Three co-located resistance genes confer resistance to leaf rust and stripe rust in wheat variety Borlaug 100
Bowi Y, Rvi P. Singh, Chn Yun, Dmi Liu, Mnp S. Rnhw, Julio Hurt-Espino,Srihr Bhvni, Evns Lguh, Cixi Ln,*
a College of Plant Science & Technology, Huazhong Agricultural University, Wuhan 430070, Hubei, China
b International Maize and Wheat Improvement Center (CIMMYT), Apdo. Postal 6-641, 06600 Mexico D.F., Mexico
c Qinghai Provincial Key Laboratory of Crop Molecular Breeding, Northwest Institute of Plateau Biology, Chinese Academy of Science, Xining 810008, Qinghai, China
d Campo Experimental Valle de México INIFAP, Apdo. Postal 10, 56230, Chapingo, Edo. de Mexico, Mexico
e CSIRO Agriculture & Food, GPO Box 1600, Canberra, ACT 2601, Australia
Keywords:Co-located resistance loci Common wheat Gene interaction Puccinia triticina Puccinia striiformis Triticum aestivum
ABSTRACT Leaf rust(LR)and stripe rust(YR)are important diseases in wheat producing areas worldwide and cause severe yield losses under favorable environmental conditions when susceptible varieties are grown. We determined the genetic basis of resistance to LR and YR in variety Borlaug 100 by developing and phenotyping a population of 198 F6 recombinant inbred lines derived from a cross with the susceptible parent Apav#1. LR and YR phenotyping were conducted for 4 and 3 seasons, respectively, at CIMMYT research stations in Mexico under artificial epidemics.Mendelian segregation analyses indicated that 3–5 LR and 2 YR genes conferred resistance in Borlaug 100.Lr46/Yr29(1BL),Yr17(2AS)and Yr30(3BS)were present in the resistant parent and segregated in the RIL population based on characterization by molecular markers linked to these genes. When present alone, Lr46/Yr29 caused average 13% and 16% reductions in LR and YR severities,respectively,in RILs.Similarly,Yr17 and Yr30 reduced YR severities by 57%and 11%,respectively. The Yr30 and the Yr17 translocation were also associated with 27% and 14% reductions, respectively, in LR severity, indicating that the 3BS and 2AS chromosomal regions likely carry new slow rusting LR resistance genes, temporarily designated as LrB1 and LrB2, respectively. Additive effects of Yr30*Yr17, Yr29*Yr17 and Yr29*Yr30 on YR and LR were significant and reduced YR severities by 56%,55%,and 45%,respectively,and LR severities by 34%,40%,and 45%,respectively.Furthermore,interaction between the three genes was also significant, with mean reductions of 70% for YR and 54% for LR severities. Borlaug 100, or any one of the 21 lines with variable agronomic traits but carrying all three colocated resistance loci, can be used as resistance sources in wheat breeding programs.
1. Introduction
Leaf rust (LR) and stripe rust (YR) are fungal wheat diseases caused by Puccinia triticina (Pt) and P. striiformis f. sp. tritici (Pst),respectively.Rusts are known to occur more frequently than other wheat diseases and pose a serious threat to wheat production worldwide. Beddow et al. [1] estimated that about 88% of world wheat production could be affected by YR due to favorable climatic conditions with expected annual production losses estimated around 5.4 million tons. LR is the most widespread and common disease of wheat and can cause yield losses of up to 70%in susceptible varieties[2].Therefore,improving rust resistance is an important objective for wheat breeding programs worldwide because growing resistant varieties is the most economical, effective, and environmentally friendly strategy to manage these diseases.
Resistance to rusts in wheat can be grouped in two broad categories based on the phenotypic expression at different stages of growth. Seedling or all stage resistance is often differentially expressed and is commonly referred as race specific resistance[3]. This type of resistance usually exhibits various degrees of hypersensitivity. Race specific resistance often fails within 3–5 years of deployment due to selection of virulent variants [4].Another type of resistance, often called as adult plant resistance(APR)is effective at post-seedling or adult plant stages and is usually manifested by a slow disease progression (slow rusting)despite a susceptible host reaction. However, some race specific adult plant resistance genes are also known. Effective levels of the slow rusting type of resistance are more commonly controlled by a small number of minor genes with additive effects [5,6] and some have pleiotropic effects in conferring resistance to other diseases [7,8]. However, the number of such resistance genes identified to date is limited, and the interactions among them are inadequately studied. Therefore, it is important to identify new APR genes and to elucidate their gene interactions for optimal utilization in wheat breeding programs aimed at developing varieties with durable resistance.
