A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
Jingmin Xu,Chenyng Pn,Hn Lin,Hnfei Ye,Sheng Wng,To Lu,Qinyu Chen,Kiru Yng,Mei Lu, Qin Qin, Deyong Ren,*, Yuchun Ro,*
a College of Chemistry and Life Sciences, Zhejiang Normal University, Jinhua 321004, Zhejiang, China
b State Key Lab for Rice Biology, China National Rice Research Institute, Hangzhou 310006, Zhejiang, China
Keywords:Xanthine dehydrogenase Leaf senescence Abiotic stresses Purine metabolism
ABSTRACT Xanthine dehydrogenase, a member of the molybdenum enzyme family, participates in purine metabolism and catalyzes the generation of ureides from xanthine and hypoxanthine.However,the mechanisms by which xanthine dehydrogenase affects rice growth and development are poorly understood. In the present study, we identified a mutant with early leaf senescence and reduced tillering that we named early senescence and less-tillering 1(esl1).Map-based cloning revealed that ESL1 encodes a xanthine dehydrogenase, and it was expressed in all tissues. Chlorophyll content was reduced and chloroplast maldevelopment was severe in the esl1 mutant. Mutation of ESL1 led to decreases in allantoin, allantoate, and ABA contents.Further analysis revealed that the accumulation of reactive oxygen species in esl1 resulted in decreased photosynthesis and impaired chloroplast development, along with increased sensitivity to abscisic acid and abiotic stresses. Ttranscriptome analysis showed that the ESL1 mutation altered the expression of genes involved in the photosynthesis process and reactive oxygen species metabolism.Our results suggest that ESL1 is involved in purine metabolism and the induction of leaf senescence.These findings reveal novel molecular mechanisms of ESL1 gene-mediated plant growth and leaf senescence.
1. Introduction
The leaves are the main organs for plant photosynthesis, respiration, and transpiration and produce organic nutrients to ensure normal growth. Leaf senescence is the final stage of plant leaf growth and development and is an environment-adaptive mechanism developed by plants during long-term natural evolution [1].Senescence is also a tightly controlled plant-regulated process by which the products of photosynthesis in senescent leaves can be decomposed and transported to the vigorously growing fruits or seeds[2].Senescence processes in plant leaves thus play important roles in ensuring the yield and quality of crops.
Leaf senescence is accompanied by many complex physiological and biochemical reactions and cellular structural changes.The disintegration of chloroplasts leads to a rapid decrease in chlorophyll content, changes in sugar levels, redistribution of nutrients, and destruction of plant cellular structures [2]. The contents of a large number of functional proteins decrease in leaves, reactive oxygen species (ROS) accumulate, and levels of ROS-degrading enzymes decrease, so that ROS cannot be degraded and removed by proteases before damage occurs. ROS can oxidize functional proteins,nucleic acids,and membrane lipids,trigger membrane lipid peroxidation, and increase the content of malondialdehyde, severely damaging plant tissues and cells. ROS are thus a driving force for the accelerated senescence of leaves [3,4]. Leaves provide various nutritional substances to ensure normal plant growth and development.In the crops,senescence is first reflected in the leaves.Cultivators understand that early senescence of leaves can cause shortening of their functional period, lead to a lack of available nutrients, and impair grain development, reducing crop yield and quality [5].
Plant senescence traits are controlled by an elaborate and complex regulatory network[1].During leaf senescence,the expression of some genes is inhibited and that of others promoted.Genes that show changes in their levels of RNA or protein expression are called senescence-associated genes (SAGs). In recent years,researchers have elucidated the molecular mechanisms of rice leaf senescence and cloned many SAGs involved in chloroplast development and degradation [6–10], protease or energy metabolism pathways [11–14], and hormone synthesis and response [15–19].
The initiation and advance of leaf senescence are regulated by a series of internal genetic factors and external environmental stress factors[2].Abscisic acid(ABA)is the main signaling molecule regulating plant growth and development, and it also regulates plant responses to a variety of abiotic and biotic stresses [20]. ABA has been suggested to promote leaf senescence [21] and inhibit the expression of chlorophyll synthesis-associated genes, promote the expression of chlorophyll degradation-associated genes and the expression of leaf senescence-associated genes [16]. When plants are subjected to various environmental stresses (such as high or low temperatures, drought or waterlogging, hypoxia, and pathogenic infection), the expression of ABA synthesis-related genes is induced in leaves,increasing the ABA content in the plants and causing the early occurrence of leaf senescence [2,22]. Genes involved in the ABA pathway in rice leaf senescence are known to be mainly OsDMI3[23],OsNAP[16],OsNAC2[17],and OsMYB102[24].However,the molecular mechanisms of the ABA regulation of rice leaf senescence and ABA signaling remain largely undescribed.
Xanthine dehydrogenase(XDH),a member of the molybdenum enzyme family and a key player in the regulation of purine metabolism, catalyzes the generation of uric acid from xanthine and hypoxanthine and forms ureides in a series of metabolic reactions involving allantoin and allantoate[25].XDH in plants and xanthine oxidase in animals are structurally similar and contain two identical subunits that have homodimeric structures, with a molecular weight of 300 kD. Each subunit contains two distinct iron–sulfur clusters (Fe2-S2), one flavin adenine dinucleotide (FAD), and a sulfurated molybdenum cofactor (Moco) [26]. Shi et al. [23] have shown that XDH is involved in several metabolic processes in addition to purine metabolism, including metabolism of nitrogen,hormones, and ROS. Ureides have a high N/C ratio, are easily transported in plants,can reduce the energy expenditure of carbohydrates,and play an important role in plant survival under stress[27]. They are also effective ROS scavengers, increasing allantoin and allantoate contents, which can compensate for ROS damage[28]. It is likely that XDH is also involved in the regulation of hormone homeostasis.XDH is involved in the oxidative breakdown of the metabolites of cytokinin and may be involved in the indirect synthesis of ABA and indoleacetic acid [29]. XDH is also involved in regulating senescence processes in plants. Arabidopsis plants accumulated large amounts of xanthine in vivo, showed accelerated chlorophyll decomposition, and experienced premature senescence after gene silencing of XDH [30]. No studies of the XDH gene in rice have been reported, and XDH’s mechanism of action during the growth and development of rice is poorly understood.
In the present study, we identified a rice early leaf senescence mutant esl1, which showed leaf senescence at the tillering initiation stage of rice and severely affected growth.Map-based cloning suggested that ESL1 encodes a xanthine dehydrogenase. Loss of function of ESL1 results in reduced production of ureides, which led to the accumulation of ROS. Our results suggest that ESL1 has a prominent role in regulating rice growth and leaf senescence in rice.
