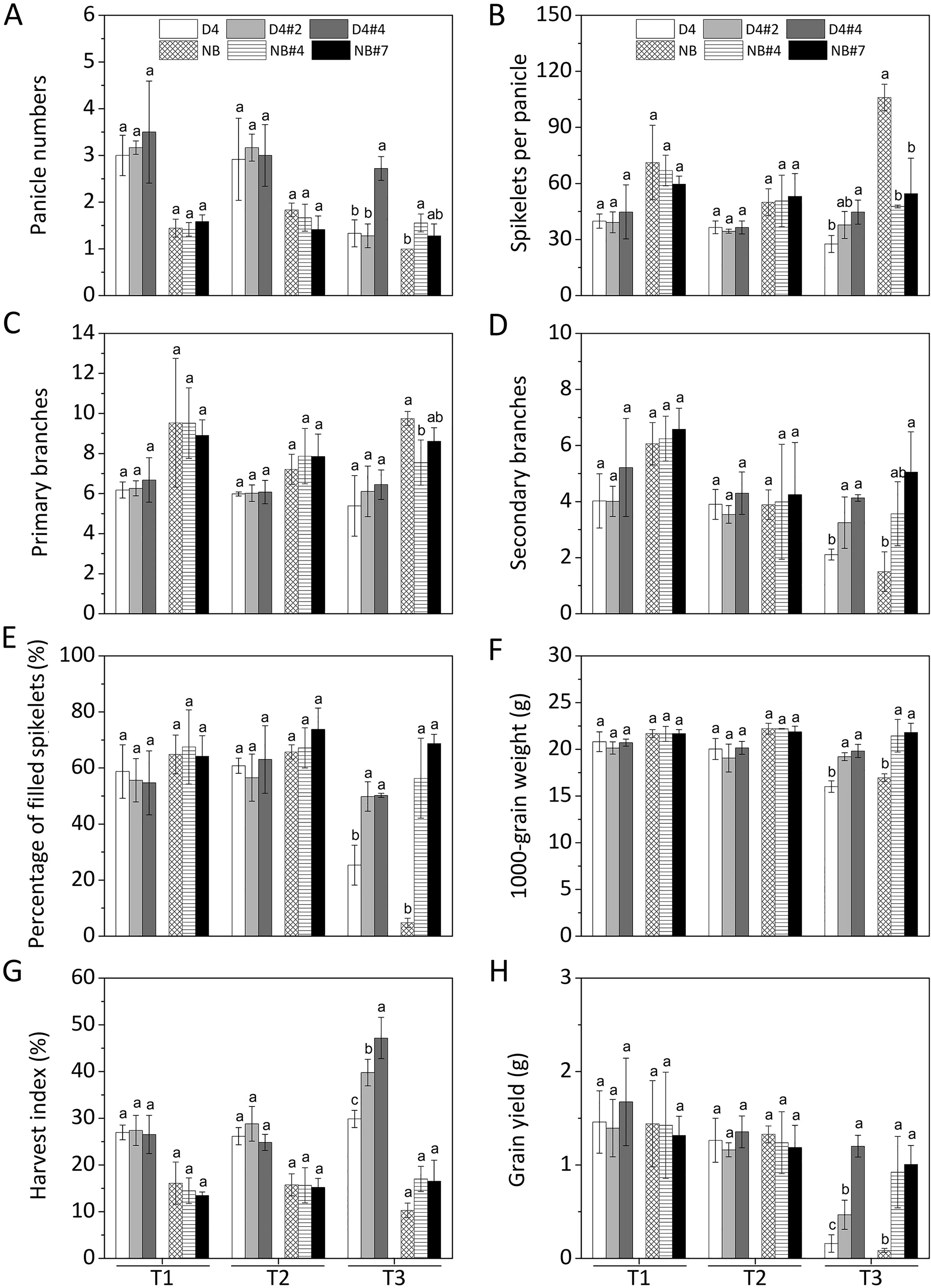
Fig. 7. Yield component indexes of OsABA8ox1-kd lines in saline–alkaline soils at the mature stage. Rice seedlings of WT and OsABA8ox1-kd plants were transplanted to saline–alkaline(SA)soils with three SA levels:moderate(T1,pH 7.59),medium(T2,pH 8.86),and severe(T3,pH 9.29).(A)Panicle number(PN),(B)spikelets per panicle(SP),(C)primary branches per panicle(PBP),(D)secondary branches per panicle(SBP),(E)percentage of filled spikelets(PFS,%),(F)1000-kernel weight(TKW,g),(G)harvest index(HI, %), and (H) grain yield per plant (GY, g) were measured at the mature stage. Values are means ± SD, n = 3. Different letters on columns represent significant (P <0.05)difference between different rice based on Duncan’s test.
