Development of high-oil maize haploid inducer with a novel phenotyping strategy
Chenxu Liu, Jinlong Li, Ming Chen, Wei Li, Yu Zhong, Xin Dong,b, Xiowei Xu, Chen Chen,Xiolong Tin, Shojing Chen,*
a College of Agronomy and Biotechnology, Beijing Key Laboratory of Crop Genetic Improvement, Laboratory of Crop Heterosis and Utilization, Ministry of Education, National Maize Improvement Center of China, China Agricultural University, Beijing 100193, China
b Chongqing Academy of Agricultural Sciences, Chongqing 401329, China
Keywords:Doubled haploid Marker-assisted selection Kernel oil content Haploid identification Haploids per ear
ABSTRACT Doubled haploid (DH) technology is important in modern maize breeding. Haploid inducers determine the efficiency of both haploid induction and identification.It has taken decades to improve the efficiency,haploid induction rate(HIR),from the ~2%of the ancestor haploid inducer,stock6,to the ~10%of modern haploid inducers.Improvement of kernel oil content(KOC)would further enhance haploid identification efficiency.Using molecular marker-assisted selection,in combine with the number of haploids per ear as phenotypic criterion,we developed a new high-oil haploid inducer line,CHOI4,with a mean HIR of 15.8%and mean KOC of 11%.High KOC of CHOI4 can achieve a mean accuracy greater than 90%in identification of haploids of different backgrounds, with reduced false discovery rates and false negative rates in comparison with the previous high-oil haploid inducer line, CHOI3. Comparison of phenotypic selection strategies suggested that the number of haploids per ear can be used as a phenotyping criterion during haploid inducer line development.CHOI4 could further increase the efficiency of large-scale DH breeding programs with lower cost.
1. Introduction
With increasing consumption of corn-based fodder, biological energy, and industrial raw materials, accelerating maize hybrid breeding is desirable. Given that heterosis is commonly exploited in maize breeding, the development of elite inbred lines with excellent agronomic traits and stress tolerance is of top priority[1,2]. In comparison with the conventional method for developing inbred lines by continuous self-pollination for at least 8 generations [3], the doubled-haploid (DH) technology has the advantage of producing completely homozygous DH lines in only two generations, substantially increasing breeding efficiency and shortening the breeding cycle. Three key procedures are involved in DH line production: haploid induction (HI), haploid kernel classification,and chromosome doubling [4]. To produce haploids efficiently,several systems have been investigated, including anther culture,interspecific hybridization, in vivo HI by Stock6-derived haploid inducers, and the mutant indeterminate-gamete materials [5–10]. Among these, in vivo HI by Stock6-derived haploid inducers has been proved to be both background-independent and efficient in maize, and has become the method of choice [11].
In vivo haploid induction was first described in 1959, when Stock6 was observed to induce ~2%maternal haploids upon pollinating female parents [7]. Because Stock6 lacks both the ability to adapt to different environments and phenotypic marker for haploid seed identification, its application is limited. The introduction of R1-Navajo (R1-nj) markers into inducers made haploid identification possible at the mature seed stage [12] and also paved the way for using HI in breeding programs and genetic studies. R1-nj is a dominant allele that causes purple pigmentation in both scutellum and aleurone. Haploid seeds do not express purple pigmentation in the scutellum, owing to lack of the male genome,but express it in the aleurone, which carries the heterozygous R1-nj allele,and thus enable the visualized identification of haploid seeds. The R1-nj marker system substantially simplified the haploid identification process and improved the efficiency of DH breeding. By 2016 more than 50 haploid inducer lines had been developed worldwide, all of which were either directly or indirectly derived from Stock6 [13]. ZMS [14], CAUHOI [15], WS14[16], and ACIR [17] were developed using Stock6 as one of the donor parents, and the haploid induction rate (HIR) of these inducer lines ranged from 3%to 5%.The agronomic traits and adaptability of these inducer lines were substantially improved relative to Stock6. These inducer lines were used as the donor parents for developing new inducer lines including KEMS [18] and MHI [19],with HIRs of 6%–8%.Based on haploid inducer lines,such as KEMS,the HIR was further improved to 8%–10%with the development of subsequent haploid inducer lines such as RWS, RWK, UH400, PHI,and CAU5[11,20–22].These lines have been widely used in breeding programs and paved the way for modern DH breeding of maize.