2. Materials and methods
2.1. Plant materials and hormone treatment
The rice early-senescence mutant esl1 was identified in an ethyl methane sulfonate mutagenesis library of the japonica rice cultivar Taipei 309.Plant materials were grown under natural conditions in Jinhua (Zhejiang province) and Lingshui (Hainan province), China.For hydroponic conditions, rice seedlings were placed in an artificial climate incubator and cultured in a 14 h light(28°C)/10 h dark(25 °C) cycle.
For dark-induced senescence,the flag leaves of wild-type plants at the beginning of tillering and similar to esl1 plants in growth were sampled under field conditions and cut into fragments about 4 cm in length.They were soaked in dishes of ddH2O in the dark for 2 h, after which the dishes were placed in an incubator and cultured at 25 °C in the dark for 5 days for observation. For ABAinduced senescence, mature seeds of wild-type and esl1 plants were mechanically shelled and disinfected with 70% ethanol solution for 2 min and 30% sodium hypochlorite solution for 30 min.They were then placed on 1/2 MS medium containing 0, 1, and 1.5 μmol L-1ABA and cultured in an artificial climate incubator for 10 days, after which root lengths and plant heights were recorded. Germinated seeds were used to measure the expression of genes involved in ABA signaling, cultured in IRRI nutrient solution for 7 days and then immersed in sterile water containing 50 μmol L-1ABA for 4 h before sampling.
2.2. Drought and salt treatments
After soaking and germinating, seeds were transplanted onto 96-well PCR plates with the bottoms removed and grown hydroponically in IRRI nutrient solution in an artificial climate incubator.Drought and salt treatments were applied by treatment of 3-weekold plants with respectively 20%(w/v)PEG 6000 and 150 mmol L-1sodium chloride (NaCl) for 3 days. For measuring ESL1 expression under drought and salt stresses,3-week-old seedlings were treated with the same solutions for 2 days and six plants were collected for RNA isolation. All stress experiments were repeated three times.
2.3. Transmission electron microscopy
Fresh leaves from the same site at the peak of tillering were taken from wild-type and esl1 plants, and fragments of 1×2 mm were cut from the main vein of the leaves with a scalpel and immersed in 2.5% glutaraldehyde solution for fixation by vacuum extraction for 20–30 min. Subsequent experimental procedures were performed as described previously [31]: refixation,rinsing,dehydration,penetration,embedding,sectioning,and uranyl acetate staining. Finally, the chloroplast morphology and microstructure of the prepared samples were observed under a Hitachi H-7650 transmission electron microscope (Tokyo, Japan).
2.4. Chlorophyll measurement and photosynthetic measurements
The concentrations of Chl a, Chl b and carotenoids (Car) in rice leaves were determined following Wang et al. [32]. Briefly, the concentration of each pigment in the leaves was determined and averaged for five each of wild-type and esl1 plants showing approximately the same growth during the same period. Leaves 2–3 cm in length were removed with scissors, and the midrib was removed and cut into fine fragments.Amounts of 2–3 mg were weighed and placed in a 10 mL centrifuge tube and 5 mL of 95%ethanol solution was added. Three replicates were used. All samples were placed in light-resistant cartons in a refrigerator at 4 °C to extract photosynthetic pigments for 24 h until all leaves turned white, with frequent shaking to prevent pigment degradation, and then centrifuged (3000×g) for 10 min. The absorbance of the supernatant was measured with a UV spectrophotometer at 665, 649, and 470 nm. The concentration of each pigment was calculated following Lichtenthaler[33]. SPAD value was measured with a SPAD Chlorophyll Meter (SPAD-502 Plus, Konica Minolta)[34].
Photosynthetic parameters were measured with a portable photosynthetic tester,LI-6400(LICOR,Lincoln,NE,USA),following the manufacturer’s instructions, and four parameters (net photosynthetic rate, stomatal conductivity, intercellular CO2concentration,and transpiration rate) of the flag leaves of wild-type and esl1 plants were measured at the peak of tillering under sunny conditions. The site selected for determination was generally the flag leaves in the middle or upper parts of the plant, and a total of six replicates were measured.
2.5. Map-based cloning and genetic complementation of ESL1
The map-based cloning population was constructed by crossing esl1 with the indica cultivars Taichung Native 1 (TN1) and Zhefu 802(ZF802).The segregation ratios of the F1generation and F2generation phenotypes were determined for genetic analysis. DNA extracted from a single plant with the mutant phenotype selected from the F2population was used for gene mapping. Twenty-one randomly selected individual plants were used for initial gene mapping, and 268 pairs of simple sequence repeat (SSR) markers evenly distributed on 12 rice chromosomes were used for linkage analysis. Subsequently, a large F2population was used for fine mapping. New polymorphic primers were also designed based on DNA sequence differences between the japonica cultivar NIP and indica cultivar 9311 to further narrow the interval containing ESL1. Sequencing primers were designed based on open reading frame information in the candidate interval to amplify the sequences of the wild type and esl1 for sequencing,and the mutation sites within candidate genes were detected by sequence alignment. Primers are shown in Table S2.
For molecular complementation experiments,the 4107-bp coding sequence of ESL1 without the termination codon from the cDNA library of the wild type was obtained by amplification using the primer ESL1-COMF/R (Table S2) and then inserted into the pCAMBIA1300::35S vector under the control of the 35S promoter,and the constructed vector was introduced into the esl1 mutant via Agrobacterium-mediated transformation.
2.6. RNA extraction and gene expression analysis
Total RNA was extracted with an RNeasy Plant Mini Kit (QIAGEN, Dusseldorf, Germany). cDNA was synthesized using 300 ng of total RNA using a ReverTra Ace qPCR-RT Kit (TOYOBO, Osaka,Japan). Real-time PCR reactions were performed using reagent SYBR Green Realtime PCR Master MIX (TOYOBO) on a qTOWER3G(Jena, Germany) instrument, and OsActin was used as an internal reference gene.Three independent biological replicates and technical replicates were performed. Primer sequences are listed in Table S2.
2.7.Determination of hydrogen peroxide(H2O2)and malondialdehyde(MDA)contents,catalase(CAT)activity,and peroxidase(POD)activity
H2O2content was determined as described by Zafar et al. [35].Fresh leaves (100 mg) were harvested and ground to fine powder in liquid nitrogen.The powder was extracted with 1 mL of 50 mmol L-1sodium phosphate buffer for 20 min on ice.After centrifugation at 12000×g for 10 min at 4 °C, the H2O2in the supernatant was quantified with a hydrogen peroxide assay kit(Beyotime,Nanjing,China).
MDA content and CAT activity were determined as described previously [36]. POD activity was determined as described previously [37].
2.8. Histochemical staining and TUNEL assay
Staining with 3,3′-diaminobenzidine(DAB)and nitroblue tetrazolium(NBT)was performed as described previously[38].The terminal deoxynucleotidyl transferase dUTP nick-end labeling(TUNEL) assay was used to detect apoptosis. When genomic DNA breaks, fluorescein-labeled dUTP can be added to the exposed 3′-OH via catalysis by terminal deoxynucleotidyl transferase, and the intensity of the signal can be observed by fluorescent microscopy. The Fluorescein In Situ Cell Death Detection Kit (Roche,Mannheim, Germany) was used following Huang et al. [39].
