The osmolyte-producing endophyte Streptomyces albidoflavus OsiLf-2 induces drought and salt tolerance in rice via a multi-level mechanism
Shuqi Niu, Yn Go, Huixin Zi, Ying Liu, Xunming Liu, Xinqiu Xiong, Qingqing Yo,Ziwei Qin, Ning Chen, Ling Guo, Yunzhu Yng, Peng Qin, Jinzhong Lin, Yonghu Zhu,*
a Hunan Province Key Laboratory of Plant Functional Genomics and Developmental Regulation, College of Biology, Hunan University, Changsha 410008, Hunan, China
b Yuan Longping High-Tech Agriculture Co., Ltd., Changsha 410001, Hunan, China
Keywords:Rice Endophytic actinomycete Osmolytes Salt tolerance
ABSTRACT Drought and salinity are major environmental stresses that impair crop growth and productivity worldwide. Improving drought and salt tolerance of crops with microbial mutualists is an effective and environmentally sound strategy to meet the demands of the ever-growing world population. In the present study,we found that the Streptomyces albidoflavus OsiLf-2,a moderately salt-tolerant endophytic actinomycete,produced abundant osmolytes,including proline,polysaccharides,and ectoine.Inoculation with OsiLf-2 increased the osmotic-adjustment ability of the rice host by increasing the proline content (by 250.3%and 49.4%)and soluble sugar(by 20.9%and 49.4%)in rice under drought and salt conditions, relative to the uninoculated control. OsiLf-2 increased stress responses in the rice host at the physiological and biochemical levels(photosynthesis efficiency,osmolytes and antioxidant content),and the gene level(osmolytes synthesis, stress-responsive and ion-transport related genes), raising rice yields under both greenhouse and saline–alkaline soil conditions. The use of endophytic actinomycetes offers a promising biotechnological approach to developing stress-tolerant plants.
1. Introduction
Plants are exposed to many abiotic stresses, including drought,salinity, high and low temperature, UV radiation, and nutrient scarcity [1]. Among these stressors, drought and salinity are the major ones,limiting the geographical distribution and productivity of crops [2]. Drought causes hyperosmotic stress as the primary signal, while salt stress imposes both hyperosmotic and ionic effects on plants[2,3].Thus,both drought and salinity may induce oxidative damage and membrane lipid peroxidation, leading to metabolic dysfunction in plants [4–6]. In response to the drought and salt stress, plants developed a series of physiological and biochemical regulation to maintain their normal growth, such as changes in the photosynthetic parameters, osmotic adjustment,production of antioxidant molecules, and metabolic modulation[7]. A common plants’ response to drought and salinity is adjustment of osmotic status by production of osmolytes,such as proline and soluble sugar, in plant cells to maintain cell turgor and stabilize plant metabolic processes[7].The accumulation of excessive amounts of reactive oxygen species (ROS) including superoxide radicals, hydroxyl radicals, and hydrogen peroxide (H2O2) [8]may trigger the plant’s detoxification mechanism to scavenge free radicals for combating oxidative damage [9]. Accordingly, antioxidative enzymes including peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT) are activated. In addition, various signaling pathways have been reported to be involved in drought and salt tolerance in plant, including the activation of stressresponsive genes, such as OsLEA3 [10] and OsDREB2A [11].
Rice(Oryza sativa L.),a staple food crop for more than half of the world’s population,is very susceptible to drought and salt stresses owing to its evolutionary origin as a wetland species [12,13]. An estimated 20%to 30%of the world’s rice cultivation land is periodically subject to drought or salt conditions [5], posing a threat to rice production and grain security. To meet the rising global demand for food supply,breeding programs and genetic manipulation have been initiated to develop drought-and salt-tolerant rice.However, these approaches are time-consuming, costly, and limited by the multigenic nature of target traits and difficulty of genetic transformation [14,15].
To establish an effective strategy for increasing drought and salt tolerance in rice, cooperative utilization of beneficial microorganisms for defense against abiotic stresses in plants has received increasing attention for its eco-friendly, costeffective, and sustainable properties [16]. Many rhizosphere microorganisms, well known as Bacillus sp. strains, have been reported to improve drought and salt tolerance in crops such as wheat [17], tomato [18], and cucumber [19]. In comparison with soil-derived microbes, endophytes that reside symbiotically in the internal tissues of plants will more positively influence plant physiological activities [20,21]. They have the potential to mitigate water-deficit or salt-excess growth conditions in plants[22,23]. However, monocots (rice, for example) benefit less than dicots from their endophytes under drought and salt stress [24].By far, the reports of endophyte-mediated alleviation of damage by drought and salt in host rice have focused on endophytic bacteria and fungi, and most of the endophytes mentioned could improve one stress resistance in their host [25]. For example,the increase in the fresh weight of rice Nipponbare (saltsensitive) seedlings by Pantoea alhagi NX-11 was 30.3% under saline conditions [26], and shoot dry weight of rice Caawa(moderately drought-tolerant) seedlings was increased by 8%following inoculating with Piriformospora indica under 15% PEG 6000-simulated drought conditions [27].
Among the beneficial endophytes, actinobacteria, especially Streptomyces, are exciting sources of bioactive metabolites with diverse biological activities [28]. Indeed, some endophytic Streptomyces have been reported to induce systemic tolerance against abiotic stresses in plants. The endophytic S. padanus AOK-30 increased drought resistance of mountain laurel seedlings grown in flasks by structural modification of the host cell wall [29,30],the drought-tolerant endophytes S. coelicolor DE07, S. olivaceus DE10, and S. geysiriensis DE27 increased the growth and yield of wheat via production of phytohormones under drought conditions in both greenhouse and field [31], and the endophyte Streptomyces sp. GMKU 336 increased salt tolerance by secretion of 1-aminocyclopropane-1-carboxylic acid deaminase in rice seedlings in the greenhouse environment [32]. However, to date,there are few reports of the use of Streptomycetes to help host plants resist both drought and salt stress, and field experiments are scanty.
In previous work, we isolated a rice endophyte S. albidoflavus OsiLf-2(hereafter OsiLf-2)that increased the host’s ability to resist biotic stress and produce a lot of secondary metabolites.OsiLf-2 is a halotolerant strain, able to tolerate up to 1000 mmol L-1(about 6%) NaCl [33]. Some plant beneficial microbes increase the tolerance of their host plants to abiotic or biotic challenges [34]. Rhizobacterium Bacillus subtilis GB03 induced salt tolerance in wheat[17]and improved cotton health by suppression of the root pathogen Fusarium spp. [35]. In the rhizosphere, Pseudomonas chlororaphis O6 induced tolerance in Arabidopsis against drought stress[36] and elicited systemic resistance in tobacco to Erwinia carotovora [37]. In view of the intimate interaction of plant and endophytes, we speculated that OsiLf-2 would also influence plant abiotic stress tolerance.
The objective of the present study was to identify the potential benefit and mode of action of OsiLf-2 in a rice host for increasing drought and salt tolerance, using the following approaches: (1) characterizing the growth and osmolyte production of OsiLf-2 under drought and salt stresses, (2) measuring effects on rice drought and salt tolerance resulting from OsiLf-2 inoculation under growth-chamber conditions, (3) measuring effects of OsiLf-2 on rice yield in saline–alkaline soil under field conditions, and (4) investigating the interaction of the OsiLf-2 symbiont with its rice host by physiological, biochemical, and genetic experiments.
2. Materials and methods
2.1. Plant material and microorganisms
Indica rice (Oryza sativa cv. indica 9311, moderately droughtand salt-tolerant)seeds were surface-sterilized as described previously [38]. Streptomyces albidoflavus OsiLf-2 (GenBank accession number NZ_MNPQ00000000.1,China General Microbiological Collection Center accession number CGMCC-11673)was isolated from rice leaves and maintained on potato dextrose agar (PDA)at 30 °C for about 5 days. Spore suspensions were prepared following Gao et al. [33]. Spores of OsiLf-2 matured on PDA were scraped off the medium with a cotton swab that was then dipped in sterile distilled water, and the concentration of the spore suspension was adjusted to 5 × 108spores mL-1using a hemocytometer.
2.2. Determination of osmolytes and growth conditions
OsiLf-2 was cultured in International Streptomyces Project 2(ISP2) [39] broth at 30 °C with shaking (170 r min-1) for 5 days.To mimic drought and salt stresses, 20% (w/v) polyethylene glycol-6000 (PEG 6000) and 150 mmol L-1(0.9%) NaCl was added in ISP2 broth, respectively. PEG 6000 is a water-binding polymer that lowers water potential and is widely used to mimic drought stress [40]. After 5 days of incubation, cell-free culture filtrate(CFC) passed through a 0.22-μm membrane filter was collected for extracellular proline and exopolysaccharide (EPS) quantification. The cultured cells were harvested by centrifugation at 4 °C and 8000×g for 10 min after three wash cycles with 200 mmol L-1PBS (pH 7.0). Intracellular and extracellular proline content was determined by Goodwin’s method [41] with modifications.OsiLf-2 cell pellets or CFC was added 3%sulfosalicylic acid solution for extracting proline in a boiling water bath, after which the extracting was reacted with ninhydrin. The resulting red prolineninhydrin product was salted out and extracted into benzene,and absorbance was measured at 520 nm. Ectoine content was determined following Nagata and Wang [42] with modifications.OsiLf-2 cell pellets were concentrated by freeze-drying and then extracted with 80% ethanol for 1 h. The supernatant was dried at 40°C and then extracted with absolute ethanol:chloroform:ultrapure water (1:1:1) for 20 min. Equal volumes of chloroform and the extraction mixture were combined and shaken for 10 min.Finally, the supernatant was lyophilized and then dissolved in ultrapure water. The extracted ectoine was determined by HPLC with UV detection at 210 nm (Shimadzu, LC-20AT, Tokyo, Japan)using an Inertial NH2column (5 μm, 4.6 × 250 mm) (Shimadzu).
For intracellular polysaccharide extraction, OsiLf-2 cell pellets were treated with ultrasound, extracted with distilled water at 90 °C for 2 h, and centrifuged [43]. The polysaccharide was measured following Bailey[44]using anthrone colorimetry.The extract was dissolved in 1 mL of distilled water and placed in a bath of cold water and agitated with slow addition of anthrone and H2SO4reagent (anthrone was dissolved in 75% H2SO4). The thoroughly mixed solution was transferred to a boiling water bath for 20 min. After cooling, absorbance was determined at 620 nm.
For extraction of EPSs, three volumes of cooled ethanol were added to the CFC and held at 4 °C for 24 h to precipitate EPSs.The pellet was then washed with 80% ethanol and dried thoroughly. The extract was quantified following Bailey [44].
Biofilm was measured following Oliveira et al. [45] using the dye crystal violet. OsiLf-2 was cultured in a 96-well plate with 200 μL ISP2 per well. After 7 days, the medium was removed and the plate was washed,stained with 0.1%crystal violet,and washed again,and the remaining stain was solubilized with 200 μL of 33%acetic acid. Absorbance was measured at 595 nm using 33% acetic acid in water as the blank.
For rice samples, proline content in shoots was determined by the sulfosalicylic acid method following Bates et al. [46]. Soluble sugar content was measured following Bailey [44] with modifications. Briefly, 1 g of fresh shoots were ground thoroughly in water and centrifuged. Then, 1 mL supernatant, 0.5 mL anthrone, and 5 mL H2SO4were mixed slowly and held for 10 min.After cooling,absorbance was determined at 620 nm.
2.3. Drought and salt treatment
In a hydroponic experiment, surface-sterilized rice seeds were coated with a spore suspension of OsiLf-2 or water (both containing 0.3%xanthan gum,a natural adhesive,for attachment of endophytes to the surface of rice seeds) following Gao et al [33]. Seeds were dried overnight and germinated in the dark at 30°C for about 4 days. Seedlings were cultured in a growth chamber with a photoperiod of 14/10 (light/dark) at 30/25 °C day/night temperature and 45% relative humidity, with 500 mL/pot of fresh hydroponic solution (0.3 mmol L-1KH2PO4, 0.35 mmol L-1K2SO4, 1 mmol L-1MgSO4·7H2O, 0.5 mmol L-1Na2SiO3·9H2O, 1 mmol L-1CaCl2-·2H2O, 9 μmol L-1MnCl2·4H2O, 20 μmol L-1H3BO3, 0.77 μmol L-1ZnSO4·7H2O, 0.32 μmol L-1CuSO4·5H2O, 20 μmol L-1NaFeEDTA, and 0.39 μmol L-1Na2MoO4·2H2O, pH 5.5) being replaced every 3 days. After 14 days, rice seedlings were transferred to a hydroponic solution containing 20% PEG 6000 or 150 mmol L-1NaCl and cultured for 7 days.They were then transferred back to normal hydroponic solution and allowed to recover for 7 days. Seedlings with green and healthy young leaves were assigned as having survived, and the survival rate was calculated as the number of surviving seedlings divided by the total number of seedlings.
In the pot experiment, germinated seedlings were planted in pots containing autoclaved soil under growth-chamber conditions.The 1-month-old rice seedlings were sprayed with a spore suspension of OsiLf-2 containing 0.2%(v/v)Tween 20.Control leaves were sprayed with water containing 0.2%(v/v)Tween 20.At the panicle booting stage, the watering of rice was halted or 1 L of water containing 1% NaCl (w/v) was added to the soil, while the control group was irrigated normally with water. After 10 days of treatment, normal irrigation was resumed.
In the field experiment,surface-sterilized rice seeds coated with the strain OsiLf-2 or water (both with 0.3% xanthan gum added)were sown following Gao et al. [33]. The field is located in Wenchang, Hainan, China (YSSRI, 20°2′′N, 110°37′′E). Three replicates per treatment were planted in each field using a block design.The element content of soil samples was measured with an X-ray fluorescence spectrometer (S8 Tiger, Bruker, Karlsruhe, Germany).
2.4. Plant growth parameter measurement
Hydroponic seedlings were collected 24 h post-treatment (hpt)for drought and salt treatments.Dry weight was measured following Cao et al. [47]. Total chlorophyll and carotenoid content were measured following Niu et al. [48]. The uppermost fully expanded rice leaves were used to measure net photosynthetic rate with a LI-6400XTP portable photosynthesis system(Li-COR Inc.,Lincoln,NE,USA). Leaves at the same or similar age and position were chosen,and measurements were made between 9:00 AM and 12:00 AM on days with sunshine. Root growth parameters were determined using the software WinRhizo Prov. 2002c (Régent Instruments,Quebec, Canada) as described by Cao et al. [47].
After rice plants were harvested from the pot experiment and field experiment, agronomic indexes, including effective tiller number, panicle length, thousand-grain weight, and percentage of filled grains were measured.
2.5. Stress-response trait measurements
Rice seedlings from the hydroponic experiment were collected at 24 hpt following drought and salt treatments. H2O2accumulation in rice leaves was measured following Luna et al.[49]. Leaves were stained in 3,3′-diaminobenzidine(DAB)at pH <3 in the dark for 8 h and then boiled in 95% ethanol until all leaves were transparent. A SMZ1000 stereoscope (Nikon Corp. Tokyo, Japan) using Image-Pro plus image analysis software was used to establish the threshold of DAB staining in the leaves and to distinguish the staining from the background. The DAB staining intensities were quantified as the number of stained pixels relative to the pixels corresponding to the leaf. Mean H2O2measurement was based on at least eight typical photographs from 10 randomly selected seedlings in each treatment.
Malondialdehyde (MDA) content was estimated following Rao and Sresty [50]. A 1-g sample of leaves was macerated in 5 mL of 0.1% trichloroacetic acid and the homogenate was centrifuged.Then 1 mL of the supernatant was mixed with 4 mL of 20% TCA containing 0.5%thiobarbituric acid.The resulting mixture was centrifuged, and the absorbance of the supernatant was measured at 532 nm.Leaf relative electrical conductivity was measured following Verslues et al. [51] and Estrada et al. [52] using an electrical conductivity meter (DDS-11A, Yueping, Shanghai, China).
For antioxidant enzyme activity, peroxidase activity was measured following Guo et al.[53].Superoxide dismutase and catalase activities were measured following Lee and Lee [54].
2.6. Determination of Na+ and K+ content
Rice seedlings from the hydroponic experiment were collected at 7 days post-treatment. Ion content was determined with an inductively-coupled plasma atomic emission spectrometer (ICPAES,JY2000-2,Horiba Jobin Yvon,Paris,France)as described previously [47].
2.7. Analysis of gene expression
Rice seedlings from the hydroponic experiment were collected at 12 and 24 hpt following drought and salt treatments. Total RNA was extracted with a Plant Total RNA Extraction Kit (Sigma-Aldrich,Louis,MO,USA)according to the manufacturer’s specifications. Then the RNA was reverse-transcribed into complementary DNA (cDNA) with the PrimScript RT reagent Kit (Takara, Dalian,China) following the manufacturer’s instructions. Reverse transcription quantitative real-time polymerase chain reaction (RTqPCR)was performed using SYBR Premix Ex Taq TM II (Tli RnaseH Plus)(Takara)according to the user’s manual.Primers are listed in Table S1. The expression levels of ectA, ectB, ectC, OsALAD, OsPSY3,OsatpE, OsP5CS1, OsLEA3, OsRab16A and OsDREB2A were determined at 24 hpt, and those for OsSOS1, OsHKT1;5 and OsNHX1 at 12 hpt. The housekeeping gene OsActin was used as an internal quantitative control,and the gene expression level was determined by the 2-ΔΔCT method [26].
After the plants were exposed to stress conditions, endophyte colonization inside the host was quantified by DNA-based qPCR as described previously [55], with slight modification. Total DNA was isolated from rice shoots and roots and extracted with an EZ-10 spin column plant genomic DNA purification kit (Sangon Biotech,Shanghai,China)according to the user’s manual.To detect OsiLf-2 and rice DNAs,specific primers for the OsiLf-2 gene RpoA(a DNA-directed RNA polymerase subunit alpha of OsiLf-2) and rice gene OsUbq (a rice genomic ubiquitin DNA) were used for real-time PCR. Relative OsiLf-2 growth was calculated as a ratio(RpoA/OsUbq)represented by the equation 2Ct(OsUbq)-Ct(RpoA).Primers are listed in Table S1.
2.8. Statistical analysis
All assays were performed with three replicates. Means were compared with ANOVA using SPSS statistical software (version 17.0, SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was used to separate means at a significance level of P <0.05.GraphPad Prism version 6.0 software was used for plotting.
3. Results
3.1. Growth and osmolyte production of OsiLf-2 under drought and salt stresses
OsiLf-2 grew very well under 20%PEG 6000-simulated drought and 150 mmol L-1NaCl-simulated salt conditions (data not shown), indicating that OsiLf-2 had the potential to withstand hypertonic stress. When OsiLf-2 was cultured under drought and salt stress, the content of intracellular proline was significantly increased by 21.6% and 38.4% (Fig. 1A), and that of ectoine was increased 2.1-fold and 167.5-fold (Fig. 1B), respectively, relative to the untreated control. Intracellular polysaccharides accumulated inside OsiLf-2 cells was increased by 38.3% under drought conditions and decreased by 15.8% under saline conditions(Fig. 1C). OsiLf-2 increased the formation of biofilm, a thick layer of prokaryotic organisms composed mainly of EPSs [56], by 42.6%under salt stress (Fig. 1D). This finding indicated that OsiLf-2 protected itself from salt stress by secreting more EPSs(Fig.S1).However, no biofilm was observed under the drought condition.
3.2.Effect of OsiLf-2 on host rice growth and yield under drought and salt conditions
OsiLf-2 inoculation (E+) increased stress adaption and restored the growth of rice facing drought(D+)and salt(S+)stress(Fig.2A).The plant dry weight of E+rice was significantly increased by more than 60.0% relative to non-inoculated (E-) rice (Fig. 2B). Suppression of the development of belowground parts of rice,as measured by root length, surface area, and volume, was observed under D+and S+ stress. But the negative effects were also mitigated in the presence of OsiLf-2(Fig.S2;Table S2).After seven days of recovery from D+ and S+ treatments, the survival rate of E+ rice was much higher than that of E- rice, by 26.3% and 130.1%, respectively(Fig. 2C). Consistently, the relative OsiLf-2 biomass was much higher both in shoot and root in E+ rice than in E- rice seedlings(Fig. S3).
The pot experiment further confirmed the drought and salt tolerance of rice induced by the presence of OisLf-2. As shown in Fig.3,the measured agronomic traits of E+rice at the reproductive stage were all improved. Thousand-grain weight (TGW) in E+ rice was significantly increased by 4.6%and 4.5%after D+and S+treatments, respectively (Fig. 3D).
In the field experiment, the total content of soluble salt in the soil was 1.7 g kg-1, and the electrical conductivity was 411 μS cm-1(Table S3), which were categorized as mildly saline [57].OsiLf-2 inoculation significantly increased rice growth, with tiller number and shoot dry weight of seedling increasing by 16.4%and 15.1%,respectively(Fig.S4).Rice yields also increased,as effective tiller number and TGW were increased by 58.4% and 4.7%,respectively (Fig. 4).
3.3.Contribution of OsiLf-2 to rice tolerance to drought and salt stress
In the present study, soluble sugar and proline content in rice were induced by drought and salt treatment. Inoculation with Osilf-2 increased these osmolyte contents by 20.9% and 49.4% for soluble sugar and by 250.3% and 49.4% for proline under D+ and S+ conditions, respectively (Fig. 5A, B). Correspondingly, the expression levels of OsP5CS1, which encoded a key enzyme in the proline synthesis pathway [58], in E+ rice were significantly induced under D+and S+treatments by more than onefold relative to E-rice(Fig.5).This finding suggested that the higher content of proline in the E+ rice might be resulted from the greater upregulation of this gene under stress conditions. The expressions of ectoine synthesis genes(ectA,ectB,and ectC)of OsiLf-2 in E+rice were stimulated under D+and S+conditions.In particular,expression of the key gene ectC was increased 14.0- and 5.8-fold relative to E- rice under D+ and S+ conditions (Fig. 5 F).
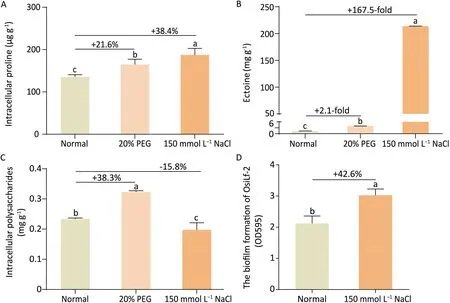
Fig.1. Production of osmolytes by OsiLf-2 under normal,drought(20%PEG-simulated)and salt(150 mmol L-1 NaCl-simulated)conditions.Normal,ISP2 broth;20%PEG,ISP2 broth with 20%(w/v)PEG 6000;150 mmol L-1 NaCl,ISP2 broth with 150 mmol L-1 NaCl.Values are means and bars indicate SEs(n=3).Different letters indicate significant differences among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).

Fig. 2. Effect of OsiLf-2 on rice seedling growth phenotype. (A) A representative photograph of E+ and E- rice at seedling stage, (B) plant dry weight, and (C) survival rate under drought and salt conditions.14-day-old OsiLf-2-inoculated(E+)and non-inoculated(E-)rice seedlings were treated with 20%PEG 6000(Drought) and 150 mmol L-1 NaCl (Salt) for 7 days and then allowed to recover for 7 days, while the control group was irrigated normally with hydroponic solution. After 7 days of stress treatment, 12 plants in each line were taken to measure whole-plant dry weight.Survival rates of rice were measured after 7 days of recovery in 48 plants of each line.Values are means and bars indicate SEs (n = 3). Different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).
Drought and salt stress can severely affect the photosynthetic system[59].Total chlorophyll content,carotenoid content,as well as net leaf photosynthetic rate of both OsiLf-2 treated and nontreated rice seedlings were severely suppressed under D+ and S+treatments.But OsiLf-2 inoculation alleviated these effects significantly(Fig.6).We also analyzed the expression of three photosynthesis associated genes (OsALAD, OsPSY3, and OsatpE) in rice. 5-aminolevulinic acid dehydratase (ALAD) catalyzes the committed step in chlorophyll biosynthesis.ALAD activity and its gene expression were reported [60] to be down-regulated in abiotic-stressed rice and wheat seedlings. Phytoene synthase (PSY) mediates carotenoid biosynthesis and a member of this family,PSY3,was considered [61] to regulate carotenoid flux in response to stress. OsPSY3 transcripts were up-regulated upon drought and salt treatment[61]. ATP synthase plays a vital role in photosynthesis during leaf development and one subunit protein AtpE in rice was suppressed by cold stress at the transcript level [62]. In the present study, all three genes were significantly up-regulated in E+ rice under D+and S+ conditions by 0.5- to 11.9-fold relative to E- rice(Fig.6D–F).These results indicated that OsiLf-2 improved the photosynthetic system of its rice host under stress.
As shown in Fig. 7, D+ and S+ stress induced overproduction of ROS (represented by the H2O2level revealed by DAB staining) in rice, resulting in lipid peroxidation (represented by product MDA accumulation), and consequent cell membrane damage (represented by increased relative electrical conductivity [REC]). However, OsiLf-2 inoculation slowed the accumulation of H2O2by 24 h (Fig. 7A) and reduced the H2O2level by up to 69.1%, REC by up to 64.2%, and MDA content by up to 42.6%, respectively, under the D+and S+conditions(Fig.7B–D).A higher level of antioxidant enzyme activities was observed in E+rice than in E-rice(Fig.7E–G). The activity of CAT was lower in E+ rice than in E- rice under normal conditions,indicating homeostasis in the rice-OsiLf-2 symbiosis. But under the D+ and S+ conditions, the activities of CAT were significantly induced by 15.3% and 55.1% respectively(Fig. 7F). Thus, OsiLf-2 induced the antioxidant stress defense system of the rice host.
OsiLf-2 significantly reduced excess absorption of Na+by 50.0%(Fig.8A and F)and increased K+uptake,resulting in a significantly higher K+/Na+ratio in shoot and root by 190.2%and 818.1%,respectively (Fig. B and G). We further investigated the Na-acquisition process of rice. The transcription of three rice genes (OsSOS1,OsNHX1, and OsHKT1;5) involved respectively in expulsion of Na+[63],Na+compartmentalization[64],and Na+retrieval from xylem[65], were all significantly up-regulated in both E+ rice shoot and root, by 16.3%–506.6% (Fig. 8C–E, H–J).

Fig.3. Effect of OsiLf-2 on rice under drought and salt conditions at the reproductive stage.Watering of rice was halted or 1 L of irrigation water containing 1%NaCl(w/v)was added to the soil,while the control group was irrigated normally with water.(A)After 10 days of treatment,the representative photograph of the E+and E-rice plants was taken,after which normal irrigation was resumed.After plants were harvested,(B)effective tiller number,(C)panicle length,(D)thousand-grain weight,and(E)percentage of filled grains were measured. Values are means and bars indicate SEs (n = 20). Columns with different letters indicate significant difference among treatments at P <0.05(ANOVA and Duncan’s multiple range test).

Fig. 4. Effect of OsiLf-2 on rice under saline–alkaline field conditions. (A) The representative photograph of E+ and E- rice was taken at the maturity stage. (B) Yield of individual plants, (C) thousand-gain weight and (D) effective tiller number were measured. Values are means and bars indicate SEs (n = 30). Columns with different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).

Fig.5. Effect of OsiLf-2 on osmotic adjustment capability of rice under drought and salt conditions.(A)Soluble sugar,(B)proline and(C)expression of OsP5CS1of rice,(D–F)ectA, B and C of OsiLf-2 in rice shoots in the hydroponic experiment were tested at 24 h post-treatment following drought and salt treatments. Values are means and bars indicate SEs (n = 3). Different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).