Haploid identification is another key procedure in DH breeding.Although the R1-nj marker system is effective and has been used for several decades, it is labor-intensive and time-consuming in large DH breeding programs and is difficult to use with some tropical germplasm[20,23].To solve this problem,Chen and Song[24]developed a method using kernel oil content (KOC) as an identification marker between diploid and haploid kernels. Kernel oil is stored mainly in embryos,high-oil pollinators could improve KOCs of hybrid kernels, i.e., the xenia effect, nevertheless, KOCs of haploid kernels is low.The determination of KOC by nuclear magnetic resonance (NMR)is fast, accurate,nondestructive, and suitable for automatic classifying kernels as diploids with high KOCs and haploids with low KOCs. This method has been proven to be feasible with the high-oil inducer CAUHOI, which has a KOC of ~7% [25],and was further confirmed to be accurate by development of the inducer line CHOI3,with a KOC of ~8%[26].The method was also successfully verified and optimized in European countries with the high-oil inducer line UH600 [27–29].
In addition to KOC, kernel weight has been tested for haploid sorting;however,the accuracy of this approach was unsatisfactory owing to a genotype effect [30]. Near-infrared reflectance (NIR),another promising method that responds to kernel components[31],has been used for haploid identification[32–34]with an accuracy of up to 90% with proper models. Except for seed stage analysis, haploid identification methods have also been tested at the seedling stage using traits including radicle length, coleoptile length, and root color, but the efficiency of these approaches is low because of the cumbersome manipulations they require and their overreliance on the experience of investigators [35].
Given the substantial role of haploid inducer lines in maize DH breeding, elucidation of the genetic and biological mechanisms of haploid induction would be beneficial for further improvement of haploid induction efficiency.Eight quantitative trait loci(QTL)that contribute to HI have been detected[36],of which two major QTL,qhir1 and qhir8, were located in bins 1.04 and 9.01, explaining respectively 66% and 20% of genetic variances [26,37,38]. Further studies revealed that loss of function of the gene ZmPLA1/NLD/MTL in qhir1 triggers HI[39,40,41].A single-nucleotide substitution in the gene ZmDMP within qhir8 significantly increased HIR [42].Knockout of ZmPLA1/NLD/MTL homologs and ZmDMP has led to the identification of effective haploid induction systems in wheat(Triticum aestivum L.),rice(Oryza sativa L.)[43,44],and Arabidopsis thaliana [45]. In addition, genetic studies have revealed abnormal phenomena including segregation distortion, kernel abortion, and increased occurrence of hetero-fertilization and twin embryos[46–49], which may shed light on the mechanism of HI.
To further increase DH efficiency in maize, it is necessary to improve the efficiency of three linked techniques:HI,haploid identification, and chromosome doubling. Here we describe a newly developed high-oil haploid inducer line, CHOI4, with excellent induction and identification properties. Meanwhile, we also introduce a strategy of phenotypic selection based on the number of haploids per ear that is intended to be more effective than the conventional method of calculating HIR.
2. Materials and methods
2.1. Materials
To generate a haploid inducer line with both high KOC and high HI efficiency,the haploid inducer line CAU2,a haploid inducer line,was chosen as one parent to cross with the Beijing High Oil population (BHO) [50]. Both CAU2 and BHO were developed by China Agricultural University. CAU2 has a HIR of ~10% and a clear R1-nj marker in seeds. BHO has a mean KOC of 15% [50]. The two parents were crossed in 2011 to generate hybrids. The singlecross hybrid Zhengdan 958(ZD958),an elite hybrid widely planted in China that displays clear R1-nj pigmentation in both aleurone and scutellum,was chosen to evaluate both HIR and KOC in candidate plants in each generation. Seven single-cross hybrids were used for testing the HIR of the candidate haploid inducer line:Xianyu 335 (XY335), Jingke 968 (JK968), Dika 516 (DK516), Dan 598/Yu 87-1 (DY), Denghai 605 (DH605), Zhongnongda 678 (ND678),and Chang 7-2/T0278 (CT). To fully evaluate the performance of the candidate haploid inducer line, three haploid inducer lines:CAU5 [42], CAU6 (previous named CAUB73), and CHOI3 [26] with KOCs of 3.5%, 3.5%, and 8.8%, respectively, were used for comparison.