2.9. Determination of allantoate, allantoin, and endogenous ABA
Allantoate and allantoin were extracted with 80% ethanol and measured following Sagi et al. [40]. Leaf samples (0.5 g) were collected from the flag leaves of three plants at the tillering stage and after treatment for 2 days under drought and salt stresses.
To determine endogenous ABA concentrations, flag leaves from 1-month-old plants were collected and weighed.ABA was assayed with a UPLC/MS/MS system (AB SCIEX/QTRAP 5500, Agilent) as previously described [41].
2.10. Phylogenetic analysis
The ESL1 protein sequence was used as a query to obtain homologous protein sequences by a BLASTP search on the NCBI BLAST website (http://www.ebi.ac.uk/Tools/sss/ncbiblast/), and the resulting protein sequences were collected and used for the construction of phylogenetic trees by the bootstrap method using MAGE version 7.0.
2.11. Transcriptome sequencing and data analysis
At the peak of tillering, flag leaves of wild type and esl1 were sampled, and RNA extraction was performed with a TRIzol total RNA extraction kit (Invitrogen, Carlsbad, CA, USA). Illumina HiSeq(Illumina,San Diego, CA, USA) library construction was performed according to the manufacturer’s instructions. Probe labeling and chip hybridization were performed, following standard protocols,by the Illumina HiSeq custom service at the Biomarker Technologies Corporation (Beijing, China). To compare samples from two sets of experiments, normalization was performed following standard Illumina HiSeq protocols. Differentially expressed genes(DEGs) were detected with the DESeq programs (http://www.bioconductor.org/packages/release/bioc/html/DESeq.html). DEGs were functionally classified using the biological process category of Rice Gene Ontology (http://www.geneontology.org/) as well as the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) enrichment. DEGs were genes of log2ratio ≥1 or ≤ - 1.
3. Results
3.1. The esl1 mutant exhibited growth retardation
The esl1 mutant was identified following mutagenesis of the japonica rice cultivar Taipei 309 by ethyl methane sulfonate.Under field growth conditions, there were no obvious phenotypic differences at the seedling stage. At the tiller initiation stage (4–5 leaf stage),growth and development of the esl1 mutant were retarded,and tillering was markedly reduced in comparison with the wild type (Fig. 1A). At maturity, the plant height and tiller number of esl1 were significantly reduced (Fig. 1B, E, F). Panicle length of esl1 was significantly less than that of the wild type (Fig. 1C). The grain of the esl1 mutant grew longer than those of the wild type,but grain width decreased at maturity (Fig. 1D), and seed-setting rate, thousand grain weight and panicle length were also significantly reduced (Fig. 1G–I). The heading date of the esl1 mutant was delayed by 10–15 days compared with the wild type. These results indicated a growth retardation phenotype.

Fig. 1. Comparison of phenotype between wild type and esl1 plants. (A) Plants at early tillering stage. Scale bar, 6 cm. (B) Plants at heading stage. Scale bar, 20 cm. (C)Phenotype of panicle in wild type (above)and esl1(below)plants. Scale bar,4 cm.(D)Mature seed and brown rice of wild type (left)and esl1(right).Scale bar,2 mm.(E–I)Statistical analysis of plant height (E), tiller number (F), seed-setting rate (G), thousand grain weight (H), and panicle length (I) in wild-type and esl1 plants. Data were recorded at panicle maturity stage. Values are mean ± SD (n = 10). **, P <0.01 (Student’s t-test).
3.2. The esl1 mutant displays early leaf senescence
The esl1 mutant also showed a phenotype of early leaf senescence.At the tillering-initiation stage,the leaf margin of the newly heading leaves of esl1 appeared yellowish and the leaf tips of the second and third leaves from the top showed early senescence,while the base remained a normal green color (Fig. 1A). At the mature stage, leaves of the entire esl1 plant at the heading stage showed chlorosis and early senescence, even reaching a state of death and curling,whereas the leaves of the wild type stayed green during the same period (Figs. 1B, 2B).
Light can regulate the process of rice leaf senescence, and dark stress can significantly promote the process of premature plant leaf senescence[16].In flag leaves from esl1 and wild type plants at the initiation of tillering for dark-induction experiments, the promotion of senescence was more pronounced after 5 days in the esl1 mutant, which showed mostly yellow leaves compared with the wild type(Fig.2A).Because esl1 showed early leaf senescence phenotype at the tillering-initiation stage, we measured the chlorophyll content of each leaf at this stage. The chlorophyll contents of the first, second, and third leaves from the top of esl1 were significantly reduced by respectively 34.4%,39.3%,and 54.8%in comparison with the wild-type, and early leaf senescence was most pronounced in the third leaves (Fig. 2B, C). At plant maturity, the chlorophyll a,chlorophyll b,carotenoid,and total chlorophyll contents of the esl1 mutant were highly significantly decreased compared with those of the wild type (Fig. S1). The SPAD value in the wild type was 40.63, while that in the esl1 mutant was only 31.05 (Fig. S2A). Typically, reduced chlorophyll content leads to reduced photosynthesis efficiency in leaves. We accordingly measured photosynthetic parameter indexes in the esl1 mutant and wild type flag leaves at the peak of tillering. The stomatal conductance and transpiration rate were significantly decreased in the esl1 mutant compared with the wild type, although there was a significant increase in the intercellular CO2concentration in the esl1 mutant (Fig. S2B–D). Specifically, the net photosynthetic rate in esl1 was decreased by 85.8%(Fig.2D),the stomatal conductance and transpiration rate were decreased by 70.8%and 59.0%,respectively, and the intercellular CO2concentration was increased by 27.9%.These results indicated that the esl1 mutant exhibited a phenotype of early leaf senescence that included reductions in photosynthetic rate and chlorophyll content.
To check for structural changes in the chloroplasts, we used transmission electron microscopy to observe flag leaf mesophyll cells from wild type and esl1 plants at the peak of tillering. The results showed that the chloroplasts of esl1 were smaller and fewer in number than those of the wild type, and there were some mutant cells in which the chloroplasts were incompletely developed(Fig.2E).The thylakoid arrangement was not markedly different between the two plants, but the osmiophilic granules were markedly increased and enlarged in the chloroplasts of the esl1 mutant, and numerous large granular starch granules filled the chloroplasts(Fig.2E).These changes may account for the dramatic decrease in chlorophyll content, the decrease in photosynthetic efficiency, and the changes in leaf color and chlorosis in the esl1 mutant.