At present,about 100 LR resistance genes have been characterized in wheat[9].Most of them are seedling resistance genes,however three pleiotropic genes Yr18/Lr34/Sr57/Pm38/Ltn1 [10], Yr29/Lr46/Sr58/Pm39/Ltn2 [11] and Yr46/Lr67/Sr55/Pm46/Ltn3 [12], as well as Lr68[13],Lr74[14],Lr75[15],Lr77[16],and Lr78[17]confer APR to LR.Likewise,83 YR resistance genes have been formally cataloged and some of them show slow-rusting APR, such as Yr16,Yr30,Yr36,Yr39,Yr52,Yr59,Yr62,Yr68,Yr71,Yr75,Yr77,Yr78,Yr79,Yr80,and Yr82[18–22].Closely linked or gene specific markers for Lr34/Yr18, Lr46/Yr29, Lr67/Yr46, Yr30, and Lr68 have been developed, such as csLv34/cssfr1-cssfr5 [23,24], csLv46G22/csLv46 [25],TM4/TM10 [26], csSr2/Xgwm533 [27,28] and cs7BLNRR/csGS [13],respectively,that are commonly used in marker-assisted selection.
Wheat varieties may have improved resistance when they carry multiple resistance genes. Singh et al. [29] found the complementary genes interaction between Lr27 and Lr31,indicating that resistance was only expressed when seedling resistance genes Lr27 and Lr31 appeared simultaneously in wheat such as in the chromosome substitution line‘‘Chinese Spring(Hope 3B)”and Australian wheat cultivar Gatcher.Klymiuk et al.[30]reported that Pst race DK92/02 was virulent on Avocet + Yr15 (IT = 5–7), and AU85569 was virulent on Avocet + Yr5 (IT = 7), while pyramiding Yr15 with Yr5 in four different backgrounds (YecoraRojoYr5Yr15, PatwinYr5Yr15,SummitYr5Yr15, and DirkwinYr5Yr15) showed full protection against both virulent isolates. Zheng et al. [31] revealed that combinations of Yr17+Yr26 and Yr9+Yr18 conferring significant resistance to Pst the field trails.Singh et al.[32,33]found that varieties with resistance gene Lr34/Yr18 in combination with 2 to 4 additional minor effect genes displayed reduced LR and YR severities under high inoculum pressure. Silva et al. [34] found that the Lr34 + Lr68 + Sr2 gene combination significantly reduced the area under the disease progress curve (AUDPC) for LR by 70% in the genetic background of Parula. Ponce-Molina et al. [35] reported that interaction between Lr46/Yr29 and Lr67/Yr46 reduced disease severity by 11%for and 5%for YR.Liu et al.[36]reported significant additive effects between gene combinations Yr26 + Yr48,Yr30+Yr64 and Yr30+Yr48.Therefore,the interaction of resistance genes in different combinations is not only important for enhanced resistance but also maintains genetic diversity in achieving stable,long-term resistance in wheat varieties.
The objectives of the present study were to: i) understand the genetic basis of LR and YR resistance in a Apav#1 × Borlaug 100 mapping population; ii) identify known and possible unknown resistance genes in Borlaug 100 that contribute to high levels of adult plant resistance to LR and YR; and iii) explore interaction effects among the identified resistance genes.
2. Materials and methods
2.1. Plant materials
We developed 198 recombinant inbred lines(RILs)from a cross between Apav#1 (CIMMYT GID 1854090, pedigree: Avocet-YrA/Pavon) and Borlaug 100 (CIMMYT name: Reedling#1, GID 7806808, pedigree: Rolf 07/4/Bow/Nkt//Cbrd/3/Cbrd/5/Fret2/Tuku ru//Fret2).Apav#1 is susceptible to LR and YR at both the seedling and adult plant stages.In contrast,Borlaug 100 showed high levels of resistance to both diseases at the adult plant stage, but susceptible and intermediate host reactions, respectively, at the seedling stage.Borlaug 100 showed 9%higher grain yield than other widely grown varieties, including its parent Roelfs F2007, good bread quality,heat and drought tolerance,and expressed good resistance to wheat blast when tested in Bolivia and Bangladesh. Because of these attributes it was also released in Australia,Bangladesh,Bolivia,and Nepal under the names Borlaug 100,WMRI#3,INIAF Tropical, and Borlaug 2020, respectively.
2.2. Field trials
The RIL population and the parents were grown and phenotyped for LR and YR response at CIMMYT research stations at Ciudad Obregon in the Yaqui Valley, and Toluca and El Batan in the Central Mexican highlands.Specifically,the population was evaluated for APR to YR at El Batan during the 2016 growing season(YR2016B) and at Toluca during the 2016 and 2017 seasons(YR2016T and YR2017T). LR assessments were made at Obregon during 2015–2016 and 2016–2017 (LR2016Y and 2017Y) and El Batan during the 2016 and 2017 (LR2016B and LR2017B) growing seasons. The high yielding, irrigated environment in Obregon,located at 28°N latitude, 39 m above sea level, is appropriate for LR phenotyping due to cool nights with good dews and warm days.The Toluca research station is located at 18°N latitude and 2640 m above sea level,receives about 800 mm of rain during the crop season and experiences cool nights that together provide a good environment for YR development.Similarly,El Batan is situated at 18°N latitude and 2200 m above sea level with about 400 mm rainfall and has conducive temperatures for LR development; however,YR can also occur if the cooler temperatures prevail for extended periods during the tillering to heading stages.