2.2. Marker-assisted selection (MAS) of the QTL qhir1
F2population was generated by self-pollination of F1plants.Young leaves from plants of the F2population were sampled in the field for DNA extraction following Murray and Thompson[51].A polymorphic simple sequence repeat(SSR)marker X18,closely linked to qhir1 was chosen for genotyping[26,37].Plants with CAU2 or BHO genotype and heterozygous at qhir1 were characterized.Plants with CAU2 genotype,and heterozygous at qhir1,showing clear pigmentation on both the scutellum and aleurone and lodging resistance were selected to be grown in the field and self-pollinated to generate the F3population. Each F3plant was genotyped with marker X18 again to 1) confirm the presence of homozygous qhir1 for progeny derived from F2plants with homozygous qhir1 allele from CAU2 and 2) genotype the F3plants derived from heterozygous F2plants. Only F3plants that were homozygous for qhir1 from CAU2 were chosen to generate the F4.From F5to F8, phenotypic selection on both number of haploids per ear and agronomic traits was applied. In F7, to verify the presence of qhir8 in candidate plants, two insertion-deletion markers,28S and 76S, flanking qhir8 [38], were used for genotyping.
2.3. Breeding strategy between selection of HIR and haploid yield
The HI ability of each plant from F2to F8was determined by pollinating at least three ZD958 ears.After harvest,ears were air dried and threshed.Kernel classification was performed using R1-nj purple pigmentation on both scutellum and aleurone [12]. Kernels with a colorless scutellum but a purple aleurone were classified as putative haploids and those with purple pigmentation on both aleurone and scutellum as hybrid kernels.Kernels with no pigmentation were discarded.During the phenotypic selection of HI ability from F2to F4, HIR was calculated as previously described[21];the mean HIR from at least three tested ears was calculated as a measure of HI ability.Plants with an HIR in the top 30%were advanced to the next generation for KOC measurement. To obtain candidate haploid inducer lines with high haploid yield, the number of haploids per ear was used as the indicator of HI ability from F5to F8.Ears of ZD958 with seed set fewer than 100 kernels were discarded. The mean number of haploids per ear for each plant was calculated. Plants with a number of haploids per ear in the top 30% were selfed to produce the next generation for KOC determination.
2.4. KOC selection
In the breeding generations from F2to F7, ears from candidate plants were first selected based on their performance in haploid induction ability and agronomic traits, candidate ears were air dried and manually threshed for single-kernel KOC determination by NMR (Niumag, Shanghai, China). Kernels with KOC in the top 30% were planted in the following season. During the evaluation of KOC-based haploid identification, haploid and diploid kernels were air dried and their KOC was assessed by NMR.
2.5. Haploid identification based on R1-nj and KOC
The efficiency of KOC-based haploid kernel identification was determined after seven consecutive self-pollinated generations.Ears pollinated with CHOI4 were harvested after maturation, and each ear was air dried and threshed. In the first round, putative haploid kernels were identified according to the R1-nj pigmentation system, and each haploid and diploid kernel was numbered.KOC of these putative haploids and diploids was then determined by NMR and further used for haploid classification. The parameters, accuracy rate (AR), false discovery rate (FDR), and false negative rate (FNR) were used for evaluating the haploid identification[27–29].
2.6. Field management and selection of agronomic traits
Field experiments were conducted in Sanya, Hainan (18°35′N,109°18′E) in winter, and in the experiment station of China Agricultural University, Beijing (39°56′N, 116°20′E) in summer.Because of the different flowering dates of CAU2, BHO, and ZD958,ZD958 was planted 5 days in advance of the others.In both Beijing and Hainan,consistent management practices were applied for weeding, irrigation, and pest control. Emasculation of ZD958 and other female parents was performed to avoid pollen contamination. Young ears of both female parents and breeding populations were covered with paper bags before silking. To guarantee a high quality of pollination, silks were cut to ~2 cm long 1 day before pollination. Agronomic trait selection was performed from F2to F8by visual scoring. Plants that had large amounts of pollen and strong resistance to lodging and diseases were considered ideal.
Data analysis was performed with Excel and R 3.1.4.Student’s ttests were used for comparing HIR of different genotype plants.The Shapiro–Wilk test was used for normality testing.
3. Results
3.1. MAS of qhir1
Genotyping of the F2population (732 plants) on qhir1 revealed that 288 had the CAU2 genotype, 86 had the BHO genotype, and the remaining 358 were heterozygous (Fig. S1). The segregation ratio of qhir1 in the F2population diverged (P <0.01) from the 1:2:1 segregation ratio.The mean HIRs of the A,H,and B genotype classes were respectively 1%,2%,and 2%(Fig.1).Based on the genotype and HIR performance of each F2plant,self-pollinated progeny of 46 plants that carried homozygous qhir1 from CAU2 and 26 that were heterozygous at qhir1 were sown in the next season. Genotyping of the qhir1 allele and phenotyping of F3plants derived from heterozygous F2plants revealed that the mean HIR of BHO genotype plants was <1%, while the mean HIRs of heterozygous and CAU2 plants were respectively 3.0% and 4.2% (Fig. 1).
3.2.Phenotypic selection responses of HIR and number of haploids per ear
Selection response revealed that the mean HIR of candidate plants increased from 1.9% in the F2to 4.0% in the F4. The mean number of haploids per ear increased from 3.6 in the F2to 7.6 in the F4(Figs.2,3).Over the next four generations,as number of haploids per ear was used as the phenotype for selection instead of HIR, the average HIR of candidate lines increased to 13.7% in the F8population and the mean number of haploids per ear increased to 27.7. Thus, using number of haploids per ear led to a stronger selection response in both the HIR and the number of haploids per ear.Comparison of the mean HIR and haploids per ear between F7and F8, revealed an increase in number of haploids per ear and decrease of HIR under selection on number of haploids per ear(Fig. 3).
After seven consecutive generations of selection, a stable haploid inducer line was generated and was referred to as CAU High Oil Inducer 4 (CHOI4). Evaluation of the HI ability of CHOI4 was performed using seven different single-cross hybrids. The HIRs of CHOI4 among the resulting hybrids ranged from 10.6% to 20.8%(Table 1). CHOI4 produced 13.4–43.4 haploids per ear across all female parents (Table 1).
3.3. Phenotypic distortion of haploid induction ability during CHOI4 development.
Further analysis of HIR in plants of each generation with quantile–quantile (Q–Q) plots and the Shapiro-Wilk test showed skewed (P <0.01) distributions from F2to F6(Fig. S2), with most plants showing low HI ability(Figs.2,S2).The skewed distribution of haploid induction ability returned to a normal distribution after the F7under phenotypic selection.
3.4. Selection response of KOC
KOC showed a typical normal distribution in the F2generation,with a mean value of 9% (Figs. S3, S4), similar to the midparent value.Using the single-kernel determination and selection method,the mean KOC increased to 11% through seven generations generated by sequential self-pollination(Fig.S3).The KOC of CHOI4 was substantially higher than the midparent value.
3.5. Accurate identification of haploids using KOC
As shown in Fig.3,among the progeny pollinated with the nonhigh-oil inducer line CAU5 (KOC, 3.5%), the KOC distribution overlapped extensively between haploids and diploids (Fig. 4).Although the accuracy of haploid identification reached 84%, it was accompanied by a high FNR of 40% and a high FDR of 12%(Table 2).For this reason,CAU5 could not be used for haploid identification by KOC.
The discriminating effect on haploids was increased in progeny pollinated by the high-oil haploid inducer line CHOI3.The haploid identification accuracy was 94.68%,with an FDR of 10.00%and FNR of 17.11% (Fig. 4; Table 2). Among progeny pollinated by CHOI4(KOC, 11%), haploids and diploids could be clearly distinguished by their KOCs, which showed very little overlap(Fig. 4). The accuracy of haploid identification using KOC was as high as 96.34%,and both FDR and FNR were <4.07%(Table 2).The efficiency of haploid identification using KOC reached its maximum at a threshold of 4.8% among the progeny of ZD958 × CHOI4.