Overaccumulation of ROS under various stress conditions is a primary damaging factor in crops, causing damage to the cellular membrane, DNA, RNA, and proteins [63]. In the present study,silencing of OsABA8ox1 increased ROS-scavenging capacity(Fig. S3), thereby reducing the accumulation of ROS (Fig. 3), under alkaline stress and in turn mitigated alkali-induced rice root damage,as shown by lower root membrane injury(Fig.2A–D)and cell death(Fig.2E–H)in OsABA8ox1-kd lines.Thus,suppression of OsABA8ox1 achieved the same benefit as the priming effect of exogenous ABA, namely increasing ABA levels in rice plants (Fig. 1C,D). Our previous studies [5,14] showed that exogenous ABA mitigated alkali-induced root damage and increased the root growth of rice seedlings. Suppression of OsABA8ox1 increased root growth in both hydroponic and severely SA soils, as shown by the greater fold change in root growth indexes (Table S3) and greater root length and root numbers in the OsABA8ox1-kd lines (Fig. 6). These results indicate that increase in endogenous ABA levels could mitigate SA-induced inhibition of the root system by inhibiting ROS overaccumulation in roots.
Upregulated expression levels of stress tolerance-associated genes are another mechanism underlying the ABA priming effect for increasing rice alkaline tolerance [5]. We investigated several stress tolerance-associated genes. SalT and OsWsi18 were superinduced in OsABA8ox1-kd lines compared with WT lines under alkaline stress conditions (Fig. S4A–D), indicating that the ABA signaling pathway is super-activated in response to alkaline stress in OsABA8ox1-kd lines. The stress tolerance-associated genes OsPEX11 and OsJRL showed an expression pattern similar to that of SalT and OsWsi18 (Fig. S4E–H), indicating that the functions of cell defense and root uptake were better in the OsABA8ox1-kd lines.Taken together, these results suggest that silencing of OsABA8ox1 contributes to increasing endogenous ABA levels (Fig. 1C, D) and then strengthens the ABA signal pathway in rice plants to resist alkaline stress.
Rice grown in severe SA paddy soils suffers from severe inhibition of growth and reduced yield,which reaches only 12.9%–90.7%of the average rice yield worldwide[64,65].It is desirable to identify an effective method for increasing rice yield in severe SA soils.We previously [15] reported that exogenous ABA primes rice for increased seedling survival,plant growth,and grain yield in severe SA paddy fields and that the grain yield increased by a maximum of 55%. In the present study, silencing of OsABA8ox1 increased seedling survival and reduced leaf withering (Fig. 4) in severely SAtreated soils compared to WT plants, contributing to the increase in grain yield[15]. OsABA8ox1-kd rice plants showed greater plant growth as shown by higher shoot length (Fig. 5A, B), chlorophyll content (Fig. 5C, D), biomass accumulation (Fig. 5E–H) and root growth (Fig. 6), than WT plants under severe SA soil conditions.OsABA8ox1-kd increased yield (Fig. 7), as shown by higher panicle weight and length(Fig.S6),more branches per panicle(Fig.7A–D),more filled spikelets(Table S4,and other yield components,including SP, PFS, HI, and TKW (Fig. 7E–G), directly contributing to rice yield formation. The WT plants rarely achieved high grain yield at only 0.1–0.2 g per plant (Fig. 7H), whereas the OsABA8ox1-kd plants showed higher yield component indexes, maintaining yield formation normally in severely SA soils.