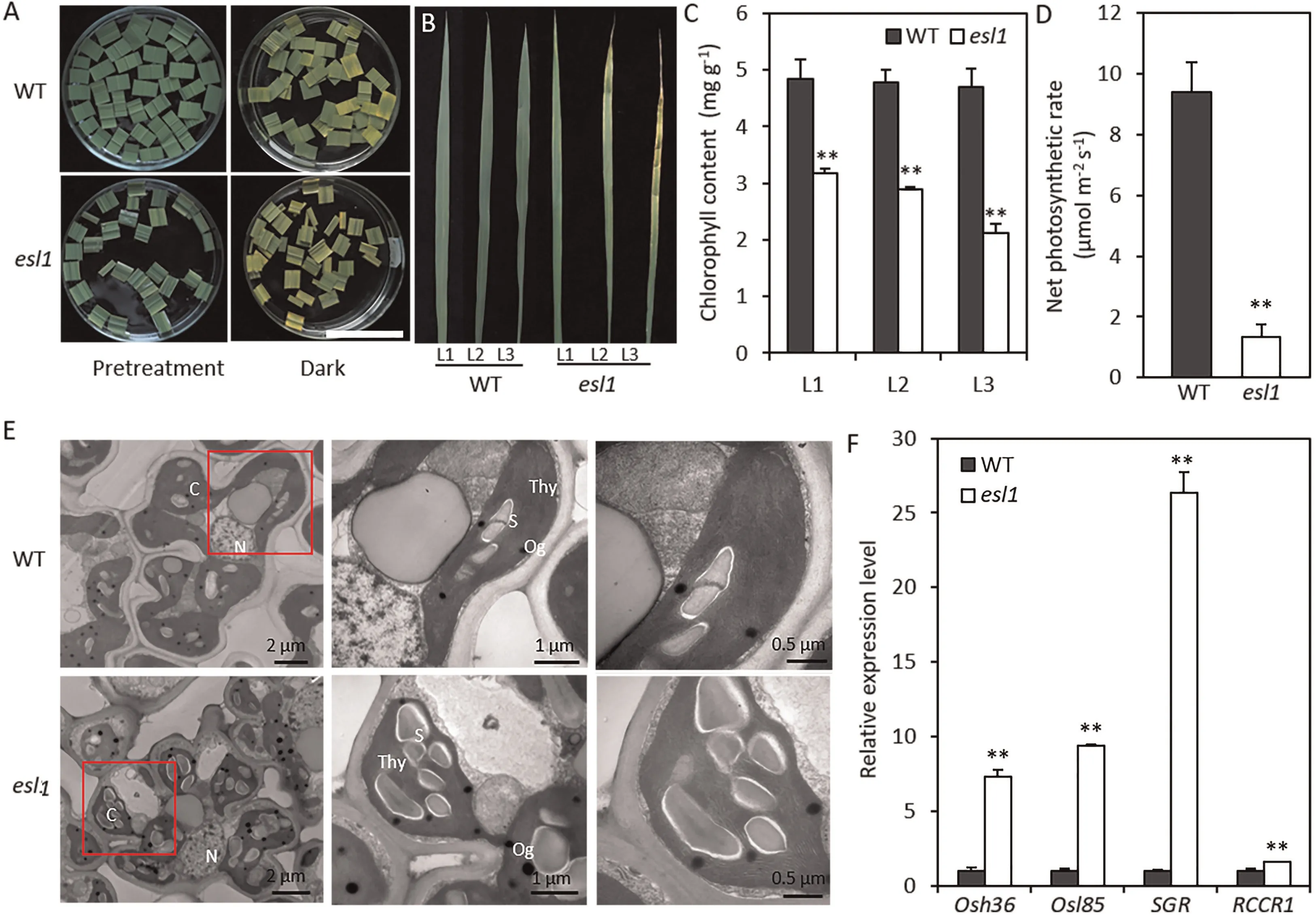
Fig.2. Identification of leaf senescence in esl1.(A)esl1 promoted dark-induced leaf senescence.Detached flag leaves from wild-type(WT)plants and esl1 at the tillering stage were incubated in water for 5 days in darkness. Scale bar, 2 cm. (B) The upper three leaves of esl1 and WT plants at the early tillering stage. (C) Chlorophyll content of the leaves shown in B. (D) Statistical analysis of photosynthesis rate in WT and esl1. (E) Ultrastructure of chloroplasts in the WT and esl1. C, chloroplast; N, nucleus, Og,osmiophilic plastoglobuli; S, starch granule; Thy,thylakoid. (F) qRT-PCR analysis of four senescence-associated genes (Osh36, Osl85, SGR, and RCCR1)in WT and esl1. Values are mean ± SD (n = 3). **, P <0.01 (Student’s t-test).
Senescence leads to changes in the expression of many senescence-associated genes. To determine whether SAGs expression differed between wild-type and esl1 leaves, we performed real-time PCR for SAGs(Osh36,Osl85,SGR,and RCCR1).The expression levels of all SAGs were significantly upregulated in the esl1 mutant(Fig.2F).These results further demonstrated that early leaf senescence occurred in the esl1 mutant.
3.3. Map-based cloning of ESL1
To determine the inheritance mode of the esl1, we crossed esl1 with the typical indica rice cultivars TN1 and ZF802. The F1plants showed normal leaf phenotypes and the F2plants showed segregating populations of two phenotypes,with normal leaves or early leaf senescence, in an approximately 3:1 ratio (Table S1). These results indicated that the early leaf senescence trait of the esl1 mutant was controlled by a single recessive nuclear gene.
To isolate and clone ESL1, we mapped the gene using F2segregating populations constructed from esl1 and TN1. First, we performed polymorphism analysis between the two parents of esl1 and TN1, and SSR markers with polymorphisms between the two parents were genotyped with a total of 116 primer pairs. We randomly selected 21 plants with the early-senescence phenotype for linkage analysis, and the results mapped ESL1 between markers B3-11 and B3-16 on chromosome 3 (Fig. 3A). To more finely map ESL1, we first developed new Indel markers located between the B3-11 and B3-16 markers and polymorphic across the parents,followed by a chromosome-walking method to narrow the mapping interval using the remaining 875 F2mutant plants.Finally,the target gene was finely mapped between markers M4 and M6,within a physical interval of 180.65 kb(Fig.3B).This region contains a total of 24 open reading frames, and sequencing of these revealed a deletion of one base (T) in the fourth exon of LOC_Os03g31550,resulting in premature termination of translation (Fig. 3C–E).
Next, we performed genetic complementation to demonstrate that the mutation of LOC_Os03g31550 was responsible for the esl1 mutant phenotype.We constructed a complementation vector containing the wild type coding sequence and used it for Agrobacterium-mediated genetic transformation of esl1.Of 15 independent transgenic lines, 11 containing the wild-type ESL1 gene showed a wild-type phenotype at the tillering stage (Fig. 3F). This result demonstrated that mutation of ESL1 accounted for the early leafsenescence phenotype of the esl1 mutant.