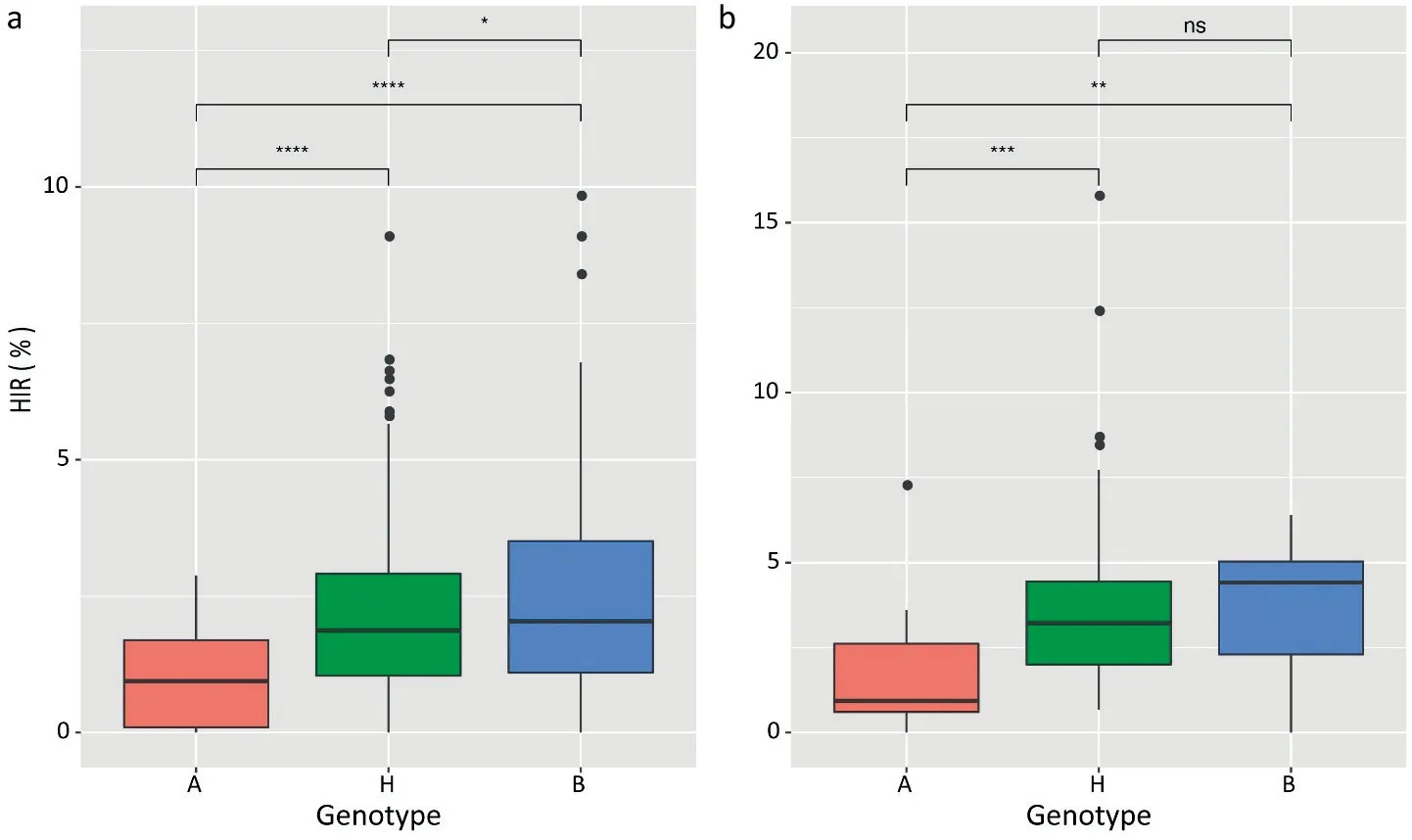
Fig. 1. Marker-assisted selection of qhir1 in F2 (a) and F3 (b). A, homozygous haplotype of qhir1 locus from BHO; B, homozygous haplotype of qhir1 from CAU2; H,heterozygous genotype.Sample sizes of A,H and B were respectively 25,291 and 131 in F2 population and 17,49 and 13 in F3 population.Significance test result for each pair of genotypes is indicated. ns, not significant. *,**, ***, and **** indicate significant at P <0.05, P <0.01, P <0.001, and P <0.0001, respectively.

Fig.2. Distribution of number of haploids per ear from F2 to F8.Each row shows the distribution of number of haploids per ear in a breeding generation.The vertical dotted line represents the mean value.
Haploid identification efficiency was tested in 4 genetic backgrounds, the single-cross hybrid JK968, and inbred lines Zheng 58, and Chang 7-2. The KOC distribution of diploids and haploids showed little overlap, and the haploid identification accuracies in JK 968, Zheng 58, and Chang 7-2 were 97.18%, 98.93%, and 99.44%, respectively, with a FDR <1.39% and FNR <5.71% (Fig. 4;Table 2).In comparison with CHOI3,the efficiency of both haploid induction and haploid identification of CHOI4 was increased.Although the KOC of hybrid kernels varied among germplasm,the difference in the KOC between crossed and haploid kernels appeared to be more stable.

Fig. 3. Response of HIR, number of haploids per ear, and total kernel number per ear under selection from F2 to F8.The left ordinate shows the value of HIR(%)or the number of haploids per ear. The right ordinate shows total seeds per ear. The strategy of selection on HIR from F2 to F4 is shown in blue background, and the strategy of selection on the number of haploids per ear from F5 to F8 is shown in orange background. Dotted lines are fit lines for both HIR and the number of haploids per ear. The slopes of dotted lines show the genetic gain in haploid induction ability under the two strategies.