2.3. Field trials and inoculation methods
The parents and RILs were planted in 0.7 m paired rows on 80 cm wide raised beds at about 60 seeds in each plot. In the LR field experiment, spreaders of susceptible line Avocet + Yr24/Yr26 was sown on one side of each plot as clumps in the middle of the 0.3 m pathways and around the experimental block.Equal proportions of the urediniospores of Pt races MBJ/SP and MCJ/SP were suspended in lightweight mineral oil (Soltrol 170R) and was sprayed on the spreaders about 6 weeks after sowing; and the same procedure was repeated over three consecutive days. The avirulence/virulence formula of MBJ/SP and MCJ/SP was described by Herrera-Foessel et al.[13].A similar field design was used for YR testing. A mixture of six susceptible wheat lines derived from the Avocet/Attila, Morocco, and near-isogenic line Avocet + Yr31 were used as YR spreaders. Pst race Mex08.13 was sprayed on the YR spreaders within and around the test areas about 4 weeks after sowing and repeated thrice. The avirulence/virulence formula for Mex08.13 is given in Lan et al. [37].
2.4. Disease evaluation
The disease evaluations on flag leaves were carried out visually and included disease severity (DS) based on the modified Cobb Scale [38] and host reaction. The first disease data were recorded when the susceptible parent Apav#1 displayed around 80% severity,whereas second scores were made one week later.The final DS was used in all analysis. The host reactions followed the description given in Roelfs et al. [39], where ‘R’ = highly resistant, or hypersensitive necrotic areas with no or small uredinia/sporulation; ‘MR’ = moderately resistant, or necrotic areas with medium sized uredinia/moderate sporulation; ‘MS’ = moderately susceptible,medium sized uredinia/moderate sporulation without chlorosis/necrosis; ‘S’ = susceptible, large size uredinia/profusely sporulating areas without chlorosis/necrosis; and various combinations of the above.
2.5. Molecular marker analysis
DNA from parents and 198 RILs were extracted using the CTAB method [25]. Functional/closely-linked molecular markers for 8 leaf rust (Lr1, Lr9, Lr10, Lr16, Lr19, Lr21, Lr26, and Lr46) and 11 stripe rust (Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26, YrSP, Yr29, Yr30,and Yr46) resistance genes (Table S1), were used for parental screening. Three polymorphic markers, csLV46G22, gwm533, and VENTRIUP/LN2, were then used for genotyping of the entire RIL population. A standard polymerase chain reaction (PCR) was performed following Dreisigacker et al.[25].A 10 μL system was used for PCR amplification,including 5 μL 2×Taq PCR Mix,2 μL 1 μmol L-1primer and 3 μL DNA.The PCR amplification procedure was as follows: pre-denaturation at 94 °C for 5 min; denaturing at 94 °C for 1 min;annealing at 50–66°C for 1 min(the temperature determined by each primer);extension at 72°C for 2 min,a total of 30–35 cycles;finally,extension for 10 min at 72°C and preservation at 4°C. Amplified products were detected by 1.5%–3.0% agarose gel electrophoresis. Cleaved amplified polymorphism (CAP) was used with csLV46G22, and amplification products were digested with BspeI endonuclease(37°C,1 h)before detection by electrophoresis.KASP markers were assayed by RT-PCR.
2.6. Genetic and statistical analysis
The SAS PROC CORR program was used to calculate Pearson’s correlation coefficients between final disease severities in tested environments. The number of resistance genes was estimated using the F6phenotypic segregation ratio by the Mendelian segregation[40]. The 198 RILs were grouped into three phenotypic categories based on DS and host reaction, including homozygous parental type resistant(HPTR)that showed a similar or lower phenotype compared to the resistant parent, homozygous parental type susceptible(HPTS),that showed a similar or higher phenotype compared to the susceptible parent,and the remaining RILs formed the‘‘Others”category[40].These results were then compared with the expected frequencies from Mendelian segregation to determine the number of resistance genes. The χ2analysis was performed using the ‘‘CHITEST” function in Microsoft Excel. The SAS PROC GLM program was used to test the interaction between LR and YR resistance loci.