To investigate the mechanism of OsABA8ox1-kd in increasing alkaline stress tolerance of rice seedlings and grain yield in SA soils,we measured the endogenous ABA contents in rice plants. The increase in ABA content was not statistically significant in the OsABA8ox1-kd lines and WT plants under low-concentration or short-term Na2CO3treatments (≤15 mmol L-1for 3 days) and moderate (pH 7.59) or medium (pH 8.86) SA soil conditions(Fig. 1C–F). However, more ABA accumulated in the OsABA8ox1-kd rice plants with increased stress intensity or time of alkaline stress and under severe SA soil conditions(Fig.1C–F).These results indicated that severe SA stress stimulated catabolism of endogenous ABA in rice, whereas silencing of OsABA8ox1 inhibited overcatabolism of ABA,implying that the ABA signal was strengthened by higher endogenous ABA levels in the OsABA8ox1-kd rice plants for an augmented response to SA stress. These findings suggest that suppression of OsABA8ox1 to increase endogenous ABA levels increases alkaline tolerance of rice and provides a new and practical approach for increasing rice yield in SA paddy fields.
The phytohormone ABA, as a common chemical compound of priming, functions in the response to stress factors via seed priming or root drenching[15,22].However,the high cost and unstable and light-sensitive characteristics of ABA make it difficult for ABA to exert efficient effects on plants under field conditions[15].Many ABA agonists have been identified for potential application in agriculture, including pyrabactin [66,67], ABA mimic-1 (AM1)/quinabactin [68,69], and ABA analogs that display ABA-like activity[70,71]. The priming effect could be activated by endogenous ABA in plants to stimulate a potential tolerance effect in response to various stress factors [72], and catabolism of endogenous ABA acts in the regulation of plant growth and improvement of stress tolerance [73,74]. Genetic and molecular modification of the ABA catabolism pathway would be a valuable approach to strengthening ABA levels in plants, as suggested by previous studies of OsABA8ox2 [75] and OsABA8ox3 [26] and the present study. However,these agonists,ABA analogs,small-molecule probes,and transgenic plants were designed according to the structural modification,biosynthesis, catabolism, conjugation, and systemic transport of ABA or ABA receptors in plants, and interactions with other endogenous phytohormones, off-target effects,environmental stability and biosafety will be key restrictive factors in the application of these strategies under field conditions [70]. These approaches,including that described in the present study, indeed improved our understanding of the molecular mechanism underlying the effect of ABA for potential agricultural use:inhibition of ABA catabolism in rice contributed to improving stress tolerance and yield under stress conditions. It is hoped that modification of genes in rice itself by genome editing based on biosafety rules will be feasible for field applications.
In summary, silencing OsABA8ox1 increased endogenous ABA levels in rice and achieved the same priming effect as exogenous ABA, increasing alkaline tolerance and grain yield in severe SA paddy soil.These results provide an effective approach for increasing plant growth and grain yield of rice in SA fields.
CRediT authorship contribution statement
Zhengwei Liang and Chang-Jie Jiang:Conceptualization.Zhengwei Liang:Funding acquisition and project administration.Xiaolong Liu, Xianzhi Xie, Chongke Zheng, Xiaowei Li, Lixing Wei,Yangyang Jin and Guohui Zhang:Investigation,data analysis and curation,and validation.Xiaolong Liu:Writing-original draft.Changjie Jiang:Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Key Research and Development Program of China (SQ2018YFD020224), Chinese Academy of Sciences STS Network Foundation (KFJ-SW-STS-141-01), the Strategic Priority Research Program of Chinese Academy of Sciences (XDA080X0X0X), and the Foundation of Innovation team International Partner Program of Chinese Academy of Sciences(KZZD-EW-TZ-07-08).We thank Xianzhi Xie,Chongke Zheng,Xiaowei Li,and Lixing Wei for their help in designing and producing the transgenic rice lines.
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.06.011.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet