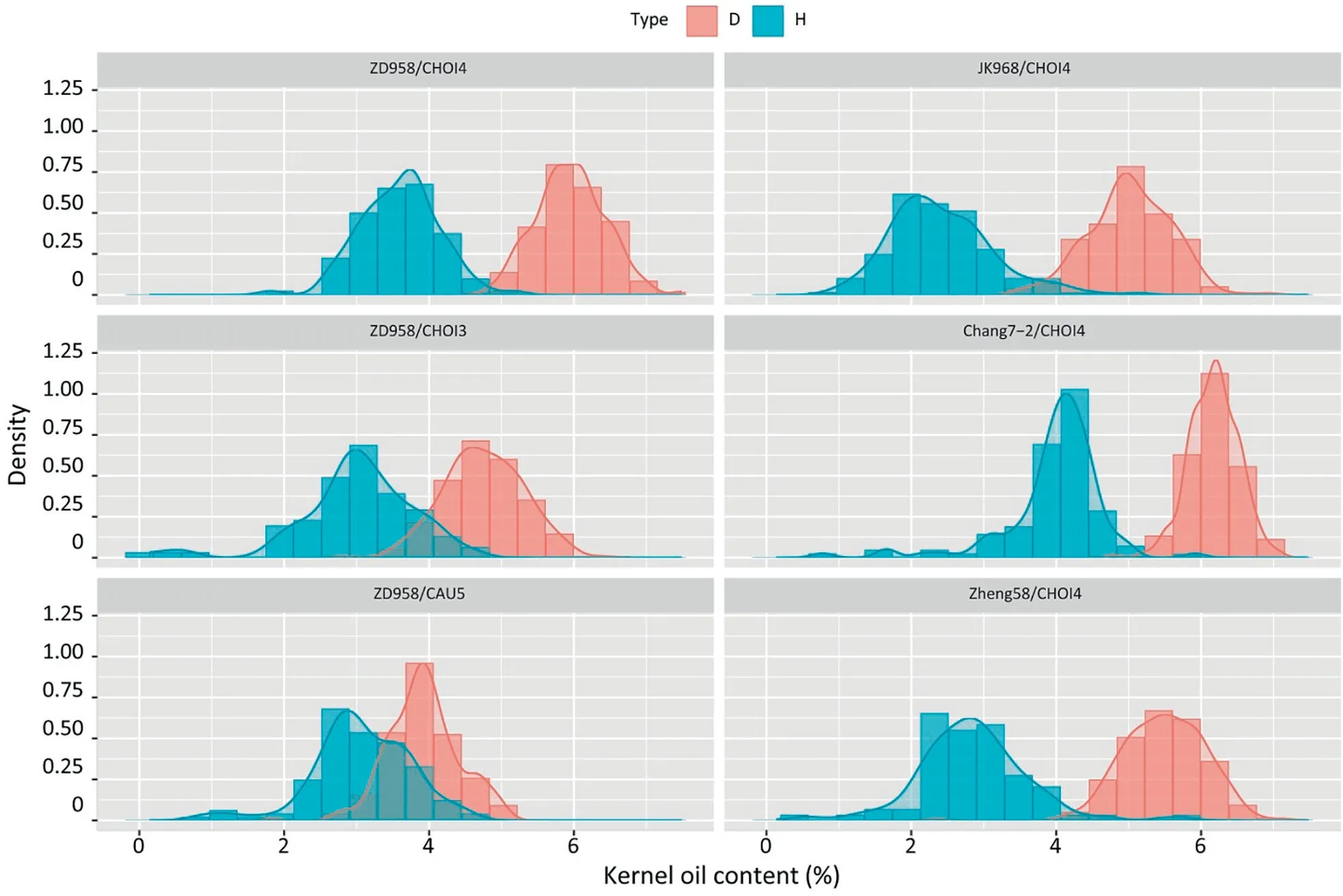
Fig. 4. Kenel oil content (KOC) of haploids and diploids from different crosses. Left side: KOC of diploid (pink) and haploid (aqua) kernels derived from ZD958 × CHOI4(upper),ZD958×CHOI3(middle),and ZD958×CAU5(lower).Right side:KOC of haploid and diploid seeds derived from crosses of JK968×CHOI4,Zheng 58×CHOI4,and Chang 7-2 × CHOI4, respectively. Types D and H represent diploid and haploid kernels, respectively.
Given that the KOC of kernels is strongly influenced by the environment, comparison of KOC-based haploid identification in the two growing environments, Beijing and Hainan, was performed.The accuracy of haploid identification was higher, but FDR and FNR were lower in Hainan than that in Beijing(Table 3).The mean KOC of both haploids and diploids was high in HN, as reflected by the increased threshold.