3. Results
3.1. Phenotypic analysis
The final LR severity (FDS) and reaction of the susceptible parent Apav#1 were 90%–100% S across four environments whereas resistant parent Borlaug 100 displayed 0–1% MS (Fig. S1A) in all test environments. The mean LR severities of the population ranged from 20.9%to 37.7%(Fig.S1A).The DS of RILs showed a continuous distribution (Fig. S1A) across four environments, indicating quantitative inheritance for LR.
The FDS and host reaction of Borlaug 100 were 1%–20%MS over three YR test environments,whereas Apav#1 showed 90%–100%S.The average DS for the entire population was 33.9% to 44.7%(Fig. S1B). FDS for YR was also continuously distributed indicating the presence of multiple resistance genes.
The correlation coefficients between disease severities of RILs across the four LR environments ranged from 0.78 to 0.86(Table 1),and from 0.70 to 0.91 for the three YR environments (Table 1).There were significant correlations ranging from 0.37 to 0.81 between LR and YR severities(Table 1).These relatively high correlation coefficients between LR and YR severities indicated the presence of segregating pleiotropic resistance genes in the population.
3.2. Estimation of gene numbers
The Mendelian genetic analyses comparing the observed and expected frequencies of RILs in the three phenotypic categories suggested the segregation of 3 to 5 LR resistance genes with additive effects in the four environments(Table 2).For YR,the observed frequencies of RILs in the three phenotypic categories conformed to an expected segregation of two resistance genes showing additive effects in all three environments (Table 2).
3.3. Known resistance gene analysis
The closely linked molecular marker csLV46G22 confirmed the presence of the pleiotropic resistance gene Lr46/Yr29 in Borlaug 100 and permitted classification of RILs for the presence and absence of this gene. The LR severity of RILs ranged from 10%to 90% when Lr46 was absent, whereas severity ranged from 1%to 70% when present (Fig. 1A). The mean final LR severity of RILs containing Lr46 was significantly reduced by an average of 17%compared to the non-Lr46 RILs (Table 3). LR severity was 1%–80% for RILs carrying the Yr30 gene from Borlaug 100 based on molecular marker gwm533, and 5%–90% in RILs without the gene(Fig. 1B). For the introgressed segment containing Yr17, also derived from Borlaug 100, the LR severity of RILs with and without the 2NS segment was 1%–70% and 5%–90%, respectively(Fig. 1C). The significant reductions in LR severities in RILs due to the presence of Yr30 and Yr17 were 17.2% and 18.3%, respectively, indicating that these genes or gene regions, conferred slow rusting resistance to LR (Table 3). We therefore provisionally designated the respective slow rusting leaf rust resistance genes as LrB1 and LrB2.
For YR, the closely linked molecular markers for resistance genes Yr29, Yr30 and Yr17 identified their presence in Borlaug 100 and RILs were also characterized for their presence or absence. Single marker analysis showed a significant correlation between these genes and YR severity reductions (Table 3). YR FDS was 1% to 90% and 10% to 90% for RILs containing and lacking Yr29, respectively (Fig. 1D). On average, RILs carrying only Yr29 showed significantly lower disease severity than RILs without it(Table 3).For the APR gene Yr30, the mean YR severity of RILs carrying this gene was 15.4% less than the RILs without it (Table 3;Fig. 1E). RILs with gene Yr17, showed mean FDS ranging from 1%to 80% whereas those without it displayed 10%–90% FDS (Fig. 1F).The disease severity for RILs carrying the introgressed Yr17 segment was significantly reduced by 32.7% compared with the RILs without it (Table 3).
3.4. Interaction between detected resistance genes
The mean severities of RILs carrying combinations of at least two resistance genes were significantly lower than the mean severities of RILs carrying only one of them.Based on combined data,the gene combinations Yr30*Yr17, Yr29*Yr17 and Yr29*Yr30 had a significant effect on YR response with reductions in YR severity by 56%, 55%, and 45%, respectively (Table 4). However, no significant interaction on YR was detected among three resistance genes compared with the two genes combinations (Table S2). The mean YR FDS of RILs pyramided with all three genes was reduced to 5.7%,i.e. approached a near-immune resistance level (Fig. 2). For LR,highly significant interactions occurred between Yr30,or the provisionally designated gene LrB1, and the other two genes as well as among all three of them (Table S2). Similarly, the combination of Yr30/LrB1*Yr17/LrB2, Lr46/Yr29*Yr17/LrB2 and Lr46/Yr29*Yr30/LrB1 reduced LR severity by 34%, 40%, and 45%, respectively (Table 4).Once again, the mean LR FDS was <5% in 21 RILs that possessed all three resistance genes (Fig. 2).