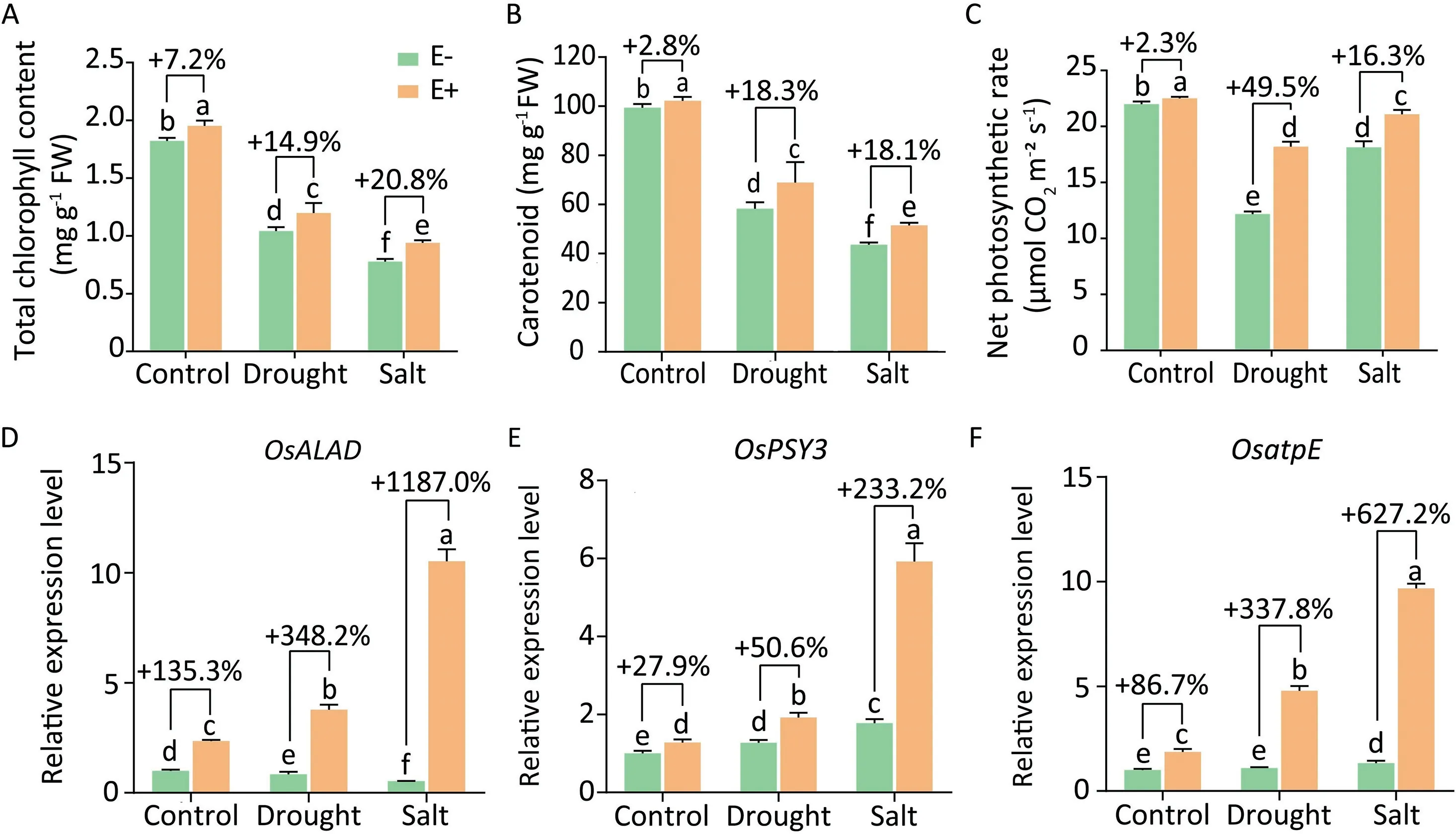
Fig.6. Effect of OsiLf-2 on rice photosynthetic system under drought and salt conditions.(A)Total chlorophyll content,(B)carotenoid content and(C)net photosynthetic rate were measured in leaves of E+and E-rice in a hydroponic experiment,collected at 24 h post-treatment with drought and salt treatments.Values are means and bars indicate SEs(n=6).(D–F)relative expression of genes(OsALAD,OsPSY3 and OsatpE)in rice shoots were measured by RT-qPCR.Values are means and bars indicate SEs(n=3).Columns with different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).
3.4. Stress-responsive gene expression
The expression levels of three genes conferring abiotic stress tolerance (OsLEA3, OsRab16A, and OsDREB2A) in rice seedlings under D+ and S+ conditions were measured. The OsLEA3 [10] and OsRab16A [66] belong to the late embryogenesis abundant gene family, whose members have been linked to abiotic stress responses of plants [67]. OsDREB2A belongs to the dehydrationresponsive element binding (DREB) transcription factors, which also function in stress adaptation in plants [11]. As shown in Fig.9,all of these genes showed markedly higher expression levels under the D+ and S+ conditions, and their induction was much stronger in E+ rice than in E- rice. For example, OsLEA3 in E+ rice was up-regulated by 5.5- and 3.1-fold relative to the E- rice control under D+ and S+ conditions (Fig. 9A).
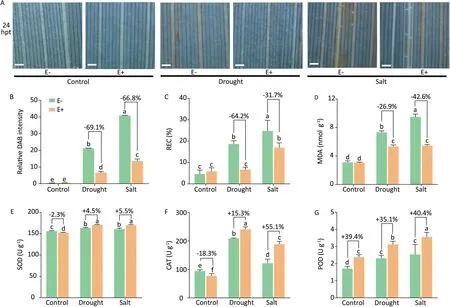
Fig. 7. Effect of OsiLf-2 on the rice antioxidant defense system under drought and salt conditions. At 24 h post-treatment (hpt) following drought and salt treatments, (A)diaminobenzidine(DAB)staining and(B)relative H2O2 intensities for H2O2 in leaves,(C)relative electrical conductivity(REC),(D)leaf malondialdehyde(MDA)content,and(E–G)antioxidant enzyme(SOD,POD,and CAT)activity of rice leaves was measured.Values are means and bars indicate SEs(n=12).Columns with different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).
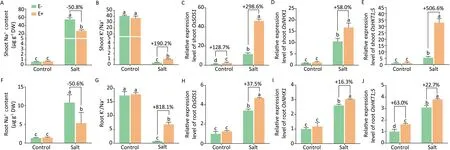
Fig.8. Effect of OsiLf-2 on rice ion homeostasis under saline conditions.(A and F)Na+content and(B and G)K+/Na+ratio under salt stress for 7 days and(C–E and H–J)relative expression levels of transporter genes(OsSOS1,OsNHX1,and OsHKT1;5)recorded by RT-qPCR under salt stress for 12 h were measured.Values are means and bars indicate SEs(n = 3). Columns with different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).
4. Discussion
Endophytes are microbial symbionts residing inside plants.Analogously to gut microflora in human hosts, ndophytes can affect the plant host responses to environmental stress. The present study confirmed the tolerance of rice to both drought and salt induced by an endophytic actinobacterium, OsiLf-2. Although various reports have focused on the effects of endophytes on improving the stress tolerance of host plants at the seedling stage, only a few studies have investigated later growth phases. Because endophytes have not been shown to improve stress tolerance and grain production in field experiments, it was unknown whether their benefits could last for the whole life cycle of host plants [24]. In this study,we investigated the long-term effects of OsiLf-2 on rice under the D+ and S+ conditions in a pot experiment.We also conducted a field trial lasting until the harvest stage for salt-tolerance detection. The results confirmed that endophytic actinomycetes have great potential as stress-mitigation tools for agriculture.

Fig.9. Effect of OsiLf-2 on stress-responsive genes of rice under drought and salt conditions.Relative expression level of(A)OsLEA3,(B)OsRab16A and(C)OsDREB2A by RTqPCR in rice shoots were measured at 24 h post-treatment following drought and salt treatments. Values are means and bars indicate SEs (n = 3). Different letters indicate significant difference among treatments at P <0.05 (ANOVA and Duncan’s multiple range test).
OsiLf-2 increased the growth and yield of host rice under normal conditions,but almost all the increased rate of growth indexes and agronomic traits were lower than those under the stress conditions(Figs.2,S4).This finding indicated that the improved stress resistance was not contributed merely by the growth-promotion potential of OsiLf-2. Plant physiology and biochemistry can be strongly influenced by drought and salt stress. We accordingly investigated the physiological and biochemical responses of the E+ rice to identify the mechanisms by which OsiLf-2 improved the drought and salt tolerance of its rice host.
High drought and salinity can cause similar damage to the plants by imposing osmotic stress [8]. Thus, plants’ response to salinity might share biochemical pathways with drought response,such as the accumulation of osmolytes [7]. Similarly, bacteria also produce osmolytes in response to elevated osmolarity conditions,such as in drought and saline environments [68]. As plant symbionts,endophytes can facilitate osmotic adjustment in host plants by producing osmotic compounds. For example, after inoculation with the endophytic bacterium strain LTYR-11ZT, wheat seedlings showed increased sugars, which could alleviate negative effects of drought stress [69]. The endophytic bacterium B. amyloliquefaciens increased proline content by 25.1% in inoculated rice, reducing osmotic stress under saline conditions [70]. Similarly, in the present study, genomic bioinformatics analysis showed that OsiLf-2 genes were involved in the biosynthesis of compatible osmolytes:proline synthetic genes(proA,proB and proC,and RocD)and ectoine biosynthetic gene cluster. Soluble sugars and proline were accumulated when rice was exposed to drought and salinity.Much higher amounts of soluble sugars and proline were found in E+ than in E- rice under D+ and S+ conditions. In particular, the proline content of E+rice was as high as 2.5-fold greater than that in E- rice under drought stress, and 6.9-fold greater than that of E- rice under normal conditions (Fig. 5B). Correspondingly, the transcript abundance of OsP5CS1, a key gene for proline synthesis,significantly increased more than 1.0-fold in E+ rice compared to E-rice under D+and S+conditions(Fig.5C).This finding indicated that OsiLf-2 increased osmolyte contents by stimulating osmolyte production by the rice host.Under saline conditions,OsiLf-2 had a strong ability to produce EPSs, as indicated by biofilm formation.Hepper [71] reported that EPSs provide a microenvironment that holds water and protects the microbe and host roots against drought.A significant increase in root-adhering soil per root tissue was observed in the sunflower rhizosphere following inoculation with the EPS-producing bacterial strain YAS34 under drought conditions [72]. Inoculation of plants with endophytes that secrete EPSs reduced Na+absorption under salt stress, owing to the polyanionic property of EPSs [26,73]. Thus, EPSs produced by OsiLf-2 might play important roles in stress defense. The mode of action of EPSs of OsiLf-2 deserves further research.
An important feature of the compatible osmolytes is that their beneficial effects are generally not species-specific [74]. That is,the osmolytes secreted by both rice and OsiLf-2 might be supplied and utilized mutually. So, the increased sugar and proline content in rice might also be contributed partly by the strain OsiLf-2, as OsiLf-2 was able to produce more of these osmolytes under D+and S+ conditions in vitro (Figs. 1, S1). This close interaction between OsiLf-2 and rice could also be observed under normal conditions,as the content of soluble sugars in rice increased as high as 1.6-fold under OsiLf-2 inoculation relative to that of the control(Fig. 5A). Sugars are major products of photosynthesis as well as the main raw materials and storage substances for metabolism.So, this result may reflect the fact that OsiLf-2 also promotes the growth of rice seedlings under normal conditions, when rice produces more available carbon resources to meet the growth requirements for an endophytic plant [75].
Another compatible solute is ectoine and its derivative 5-hydroxyectoine [76]. They are synthesized mainly in bacteria,including Streptomyces, in response to osmotic stress. Owing to its superior osmoprotectant properties, ectoine has been widely applied in biotechnological industries for biochemical, medical and cosmetic products [76]. In agriculture, to our knowledge,ectoine has never been considered as a plant-derived product.Ectoine-transgenic tobacco [77] and tomato [78] plants showed improved salt tolerance. The ectoine biosynthetic genes (ectABC)used for the transgenic study were derived from the moderate halophile Halomonas elongata OUT30018.The OsiLf-2 genome contains two ectA and three ectB genes,an ectC,and an ectD gene,and the ectC is the core biosynthetic gene of ectoine. Noteworthily,when comparing with the 38 ectoine-producing strains mentioned in the review of Czech et al.[76],the number of ectoine biosynthesis genes in OsiLf-2 (seven) is greater than in most strains, and barely fewer than the eight in Marinobacter hydrocarbonoclasticus and the nine in S.reticuli.Thus,OsiLf-2 has the potential to produce a high amount of ectoine. Indeed, when the ectoine produced by OsiLf-2 under saline conditions was quantitatively determined,the concentration reached 200 mg ectoine g-1dry cells (Fig. 1B),as much as that in the family of Halomonadaceae which are considered potential candidates for commercial production, with yields of 150–200 mg ectoine g-1dry cells [79,80]. Consistently, the expression level of ectoine synthesis genes of OsiLf-2 was increased up to 26.4-fold in E+ rice in comparison with E- rice under D+ and S+ conditions (Fig. 5D–F). The expression level of ectoine synthesis genes was almost the same in E+ and E- rice under normal conditions. This result indicates that the upregulation of these genes in E+ under stress conditions was due not only to the more OsiLf-2 mass inside the E+ rice. That is, the increases of the transcription of the ectoine biosynthetic genes were osmotically responsive, thereby contributing to the induced cellular ectoine pools in E+rice and play a role in the stress alleviation. In comparison with a transgenic approach, using plantrelated microbes to improve the stress tolerance of a host is much more economical, practical, and environmentally sound.
As the earliest signal induced by drought and salinity, osmotic stress leads to the excessive swelling of chloroplasts, and subsequent impairment of photosynthetic capacity, eventually slowing the plant growth. Thus, osmotic adjustment plays an important role in protecting photosynthesis. In the photosynthetic system,photosynthetic pigments are essential components for lightharvesting, photosynthetic capacity and primary plant production[81]. Our study showed that osmolyte-producing OsiLf-2 inoculation mitigated the decrease in chlorophyll and carotenoid contents as well as the net photosynthetic rate in E+rice seedlings under D+and S+ treatments. The transcript abundance of genes involved in the chlorophyll and carotenoid biosynthesis as well as photosynthetic pathways in rice were all increased up to 11.9-fold in E+rice relative to E- rice under D+ and S+ conditions. This induction between E+and E- rice under stress was much stronger than that under normal conditions (Fig. 6D–F). Thus, in addition to osmotic adjustment, OsiLf-2 stimulated the synthesis of photosynthetic pigments in the rice host to protect the photosynthetic capacity of rice in coping with the stress. Many reports have indicated the positive effects on the photosystem system of the host by endophytes. The endophytic bacteria Pantoea alhagi LTYR-11ZT [69]and P. fluorescens YsS6 [82] increased the chlorophyll content of wheat and tomato under water and salt stress, respectively, and Azospirillum lipoferum FK1 inoculation increased total chlorophyll and carotenoid content in chickpea under salt treatment [83].However, few reports have presented evidence that endophytes might regulate the synthesis of photosynthetic pigments during stress conditions at the transcriptional level.Based on our findings,the signaling cascade of endophytes on the photosynthetic system of host cells deserves further research.
ROS burst is considered to be a primary consequence of photosynthetic impairment. As expected, OsiLf-2 inoculation increased antioxidant enzyme activities under D+and S+conditions,protecting rice.Proline,apart from acting as a compatible osmolyte,is also regarded as a scavenger contributing to ROS scavenging[84].Thus,the increased proline content of E+ rice may also function in the detoxification of ROS, which might explain why proline content and proline biosynthesis related gene expression exhibited at a particularly high level under stress (Fig. 5B, C). Carotenoid is considered [85] an important lipid-soluble antioxidant that can remove ROS. Increased carotenoid production by OsiLf-2 might also participate in ROS scavenging. Thus, our study indicates that the mutualistic relationship between OsiLf-2 and rice could induce an efficient non-enzymatic and enzymatic antioxidant defense system to maintain cell function, contributing to plant resistance to drought or salt stress.
Salt stress is commonly caused by high concentrations of Na+in the environment [86]. Excessive accumulation of Na+by plants results in disrupting the uptake of K+into the plant cell,disturbing K+/Na+balance and ultimately plant metabolism[87].Both osmotic stress and ionic stress lead to damage to the molecular structure and function of the photosynthetic apparatus,and thereby the productivity of plants[88].In our study,OsiLf-2 improved the ion(K+/Na+)balance in rice by Na+exclusion and partition.Combining this approach with the induction of pigment biosynthesis,OsiLf-2 could ensure the high-performance photosynthesis of its rice host under stress. Given that an induced ion transporter gene, NHX1, has also been found[89]to function in acquiring solutes for osmotic water uptake,OsiLf-2 might also alleviate osmotic stress during exposure to salinity by regulating NHX1.
5. Conclusions
The endophytic actinobacterium OsiLf-2 promoted plant growth under drought and salt stress under greenhouse and field conditions. OsiLf-2 functioned as a stress controller in its rice host at multiple interacting levels, including osmotic adjustment,antioxidant production,and pigment synthesis.Its positive regulation countered the negative effects of stress on rice. Endophytes may emerge as a tool for sustaining crop production in droughtand salt-affected soil.
CRediT authorship contribution statement
Shuqi Niu:Methodology,Investigation,Writing-original draft.Yan Gao:Investigation, Methodology.Huixian Zi:Investigation,Methodology.Ying Liu:Investigation, Methodology.Xuanming Liu:Funding acquisition, Supervision.Xianqiu Xiong:Methodology.Qingqing Yao:Investigation, Methodology.Ziwei Qin:Methodology, Writing - review & editing.Ning Chen:Investigation.Liang Guo:Investigation.Yuanzhu Yang:Investigation.Peng Qin:Investigation.Jianzhong Lin:Supervision.Yonghua Zhu:Conceptualization, Funding acquisition, Supervision, Writing -review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (31672093 and 31871595), National Key Research and Development Program of China(2018YFD1000603),Hunan Provincial Important Science and Technology Specific Projects (2018NK1010), and Key Research and Development Project in Hunan Province, China (2019NK2192).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.06.008.
- The Crop Journal的其它文章
- Origin, evolution, and molecular function of DELLA proteins in plants
- Far-red light: A regulator of plant morphology and photosynthetic capacity
- A rice XANTHINE DEHYDROGENASE gene regulates leaf senescence and response to abiotic stresses
- Dissection of heterotic loci for grain yield using interconnected chromosome segment substitution lines in rice
- A soybean NAC homolog contributes to resistance to Phytophthora sojae mediated by dirigent proteins
- The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet