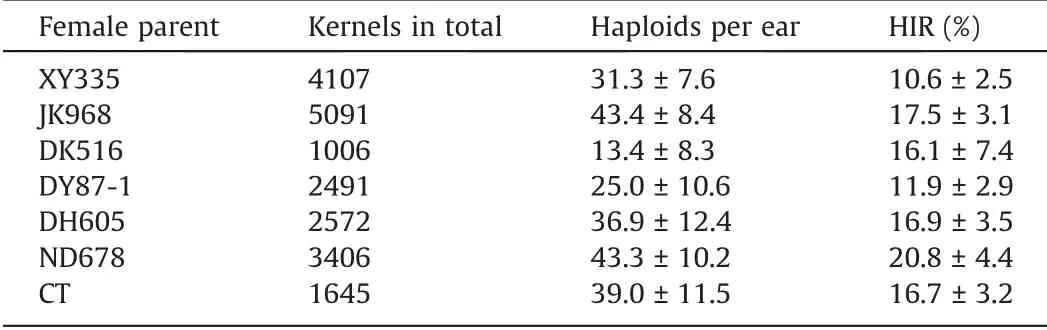
Table 1 Mean (± standard deviation) of haploid induction ability evaluation of CHOI4 using female parents of different genetic backgrounds.
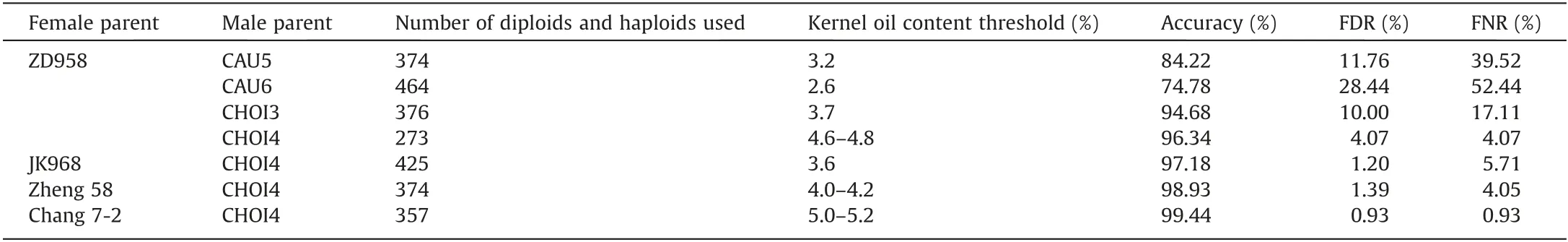
Table 2 Kernel oil contents based haploid kernel identification accuracy in different crosses.

Table 3 Efficiency of kernel oil content-based haploid identification of ZD958 × CHOI4 in Beijing and Hainan.
4. Discussion
4.1. Development of high-oil haploid inducers
In this study,we further improved the efficiency of haploid production by developing CHOI4,which showed a mean HIR of 15.78%across diverse female parents and a KOC of 11%. In comparison with the HI donor parent, CAU2, the increased HIR of CHOI4 suggests that new loci that contribute to HIR were fixed from the BHO population. Application of MAS to qhir1 increased breeding efficiency by removing heterozygous and non-inducer genotypes that accounted for more than 70% plants in the F2. qhir8 was not selected with MAS in the early generations because this study preceded the fine mapping of qhir8.Subsequent fine mapping of qhir8 has provided marker information verifying the presence of qhir8.A genetic analysis performed in the F7population revealed that qhir8 had been fixed in early generations under strong phenotypic selection (data not shown). Development of new markers that enable MAS on multiple QTL contributing to haploid induction ability could further simplify the development of haploid inducer lines.
In addition to genetic factors,HIR was affected by environment and genotype-by-environment interaction. Fortunately, the effect of interaction of genotype and environment on HIR was not that of a complex interaction, but instead was a simple increase of 1%–2% in Hainan, and thus did not affect selection [52]. The KOC of diploid kernels harvested in Hainan was higher than that of kernels harvested in Beijing,possibly accounting for the superior haploid identification accuracy.
4.2. Segregation distortion of genetic loci and HIR
Owing to segregation distortion of qhir1,the HIR of F2individuals showed an expected skewed distribution,in which most plants showed a low value of haploids per ear(Figs.2,S3).Although qhir8 explains 20% of the genetic variance of HIR, it alone does not lead to segregation distortion [21,36,46]. The segregation distortion of qhir1 could hinder the development of conventional haploid inducer lines.MAS appears to be particularly useful under such circumstances, as it can fix qhir1 effectively to counteract segregation distortion.The qhir1 genotype was homozygous in F4plants;however,the skewed distribution of HIR was still present in the F4and F5generations, suggesting the presence of minor QTL that contribute to HIR and may also lead to segregation distortion (Figs. 2,S2).The transition of HIR from a skewed to a normal distribution at the F7shows that these loci that contributed to HIR and exerted a segregation-distortion effect were fixed in the population.Considering the improvements in DH breeding worldwide, haploid inducer line maintenance is important for commercial breeding. The presence of loci that may lead to a skewed distribution underscores the importance of individual phenotypic selection through 7 or 8 generations; otherwise, HIR can decrease because gametophytes with low HI ability have strong competition during fertilization.
4.3.Selection of haploids per ear results in favorable selection response
Generally, a haploid inducer line is evaluated based on the calculation of HIR [21]. Haploid induction is a kind of abnormal double fertilization that leads to a series of kernel abortions[21,36,39–42,53].According to the formula that HIR(%)=the number of haploid seeds/(the number of haploid seeds+the number of diploid seeds)×100%,HIR can be improved by not only an increase in haploids per ear but also a decrease in the seed setting rate of an ear.The balance between HIR and seed setting rate influences haploid yield. In this study, we concentrated on the HIR of candidate plants in the first three generations, and the selection response showed that haploid induction ability of plants from the F2to the F4increased slowly (Figs. 2, 3). When we shifted the phenotyping strategy from the HIR to haploids per ear after the F4generation,both HIR and haploids per ear increased rapidly from F5to F8,suggesting that number of haploids per ear is a valuable criterion for haploid inducer line development.
Although haploid induction is usually accompanied by kernel abortion,which can thus be used as an indicator of HIR[26,54],this characteristic may not be an appropriate selection standard in haploid inducer line breeding programs. If it is used as such, loci that lead to poor seed set may accumulate over generations,disfavoring both seed setting and yield of haploids.On the other hand,several environmental factors,such as extremely high or low temperature and humidity, could lead to low pollen viability and eventually to poor seed set [55,56]. Use of number of haploids per ear should exclude plants that are sensitive to these extreme environmental conditions and increase the seed setting rate, resulting in the parallel and rapid improvement of both HIR and haploids per ear. In view of the selection response shown in the F7and F8,use of number of haploids per ear as the selection criterion may be helpful for breaking the balance between HIR and seed set, leading to a high yield of haploids.
4.4. Improved haploid identification with CHOI4 with higher KOC
As one of the key procedures in DH breeding, the efficiency of haploid identification has a substantial effect on DH breeding.KOC-based haploid identification has made this process more objective and independent of investigator experience.The development of an NMR-based haploid identification system has made the process more efficient [57]. KOC-linked DNA markers were not used during the development of CHOI4 because the genetic basis of KOC is complex,with more than 70 loci throughout the genome[58]. As compared with HIR, which is controlled by a few major QTL, traits like KOC are more suitable for genome-wide selection.By developing CHOI4 with a higher KOC, the efficiency and accuracy of haploid identification were further improved as compared with CHOI3 [26]. Even under the present conditions, further improvement of the KOC of inducers is expected, as higher KOC levels improve the throughput of haploid identification by shortening the time of KOC determination,making automatic haploid kernel identification more accurate.
In conclusion,we have used MAS to develop the haploid inducer line CHOI4, which has both a high KOC and a high HIR. We have developed a new phenotyping strategy for haploid inducer line breeding. The efficiency of both induction and identification of haploids can be improved using CHOI4,which represents a genetic resource with high application potential in DH breeding.
CRediT authorship contribution statement
Chenxu Liu:Investigation, Data curation, Writing - review &editing.Jinlong Li:Investigation, Data curation, Writing - review& editing.Ming Chen:Investigation.Wei Li:Investigation.Yu Zhong:Investigation.Xin Dong:Investigation.Xiaowei Xu:Investigation.Chen Chen:Investigation.Xiaolong Tian:Investigation.Shaojiang Chen:Conceptualization, Funding acquisition, Project administration, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (2016YFD0101200), the China Agriculture Research System (CARS-02), 2020 Research Program of Sanya Yazhou Bay Science and Technology City (SKJC-2020-02-03), and the National Natural Science Foundation of China(91935303, 32001554).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.07.009.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet