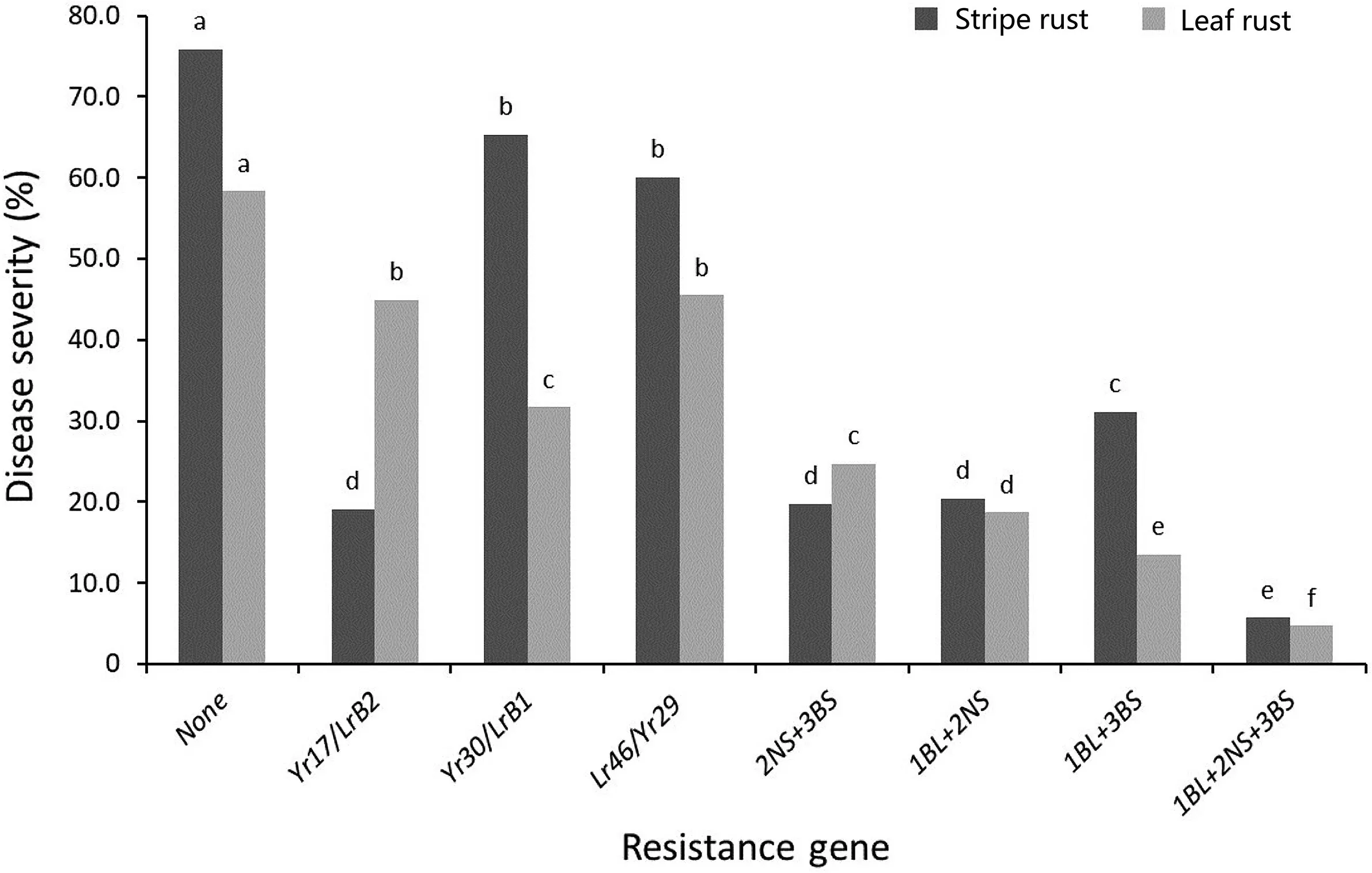
Fig. 2. Additive interaction between detected resistance gene Lr46/Yr29 (1BL), LrB1/Yr30 (3BS) and LrB2/Yr17 (2AS) and respective mean diseases severities in the Apav#1 × Borlaug 100 RIL population.
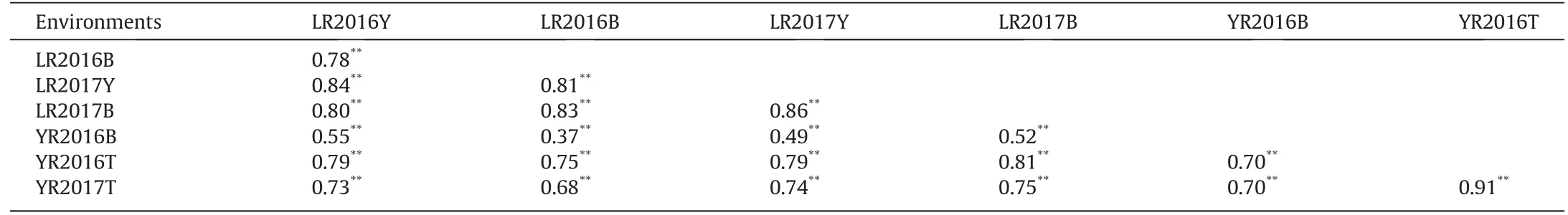
Table 1 Phenotypic correlations between leaf rust(LR)and stripe rust (YR)disease severities in 4 leaf rust and 3 stripe rust environments in Apav#1 ×Borlaug 100 recombinant inbred lines population.