We measured the pigment content of the wild-type and the esl1 mutant,and complemented line COM at the peak of tillering.There was no significant difference in pigment content between the wild type and COM lines, but pigment content was significantly higher than that of the esl1 mutant (Fig. S3A). By qRT-PCR, ESL1 was significantly less expressed in the esl1 than in the wild type,whereas ESL1 expression was higher in the COM line than in the wild type(Fig.S3B).These findings suggested that the ESL1 mutation results in decreased expression of ESL1 and was responsible for the earlysenescence phenotype of the esl1 mutant.
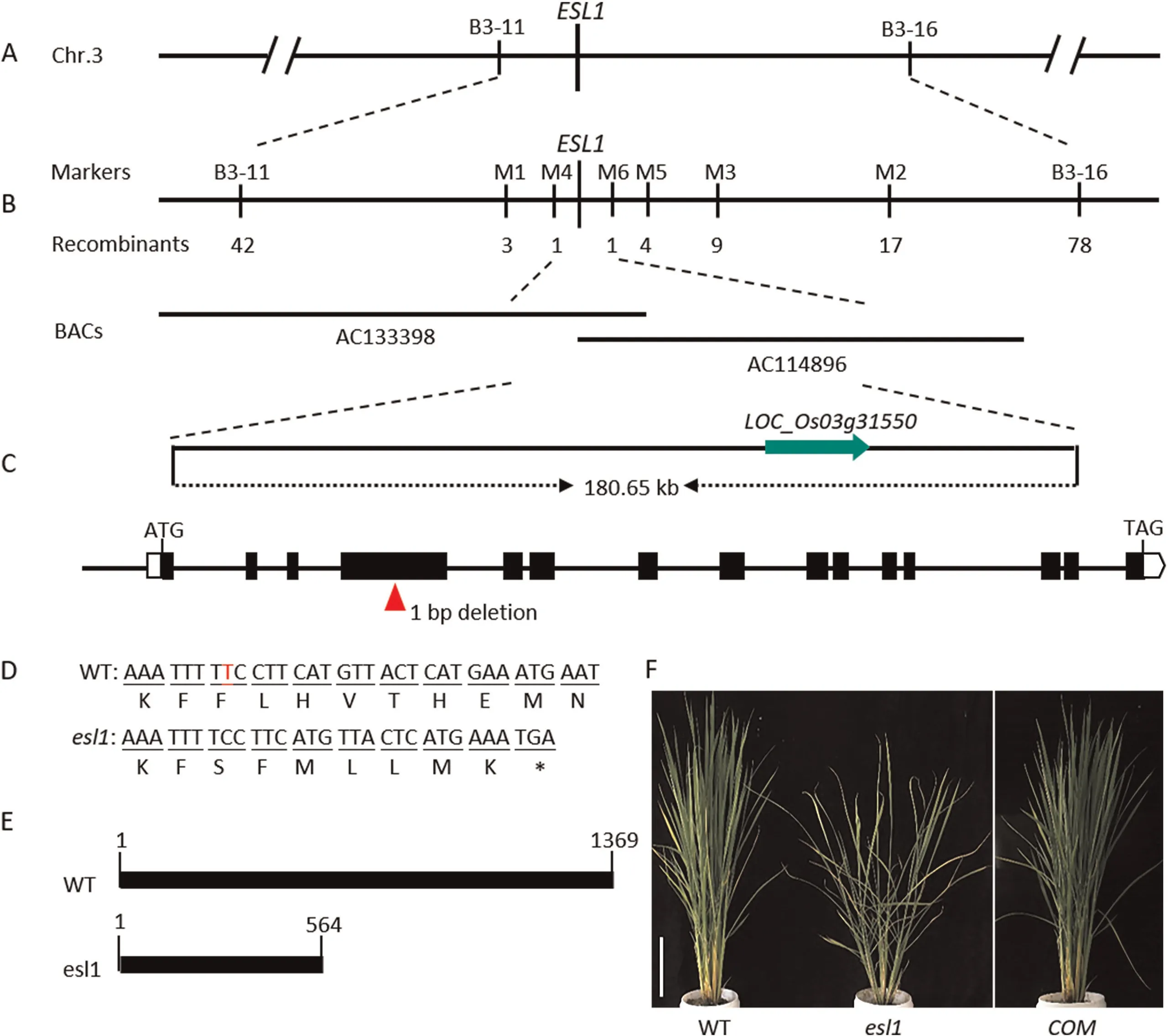
Fig. 3. Map-based cloning of the ESL1 gene. (A) Preliminary mapping of the esl1 mutant using 21 recessive F2 plants with 116 SSR markers. The gene mapped to a region between molecular markers B3-11 to B3-16 on chromosome 3.(B)Fine mapping of ESL1 further localized the mutation to the 180.65 kb genomic region between M4 and M6.The numbers under the linkage map indicate the number of recombinants.(C)ESL1 gene structure.Red arrows indicate mutation site.(D)The esl1 mutant has one bp deletion in the four exon of LOC_Os03g31550. (E) Schematic diagram of proteins encoded by WT and esl1. (F) Phenotype of the complementation transgenic line. Scale bar, 18 cm.
3.4.ESL1 encodes a xanthine dehydrogenase involved in the synthesis of ureides, expressed in various tissues of rice
According to the RAP-DB database (https://rapdb.dna.affrc.go.jp/#), the cDNA of ESL1 is composed of 4110 nucleotides and encodes 1370 amino acids. Comparative amino acid sequence has revealed that ESL1 encodes xanthine dehydrogenase (XDH). We performed phylogenetic analysis to further investigate the evolutionary origin and possible function of ESL1. The results revealed high similarity between ESL1 and homologs in Triticum aestivum,
Brachypodium distachyon, Setaria viridis, Sorghum bicolor, and Zea mays (Fig. 4A). Multiple sequence alignment revealed that the amino acid sequence of the ESL1 protein is highly conserved in plants, and the ESL1 protein of rice had the shortest evolutionary distance from Triticum aestivum and Setaria viridis (Fig. S4).
The XDH enzyme produces the downstream products allantoin and allantoate by reacting with xanthine and hypoxanthine [25].At the tillering stage, the contents of both allantoin and allantoate were significantly lower in the esl1 mutant than in wild-type leaves(Fig.4B, C).Thus,the ESL1 mutation may affect XDH enzyme activity, leading to a decrease in the content of its downstream metabolites allantoin and allantoate.
To investigate the expression pattern of ESL1 genes in diverse tissues,we extracted RNA from several tissues of wild-type plants:roots, stems, leaf blades, leaf sheaths, and panicles. By qRT-PCR,ESL1 was expressed in all tissues, with higher expression in leaves than in other tissues (Fig. 4D).
3.5. Mutation of ESL1 causes excess ROS accumulation and DNA damage
The continuous accumulation of ROS results in plant senescence[42]. To examine the ROS content of esl1 and wild type plants, we stained leaves with DAB and NBT to detect respectively H2O2and superoxide anions (O2-) in cells, and then measured H2O2and MDA contents and POD and CAT activities to quantify the concentrations of senescence-associated substances. The esl1 mutant accumulated a large amount of H2O2and O2-(Fig. 5A, B). H2O2,MDA contents, and POD activity in the esl1 mutant were significantly higher and CAT activity was significantly lower than in the wild type(Fig.5C–F).Thus,mutation of ESL1 caused accumulation of ROS in the esl1 mutant, producing a phenotype of early leaf senescence.
During apoptosis,cells activate various DNA endonucleases that produce a series of 3′–OH ends by double-strand or single-strand breakage of DNA between nucleosomes. The TUNEL assay labels the deoxyribonucleotides and derivatives formed by fluorescein,peroxidase, alkaline phosphatase, or biotin to the 3′-terminals of DNA to facilitate the detection of apoptotic cells. In TUNEL assays of the leaves of wild type and esl1 plants at the peak of tillering,most wild-type cells were negative (Fig. 5G, I), whereas a large number of DNA breaks appeared in the esl1 leaves (Fig. 5H, J).The results suggested that the ESL1 mutation induced DNA fragmentation in mesophyll cells, thereby accelerating the senescence process.
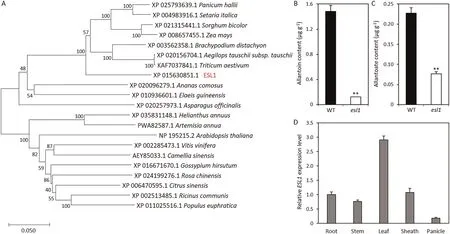
Fig.4. ESL1 is a xanthine dehydrogenase.(A)Phylogenetic tree of the ESL1 protein.(B)Allantoin content of leaves at the tillering stage.(C)Allantoate content of leaves at the tillering stage. (D) Expression pattern of ESL1 in five tissues. Values are mean ± SD (n = 3). **, P <0.01 (Student’s t-test).

Fig.5. ROS accumulation and increase of genomic DNA fragmentation in esl1 leaves.(A,B)NBT and DAB staining of leaves in wild-type(WT)and esl1 plants.(C–F)Statistical analysis of H2O2 content(C),MDA content(D),CAT activity(E),and POD activity(F)in leaves of WT and esl1 plants.Values are mean±SD(n=3).FW,fresh weight.**,P <0.01(Student’s t-test). (G–J) TUNEL assay of leaves. DAPI staining of WT (G) and esl1 plants (H). Positive results of WT (I) and esl1 plants (J). Scale bars, 50 mm.
3.6. Mutation of ESL1 resulted in abnormal sensitivity to ABA
Leaf senescence is a genetically controlled developmental process that can be regulated by a variety of phytohormones,of which ABA may be one of the most important [16]. We examined the expression of key genes associated with ABA biosynthesis and degradation metabolic pathways during the tillering initiation phase. Compared with the wild type, the ABA biosynthesisassociated genes OsNCED3, OsNCED5, and OsZEP were downregulated, whereas the degradation metabolism-associated gene OsABA8ox3 was upregulated in the esl1 mutant (Fig. S5A). To test whether the esl1 mutant altered endogenous ABA concentration,we measured the ABA contents of WT and esl1. The ABA content of esl1 was lower than that of wild-type plants (Fig. S5B). These results indicated that esl1 affected the expression of ABA metabolism genes and then affected ABA concentration, and may affect response to ABA. To investigate the response of the esl1 mutant to ABA,we used 1/2 MS medium containing several concentrations of exogenous ABA for growth and measured plant height and root length 7 days later. After 1 μmol L-1ABA treatment, the plant height and root length of the esl1 mutant decreased by respectively 75.0% and 84.8% relative to the wild type. After 1.5 μmol L-1ABA treatment, the plant height and root length of the esl1 mutant decreased by respectively 63.8% and 97.6% relative to the wild type, revealing that the plant and root growth of the esl1 mutant was significantly inhibited (Fig. 6A–C). When we investigated the effect of exogenous ABA on chlorophyll degradation, the results showed that the yellowing rate in detached esl1 leaves was accelerated(Fig.6D),and the chlorophyll content was 0.23 mg g-1fresh weight after treatment,only 11.2%of that of the wild type(Fig.6E).The senescence genes OsNAP and SGR, which can be induced by ABA, were significantly upregulated by 1.7-fold and 1.6-fold in the wild type.In contrast,the upregulation levels was significantly greater, respectively 4.9-fold and 4.6-fold in the esl1 mutant(Fig. 6F–G). Thus, the esl1 mutant showed increased sensitivity to ABA treatment.
3.7. The esl1 mutant was sensitive to drought and salt stresses
When the plants were stressed by drought, salt, or darkness,XDH activity and uric acid and ureide contents in plant tissue increased while ROS content decreased, increasing their tolerance to environmental stress and reducing their mortality rate [43,44].To investigate the responses of wild type and esl1 to drought and salt stresses, they were subjected to drought and salt conditions at the trefoil stage. After treatment with 20% PEG 6000 for 3 days,esl1 showed greater sensitivity to stress than the wild type(Fig. 7A). Allantoin and allantoate contents were significantly elevated after treatment in the wild type, but not significantly changed in the esl1 mutant (Fig. 7B, C). After 3 days of salt treatment,esl1 showed a more sensitive response than the wild type, as the leaves were yellow and withered (Fig. 7D). The allantoin content in the esl1 mutant did not change significantly, and the allantoate content showed a significant increase, whereas in the wild type,both allantoin and allantoate contents showed a significant increase (Fig. 7E, F). Next, we assessed the transcript levels of ESL1 in wild type and esl1 mutant upon drought and salt treatments using qRT-PCR. Its levels were significantly increased in the wild type after drought and salt treatments, but not significantly changed in the esl1 mutant(Fig.S6).These results suggested that ESL1 might mediate response to drought and salt stresses by regulating the content of ureides.
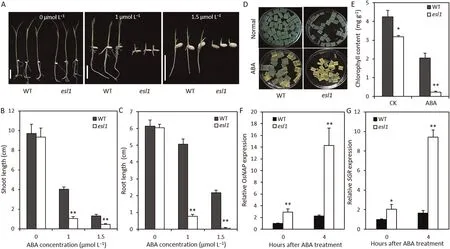
Fig.6. The esl1 mutants showed sensitivity to ABA treatment.(A)ABA effect on seedling growth on 1/2 MS medium.The photograph was taken after treatment for 10 days.The experiment was repeated three times. Scale bars, 2 cm. (B, C) Shoot and root lengths of plants grown on ABA medium. The shoot and root lengths were recorded after growth for 10 days.Values are mean±SD(n=6).(D)Phenotype of detached leaves from wild-type(WT)and esl1 plants in the presence of 5 μmol L-1 ABA.(E)Chlorophyll content of leaves in WT and esl1 plants treated with 5 μmol L-1 ABA for 5 days. (F, G) Relative expression of OsNAP and SGR after 50 μmol L-1 ABA treatment. Values are mean ± SD (n = 3). *, P <0.05; **, P <0.01 (Student’s t-test).
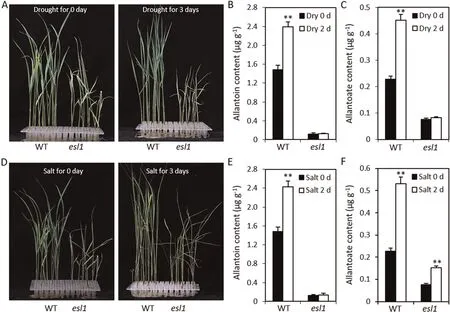
Fig.7. The esl1 mutant showed increased sensitivity to drought and salt stresses.(A)Wild-type(WT)and esl1 seedlings treated with 20%PEG 6000 for 3 days.(B,C)Allantoin and allantoate content of WT and esl1 treated with 20% PEG 6000 for 2 days. (D) WT and esl1 seedlings treated with 150 mmol L-1 NaCl for 3 days. (E, F) Allantoin and allantoate content of WT and esl1 treated with 150 mmol L-1 NaCl for 2 days. Values are mean ± SD (n = 5). **, P ≤0.01 (Student’s t-test).
3.8. Transcriptome analysis suggested the role of ESL1 in regulating photosynthesis and stress responses
To investigate the potential regulatory mechanisms of early senescence traits in the esl1 mutant, we sampled flag leaves from esl1 and wild-type plants at the tillering stage with the most pronounced early-senescence phenotypes, and performed transcriptome sequencing to compare global gene expression changes between esl1 and wild type.Of 3130 differentially expressed genes(DEGs), 1916 were upregulated (log2ratio ≥1) and 1214 were downregulated (log2ratio ≤ - 1). To identify the biological processes associated with these DEGs, we performed gene ontology(GO) analysis and found that chlorophyll biosynthesis was the most significantly enriched biological process (Fig. 8A). DEGs involved in several metabolic processes associated with photosynthesis pathways, ribosome synthesis processes, response to external stimuli and abiotic stresses, purine metabolism, and ROS homeostasis were significantly abundant (Fig. 8A). These results suggested that the ESL1-mediated increase in ROS levels affects various biological processes associated with chloroplast development, ROS homeostasis, and photosynthesis pathways.
To investigate the role of these DEGs in photosynthesis-related pathways,we assigned 3130 DEGs by Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, which revealed that these genes are involved mainly in physiological metabolic pathways and signaling pathways including carbon metabolism,amino acid biosynthesis, phenylpropanoid biosynthesis, photosynthesis pathways, glutathione metabolism, and terpenoid metabolism(Fig.8B).Among them,12 DEGs were associated with photosystem II(11 downregulated and 1 upregulated),1 was associated with the cytochrome b6f complex and downregulated, 4 were associated with photosystem I and downregulated, and 4 were associated with ATP synthase and downregulated(Table S3).Gene expression assessed by qRT-PCR in the wild type and esl1 mutant was consistent with that measured by transcriptome sequencing (Fig. 8C).Thus, ESL1 affects photosynthesis in leaves.
GO enrichment analysis revealed that the DEGs were significantly enriched in biological processes that respond to external stimuli and abiotic stresses, and the metabolic pathways closely associated with stress included ROS metabolic pathways [45],ER-protein processing metabolic pathways[46,47],and chloroplast metabolism[48,49].Therefore,we performed KEGG analysis of the DEGs involved in these pathways(Table S4).Among them,35 DEGs were involved in the ROS metabolic pathway, mainly in peroxisome metabolism (8 downregulated and 5 upregulated), ascorbic acid and aldehydic acid metabolism (4 downregulated and 4 upregulated), glutathione metabolism (10 downregulated and 12 upregulated), and flavonoid biosynthesis processes (10 upregulated). The ER-protein processing metabolic pathway was associated with 10 DEGs,2 of which were upregulated and 8 downregulated. In contrast, 9 DEGs involved in chloroplast metabolism all showed downregulation. These results suggested that ESL1 may play an important role in rice plant responses to stress.

Fig.8. ESL1 affects photosynthesis and stress response.(A)Gene Ontology(GO)enrichment analysis of DEGs in wild type(WT)and esl1(P ≤0.05).(B)Kyoto Encyclopedia of Genes and Genomes(KEGG)enrichment analysis of DEGs in WT and esl1(P ≤0.05).(C)Expression analysis of photosynthesis-associated genes in the WT and esl1.Values are mean ± SD (n = 3). **, P ≤0.01 (Student’s t-test). (D) A model describing ESL1-mediated leaf senescence and abiotic stresses response in rice.
4. Discussion
4.1. ESL1 may affect plant growth and development by regulating the contents of ureides
XDH is a typical and complex molybdenum-containing flavoenzyme whose biochemical and structural properties have been extensively studied, mainly in animal systems, for more than 80 years [50]. XDH is present in plants as a dehydrogenase in a variety of tissues and organs, usually with xanthine and hypoxanthine affinity. It also has purine and pterin as substrates but with low affinity [51]. XDH is an important enzyme in plant growth and development and is involved in a series of important physiological processes. However, to date, no natural higher plant mutants without XDH function have been found, and some mutations resulting in reduced XDH activity, often due to sulfurated molybdenum cofactor (Moco) deficiency, cause molybdenum enzyme activity changes and produce phenotypes with multiple trait changes [52]. In the present study, an XDH-inactivated mutant esl1 generated by ethyl methane sulfonate mutagenesis showed a phenotype of growth retardation and leaf senescence at the tiller initiation stage (Fig. 1).
Sequence analysis revealed that ESL1 harbored a conserved domain typical of XDH. Nakagawa et al. [30] have shown that XDH silencing can cause altered levels of purine metabolism, and defects in the production of purine metabolites can lead to delayed plant growth or the appearance of other unfavorable phenotypes,rather than being a result of the accumulation of purines themselves. Therefore, we measured the allantoin and allantoate contents in both wild type and esl1, and found that they were both significantly lower in esl1 than in wild-type plants (Fig. 4B, C).These results suggest that the esl1 mutant may be unable to produce enough purine metabolites, resulting in a phenotype of slow growth and development and early leaf senescence.
4.2. ESL1 mediates ROS homeostasis and abiotic stresses response by maintaining the balance of ureides
Studies [28,53] have demonstrated that downstream products of purine metabolism, ureides, are effective ROS scavengers. Leaf senescence is accompanied by the accumulation of large amounts of ROS, which cause oxidative damage to thylakoid membranes and other cellular components. Therefore, the production of ROS is one of the indicators of plant senescence [54]. In this study,the NBT and DAB staining indicated the accumulation of O2-and H2O2in esl1 (Fig. 5A, B), as well as a simultaneous significant elevation of H2O2and MDA contents (Fig. 5C, D). These results suggested that more ROS were accumulated in the leaves of esl1.Under normal physiological conditions, the excessive production of intracellular ROS induces the ROS scavenging system to neutralize ROS and further control ROS homeostasis[31].ROS scavenging enzyme CAT activity was significantly reduced in the esl1 mutant(Fig. 5E). Transcriptome analysis revealed that 53 DEGs of ROS metabolic pathways were significantly altered in the esl1 mutant,and the genes differentially expressed occurred in key metabolic pathways involved in maintaining ROS homeostasis,including peroxisome metabolism,ascorbic acid and aldehydic acid metabolism,and glutathione metabolism (Table S4).
Increased allantoin and allantoate contents can offset ROS damage and reduce seedling death[55].To investigate the role of ureides in abiotic stress,we determined the changes in ureide content after drought and salt stresses.After treatment for 2 days,allantoin and allantoate contents were increased in the wild type, whereas production was not significantly increased in the esl1 mutant(Fig. 7). At the same time, esl1 was more sensitive to drought and salt stresses. We speculate that ESL1 plays a key role in regulating ROS homeostatic balance and abiotic stresses response by regulating the balance of ureides (Fig. 8D).
4.3. Mutation of ESL1 affects photosystem processes and chloroplast development through ROS accumulation
As signaling molecules, ROS play regulatory roles during plant growth and development, stress adaptation, and programmed cell death [56,57]. High concentrations of ROS are harmful to organisms, and when levels rise beyond the control of homeostatic mechanisms, cells enter an oxidative stress state, which triggers lipid peroxidation, protein oxidation, nucleic acid damage, and enzyme inactivation, and leads to reduced photosynthesis efficiency and impaired chloroplast development [58,59]. Photosynthesis is the key to plant survival, as it directly regulates plant growth and development, and almost all life on Earth depends directly or indirectly on it. The process begins with the thylakoid membrane, where two photoreactions occur simultaneously in the photosystem II and photosystem I reaction centers,after which the light energy absorbed by the pigment-protein antenna complex of the photosystems is converted to redox chemical energy with high efficiency[60].In the present study,Transcriptome analysis revealed that all genes closely associated with photosynthesis were downregulated in the esl1 mutant, except for Os07g0147500(Table S3). In addition, the net photosynthetic rate was significantly reduced in the esl1 mutant(Fig.2D).These results illustrate that ESL1 is essential for rice photosystem processes.
We measured the chlorophyll content at the tillering initiation stage of the rice plants and found that each leaf of esl1 showed decreased chlorophyll content, which was dramatically reduced at the heading stage (Figs. 2C, S1). The microstructure of chloroplasts was observed at the peak of tillering, and the chloroplasts of esl1 were found to be incompletely developed and filled with numerous osmiophilic granules and large starch granules(Fig. 2E). Furthermore, expression of the chlorophyll degradation gene SGR was dramatically upregulated in the esl1 mutant(Fig. 2F). Consistently, transcriptome analysis revealed that all 9 DEGs of chloroplast metabolic processes appeared downregulated in the esl1 mutant.In addition,37 DEGs of ribosomal protein metabolic pathways were downregulated, except Os11g0135400(Table S4).Ribosomes are essential for protein synthesis.In plants,ribosomes catalyze protein synthesis in the cytoplasm, plastids,and mitochondria, and plastid ribosomal proteins play an important role in chloroplast development and the translation of key proteins for photosynthesis [61,62]. These findings indicate that ESL1 regulates photosynthesis and chloroplast development and degradation pathways by maintaining ROS homeostasis, and ROS acts as a signaling molecule in the regulation of ribosome-related gene expression.
4.4. ABA metabolism is involved in ESL1-mediated regulation of leaf senescence
Although ABA is generally considered to be a promoter of senescence [20], the transcription factor OsNAP is specifically induced by ABA and promotes senescence in rice [16], whereas the transcription factor OsNAC2 promotes senescence by inducing ABA biosynthesis [17]. In the present study, key genes in ABA biosynthesis showed downregulated expression in the esl1 mutant,whereas the expression of key genes for degradation appeared to be upregulated(Fig.S5A).Furthermore,the content of endogenous ABA in the esl1 mutant was lower than that in the wild type(Fig. S5B). Therefore, the early leaf senescence in esl1 could not be attributed to an excess accumulation of ABA. To investigate the role of ABA in the regulation of leaf senescence by ESL1, we treated wild-type and esl1 seedlings with different concentrations of ABA and found that root length and plant height were more significantly inhibited in the esl1 mutant (Fig. 6A–C). Thus, the esl1 mutant was more sensitive to ABA. In addition, ABA significantly promoted the senescence of esl1 leaves and the expression of OsNAP and SGR in the esl1 mutant (Fig. 6D–G). ABA prevents H2O2accumulation by inducing the expression of OsCATB, a key CAT gene, and protects cells from ROS oxidative damage [63].ROS are key signaling molecules in the acclimation process of plants to abiotic stress [3]. We hypothesize that mutation of ESL1 affects the expression of ABA metabolism-related genes and further alters the endogenous ABA concentrations, possibly affecting the ability to scavenge ROS and thereby promoting an abnormally sensitive response to ABA and abiotic stresses.
In summary, we propose that ESL1 plays a role in ureidesmediated leaf senescence and abiotic stress response in rice(Fig. 8D). ESL1 can regulate the levels of ureides and the endogenous ABA concentration,which,in turn,maintains ROS homeostasis. The content of ureides is involved in the response to stress.Thus, as important signaling molecules, ROS are involved in regulating leaf senescence and abiotic stress response.
CRediT authorship contribution statement
Jiangmin Xu and Yuchun Raodesigned the study and wrote the manuscript.Jiangmin XuandChenyang Panperformed most of the experiments.Han Lin,Hanfei Ye,Sheng Wang,Tao Lu,Qianyu Chen,andKairu Yangperformed part of the work.Mei Lu,Qian Qian,andDeyong Renprovided technical assistance.Jiangmin Xu, Yuchun RaoandDeyong Renconceived the project and revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research study was supported by the Key Transgenic Breeding Program of the Ministry of Agriculture and Rural Affairs of China(2016ZX08009003-003-008),the National Natural Science Foundation of China (31971921, U20A2030), and the State Key Laboratory of Rice Biology, China (20200102).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.05.011.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet
- RNAi-mediated suppression of the abscisic acid catabolism gene OsABA8ox1 increases abscisic acid content and tolerance to saline–alkaline stress in rice (Oryza sativa L.)