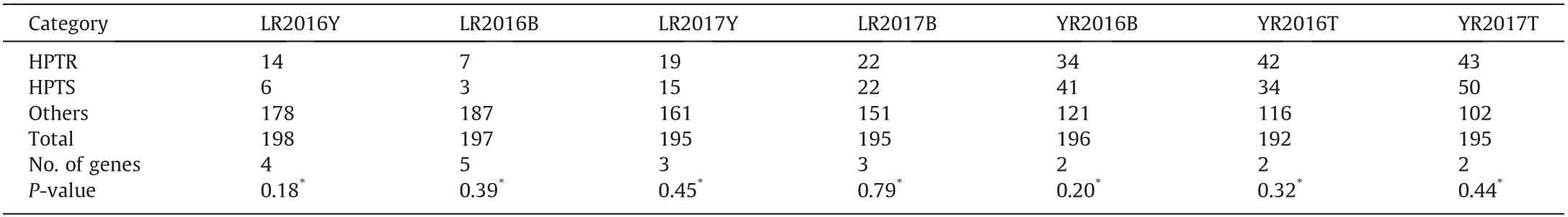
Table 2 The number of resistance genes that confer adult plant resistance to leaf rust and stripe rust calculated by Mendelian segregation ratios in Apav#1 × Borlaug 100 recombinant inbred lines population.

Table 3 The final mean leaf rust and stripe rust severities for Apav#1×Borlaug 100 recombinant inbred lines(RILs)possessing and lacking resistance genes Lr46/Yr29,LrB1/Yr30 and LrB2/Yr17 determined through respective molecular marker analysis and their comparison using t-tests.

Table 4 Mean leaf rust and stripe rust severities of Apav#1 × Borlaug 100 RILs with and without resistance genes Lr46/Yr29, LrB1/Yr30 and LrB2/Yr17 in the Apav#1/Borlaug 100 population.
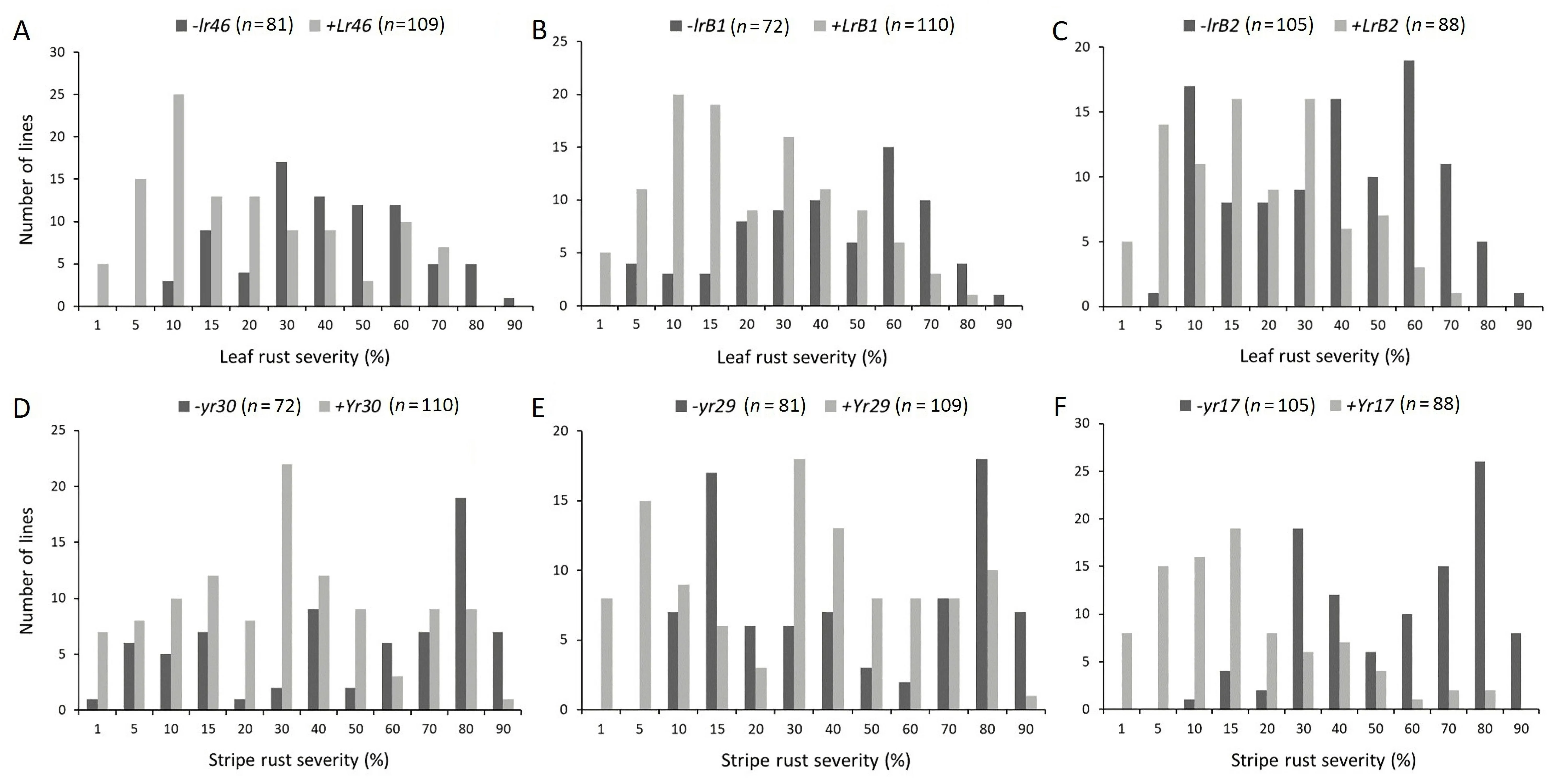
Fig.1. Comparison for the mean leaf rust and stripe rust severities in the presence and absence of individual resistance genes in Apav#1×Borlaug 100 recombinant inbred lines(RILs)population.(A)Leaf rust severities for RILs with and without Lr46.(B)Leaf rust severities for RILs with and without LrB1.(C)Leaf rust severities for RILs with and without LrB2. (D) Stripe rust severities for RILs with and without Yr30. (E) Stripe rust severities for RILs with and without Yr29. (F) Stripe rust severities for RILs with and without Yr17.
4. Discussion
YR and LR are devastating wheat diseases and occur worldwide.They often weaken the growth and development of wheat plants resulting in grain shriveling,and may lead to no harvest in serious cases [41]. CIMMYT plays an important role in mitigating the threat of these diseases by developing and sharing high yielding,stress tolerant and disease resistant wheat germplasm withpartners worldwide.This improved germplasm is the major source of new wheat varieties in Asia,Africa,and Latin America.Since the 1970s, CIMMYT has pioneered research on APR to rusts, and successfully applied the strategy to build durable resistance in varieties that has remained effective for over 50 years [42]. Borlaug 100,the variety used in this study,showed high levels of resistance to LR and YR in field trials under Mexican environments despite being susceptible to LR and showing only moderately resistant to YR in the seedling stage. The distribution of Apav#1 × Borlaug 100 RILs was continuous for both LR and YR severities in all trials.Mendelian analyses of the observed phenotypic frequencies of RILs in the three categories indicated that a minimum of three to five genes with additive effects could be involved in LR resistance and two genes conferred YR resistance. RILs were genotyped with three polymorphic molecular markers known to be closely linked to resistance genes Lr46/Yr29, Yr17 and Yr30 to determine their contribution to resistance.
The pleiotropic,slow rusting resistance gene Lr46/Yr29 plays an important role in wheat varieties with durable resistance to LR and YR and has remained effective for over 40 years. This gene conferred resistance in various mapping populations and under different experimental conditions [43–45]. For example, Lillemo et al. [46] reported that Lr46/Yr29 reduced the severity of LR and YR by 78% and 24%, respectively, and Lan et al. [47] found that Lr46/Yr29 had similar effects on LR and YR responses with 40%and 20% reductions in disease severity, when Lr46/Yr29 alone was segregating in a population. The presence of Lr46/Yr29 in our RIL population on reduced average LR and YR severities by 13%and 16%, respectively.
Yr30 on chromosome 3BS is closely linked/pleiotropic to stem rust (SR) resistance gene Sr2 and linked to LR gene Lr27. Crossa et al. [48] reported that most CIMMYT spring wheat varieties carrying Sr2 showed moderate resistance to YR in multiple environments. In our population, Yr30 reduced mean YR severity by 7.2%and LR severity by 17.2%indicating either that Yr30 had pleiotropic effect on LR, or that a co-located slow rusting gene conferred LR resistance. We provisionally designated the LR gene as LrB1. The combination of LrB1 and Lr46 reduced disease severity by 45%,indicating the importance of pyramiding them to achieve higher resistance levels.Basnet et al.[49]also detected the same additive effect in a segregating Avocet×Quaiu#3 population.The complementary seedling resistance gene combination,Lr27+Lr31,confers LR resistance to avirulent races, but Pt races MBJ/SP and MCJ/SP used in our study were virulent on plants with this gene combination in both seedling and adult plants. The Mexican cultivar Jupateco 73S, known to possess Lr27 + Lr31, is highly susceptible to these races in field trials. The susceptible seedling reaction of Borlaug 100 further demonstrates that the slow rusting resistance attributed to Yr30 was not due to Lr27 + Lr31. Ingala et al. [50]found an APR gene LrSV2, which is also located on chromosome 3BS, and is closely linked to SSR marker gwm533 [51]. In addition,the high-resolution mapping of Lr27 and LrSV2 showed that both genes were in adjacent intervals in chromosome 3BS [52]. Therefore, it is necessary to perform allelism tests between Lr27, LrB1 and LrSV2 to confirm their relationship,however we postulate that the slow rusting resistance to stem rust, YR and LR conferred by Sr2, Yr30 and LrB1, respectively, is due to the same pleiotropic resistance gene.
Yr17 was reported as a seedling resistance gene to YR showing a high level of resistance[53,54].Milus et al.[55]suggested that the expression of Yr17 resistance at the seedling or adult plant stage varied with the genetic background and environmental conditions,mainly affected by temperature and light intensity. Combining Yr17 with other minor resistance genes can play an effective role in imparting improved resistance levels. For example, the wheat variety ‘Jagger’ has long-lasting resistance due to the combination of Yr17 and the pleiotropic slow rusting resistance gene Yr18/Lr34 in North America[56].Moreover,Yr17 was the most effective resistance genes in the Jagger mapping population explaining 80% of the phenotypic variation. These results are consistent with our finding where the combination of Yr17 and APR genes Yr29 and Yr30 significantly improved the resistance levels to the Pst races used. However, we have observed that the effect of Yr17 is lost to Pst races that carry virulence to it (the infection type (IT) was ‘8′for Avocet + Yr17 near isogeneic line against Pst race Mex08.13 based on ‘0–9 Scale’).
Yr17 was identified in an introgressed segment of Aegilops ventricosa chromosome 2 N in the wheat line VPM1.The translocation also carried genes Lr37 and Sr38[57].The Pt races MBJ/SP and MCJ/SP used in our study are virulent to the race-specific gene Lr37(the IT was 3 + against MBJ/SP and MCJ/SP, respectively, based on 0–4 Scale and the leaf rust severity of NIL-THATCHER-LR37-VPM was 80% against the both races in the adult plant stage in BV2014, El Batan,Mexico),indicating the presence of a new slow rusting gene for leaf rust, provisionally designated as LrB2. Although LrB2 had only small effect in reducing LR severity when present alone, its combination with Lr46 and LrB1 resulted in significant increase in resistance (Table 4).
Our study again shows that pyramiding small to intermediate effect APR genes is important for improving resistance to wheat rusts.Lan et al.[58]found that Lr46/Yr29 and YrF had a significant additive effect on reducing stripe rust disease severity and played an important role in the resistance of wheat variety Francolin#1.Herrera-Foessel et al. [59] reported that wheat line Lalbahadur(Pavon 1B), which was one of the parents of Almop and possessed both Lr46/Yr29 and Yr60 was a superior donor parent for breeding because it displayed a much higher level of resistance in field trials than lines carrying these genes independently.In addition,there is compelling evidence that a high level of resistance or nearimmunity can be achieved through combining multiple minor/intermediate effect APR genes [45,60,61]. The mean LR and YR severities in field trials for the resistant parent Borlaug 100 in our study were 1% and 10%, respectively; and the 21 RILs carrying all three resistance genes showed mean severities of 4.7% for LR and 5.7%for YR(Table 4).Borlaug 100,or selected RILs,can be used as resistance sources for breeding.
CRediT authorship contribution statement
Bowei Ye:Formal analysis, Writing – original draft.Ravi P.Singh:Funding acquisition, Project administration, Writing –review & editing.Chan Yuan:Investigation, Writing - review &editing.Demei Liu:Investigation.Mandeep S. Randhawa:Data curation.Julio Huerta-Espino:Data curation.Sridhar Bhavani:Data curation.Evans Lagudah:Resources.Caixia Lan:Conceptualization, Data curation, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the International Cooperation and Exchange of the National Natural Science Foundation of China(31861143010), Huazhong Agricultural University Scientific &Technological Self-innovation Foundation, Australian Grains Research and Development Corporation (GRDC) with funding to the Australian Cereal Rust Control Program (ACRCP), CGIAR Research Program WHEAT (CRP-WHEAT), the Open Project of Qinghai Provincial Key Laboratory of Crop Molecular Breeding(2021-ZJ-Y05), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA24030102).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.07.004.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet

