京尼平保护高糖诱导损伤小鼠胰岛MIN6细胞的机制
徐 晶, 赵正林, 师 岩, 高 涵, 刘哲丞, 武 爽, 朱 谋, 张春晶
(齐齐哈尔医学院 生化教研室,黑龙江, 齐齐哈尔 161006)
2型糖尿病(type 2 diabetes mellitus,T2DM)是一种慢性代谢性疾病,其特点是由综合性胰岛素抵抗引起的高血糖症,胰岛素分泌异常是高血糖的主要原因。在临床治疗中,虽然采用西药或胰岛素直接注射治疗糖尿病可显著降低患者的血糖,但是长期服用药物会增加患者的心血管负担,同时西药副作用也不可忽视[1-3]。保护胰岛细胞功能促进胰岛素分泌是治疗2型糖尿病的关键。因此,研发副作用小且能有效预防并发症的天然药物是改善胰岛素抵抗,恢复胰岛细胞功能,进行有效的干预和治疗受到研究人员的关注。
京尼平(genipin, Gen)是传统中药茜草科植物中栀子果的有效成分京尼平苷,经过β葡萄糖苷酶水解而成的化合物[4-5]。研究表明,京尼平具有多重功效,例如抗氧化应激损伤、保护神经细胞、保肝利胆和抗糖尿病等[6-7]。研究发现,胰腺β细胞长时间处于高葡萄糖环境中,细胞会出现葡萄糖刺激的胰岛素分泌(glucose-stimulated insulin secretion,GSIS)缺陷和细胞凋亡,影响β细胞对胰岛素的释放量,进而影响体内对血糖的吸收和利用[8]。因此,本研究将系统分析京尼平对高葡萄糖培养下的小鼠胰岛MIN6细胞的细胞活力、胰岛素释放量和抗氧化及氧化应激方面的影响,为京尼平在糖尿病预防和治疗方面提供实验依据。
1 材料与方法
1.1 细胞及主要试剂
1.2 仪器
酶标仪(Spark);离心机(eppendorf);LSM 710 激光扫描共聚焦显微镜(ZEISS);凝胶成像系统(BIO-RAD)。
1.3 细胞培养与分组
小鼠MIN6细胞在含10% 胎牛血清的1640培养基中培养,置于37 ℃ 5% CO2细胞培养箱中,按照实验分组培养48 h。实验分组:对照组(normal control, NC),不含葡萄糖1640培养基培养;京尼平组(Gen),1640培养基中加入京尼平,终浓度为10 μmol/L;高糖损伤组(HG),葡萄糖浓度为33 mmol/L;高糖和京尼平组(HG+Gen),葡萄糖浓度为33 mmol/L,京尼平浓度为10 μmol/L。
1.4 CCK-8 检测细胞存活率
将培养的90 μL小鼠胰岛MIN6细胞,以5×104/mL密度的悬液接种在96孔细胞培养板中,置于37 ℃, 5% CO2的细胞培养箱中培养24 h。弃培养基,PBS清洗3次,对照组(normal control, NC)加入10 μL培养基;京尼平组(Gen)加入10 μL京尼平使孔内终浓度为10 μmol/L ;高糖损伤组(HG)加入10 μL葡萄糖,使孔内终浓度为33 mmol/L;高糖和京尼平组(HG+Gen)加入10 μL使孔内葡萄糖终浓度为33 mmol/L,京尼平终浓度为10 μmol/L,设置5个复孔和空白孔(只加培养基),培养48 h。每孔加入10 μL CCK-8试剂,继续孵育2 h后,在450 nm处检测光密度(OD)值,根据如下公式计算细胞存活率。
细胞存活率(%)=(实验孔-空白孔)/(对照孔-空白孔)×100%
1.5 胰岛素分泌检测
将细胞以4.5×105万/孔的密度接种在6孔细胞培养板中,培养24 h。按照上述分组,分别加入葡萄糖和京尼平,使葡萄糖终浓度为33 mmol/L,京尼平终浓度为10 μmol/L,培养48 h,收集上清,短暂离心后弃掉脱落的细胞。按照小鼠胰岛素(insulin)酶联免疫检测试剂盒说明书操作,在450 nm波长处检测光密度值,根据标准曲线计算各组胰岛素释放量。
1.6 ATP含量检测
按照上述实验分组收集细胞沉淀,用PBS清洗1次。按照ATP含量检测试剂盒说明书,将细胞沉淀悬浮在400 μL细胞提取液中,超声破碎1 min(冰浴,200 W,超声2 s,停1 s),10 000 g,4 ℃,离心10 min,收集上清至另1个EP管中,加入500 μL氯仿,漩涡震荡混匀,10 000 g, 4 ℃,离心3 min,收集上清,置于冰上。按照测定要求,在340 nm处分别读取吸光度值A1和A2,根据说明书计算公式,计算各实验组ATP含量。
1.7 ROS水平检测
按照上述实验分组收集细胞沉淀置EP管中,PBS清洗1次。每样加入500 μL培养基配制的浓度为10 μmol/L的DCFH-DA探针,重悬细胞,置于37 ℃, 5% CO2细胞培养箱中30 min,PBS清洗2次。最后,用500 μL PBS重悬细胞置流式管中。流式细胞仪测定荧光强度(fluorescence intensity, FI)。
陈少平:质量是企业和产业核心竞争力的体现。农垦是国有农业经济的骨干和代表,理所当然要在质量兴农、绿色兴农和品牌强农上走在全国农业农村系统的前列。为此,必须立足转变农业发展方式,推进农业供给侧结构性改革,全面提升农业优质化、绿色化、品牌化水平。
1.8 GSH/GSSG比值检测
按照上述实验分组收集细胞沉淀。每个样品加入500 μL试剂盒中的试剂4悬浮细胞沉淀,超声破碎(冰浴,300 W,超声5 s,间隔30 s,4次),按照谷胱甘肽测定试剂盒说明书,测定T-GSH和GSSG含量,并计算GSH/GSSG比值。
1.9 细胞内丙二醛含量,超氧化物歧化酶和过氧化氢酶以及细胞上清液中乳酸脱氢酶活性的测定
按照上述实验分组收集2×106个细胞沉淀,PBS或提取液重悬沉淀超声破碎(冰浴,300 W,超声5 s,间隔30 s,4次)。按试剂盒说明书操作,酶标仪测定各组细胞光密度(A) 值和测定蛋白质浓度,根据说明书换算成细胞内丙二醛(malondialdehyde,MDA )含量、超氧化物歧化酶(superoxide dismutase,SOD) 、过氧化氢酶(catalase,CAT)活性和细胞培养上清液中乳酸脱氢酶(Lactate Dehydrogenase,LDH)活性。
1.10 线粒体膜电位检测
将小鼠胰岛MIN6细胞配置成密度为5.0×104个/mL的细胞悬液,取1 mL接种在20 mm激光共聚焦专用玻璃底培养皿中。按照上述分组,分别加入葡萄糖和京尼平,使葡萄糖终浓度为33 mmol/L,京尼平终浓度为10 μmol/L,培养48 h。加入250 μL JC-1工作液,按照线粒体膜电位(Mitochondrial membrane potential,MMP)检测试剂盒说明书避光染色20 min,弃掉染色液,加入500 μL清理液,应用激光共聚焦显微镜获取图像和细胞荧光强度。
1.11 Western 印迹检测蛋白质表达
将收集的各实验组细胞经蛋白质裂解液冰上裂解15 min,12 000 r/min离心15 min,收集上清液获取总蛋白质。采用考马斯亮蓝蛋白质定量试剂盒进行蛋白质浓度测定,以最低浓度组为标准调整其他各实验组蛋白质的总浓度。以40 μg总蛋白质进行5%浓缩胶、15%分离胶的SDS-PAGE。电泳完毕后,采用湿法将凝胶转至PVDF膜(300 mA,1 h),5%脱脂奶粉封闭2 h。一抗4 ℃过夜、二抗37 ℃孵育2 h,ECL化学发光显色。选用GAPDH为内参照,GR一抗1∶2 000,Grx1一抗1∶250,二抗1∶2 500。结果采用凝胶成像仪扫描并分析条带光密度。
1.12 统计学处理
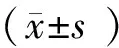
2 结果
2.1 京尼平提高高糖损伤的小鼠胰岛MIN6细胞的活力
小鼠胰岛MIN6细胞在经不同分组培养48 h,各培养组细胞形态饱满、贴壁牢固、伸展清晰,生长的状态无明显差异,结果见Fig.1。但是与对照组(0.97±0.03)比较,京尼平组(0.88±0.08)胰岛细胞活力的差异无统计学意义(P>0.05),高糖损伤组(0.80±0.06)胰岛细胞活力明显下降(P<0.05);与高糖损伤组比较,京尼平(10 μmol/L)作用于高糖损伤的胰岛细胞48 h后,细胞活力(1.00±0.05)显著提高(P<0.05),结果见Fig.2,表明京尼平(10 μmol/L)对高糖损伤的胰岛细胞具有一定的保护作用。
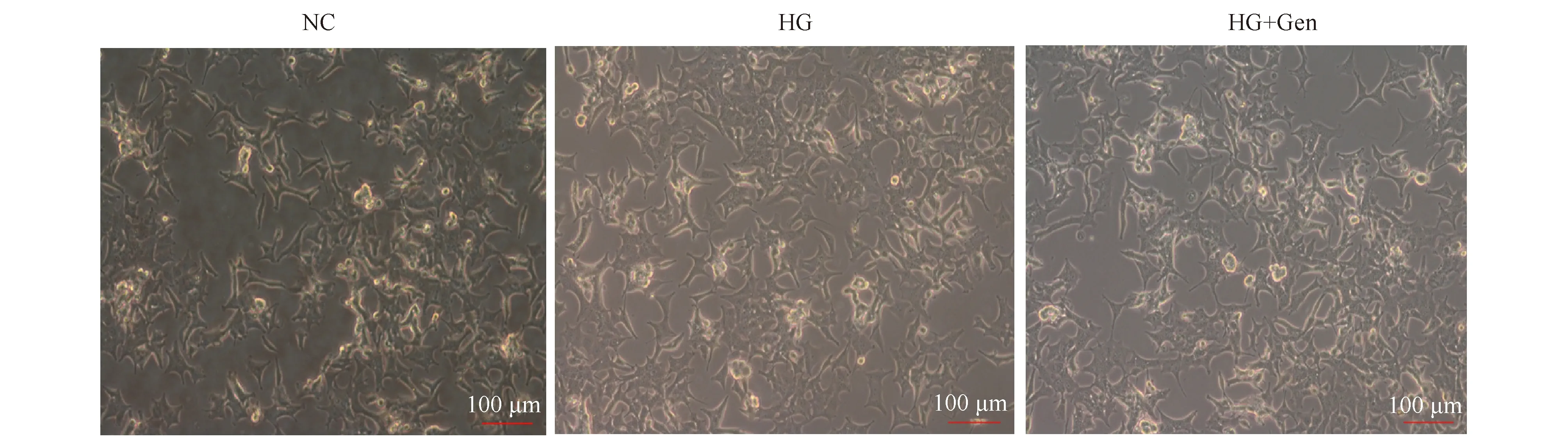
Fig.1 The effects of Genipin on MIN6 cell growth (10×) The control group (NC) was cultured under normal conditions. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG+Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin
Fig.2 CCK-8 assay analysis on the MIN6 cells treated with Genipin The control group (NC) was cultured under normal conditions. The genipin group (Gen) was treated with 10 μmol/L genipin. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG + Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. Data are presented as the Mean ± SD.n = 3.Statistical significances were calculated using Student’s t-test or two-way ANOVA, *P <0.05 compared with NC group; #P <0.05 compared with HG group
2.2 京尼平促进高糖损伤的小鼠胰岛MIN6细胞内胰岛素的释放
与对照组(0.26 ± 0.01 ng/mL)比较,京尼平组(0.24 ± 0.01 ng/mL)MIN6细胞胰岛素释放量的差异无统计学意义(P>0.05),高糖损伤组(0.19 ± 0.01 ng/mL)胰岛素释放量显著下降(P<0.001);与高糖损伤组比较,京尼平(10 μmol/L)作用于高糖损伤组(0.30 ± 0.01 ng/mL)的胰岛素释放量显著提高(P<0.01),结果见Fig.3和Table 1。
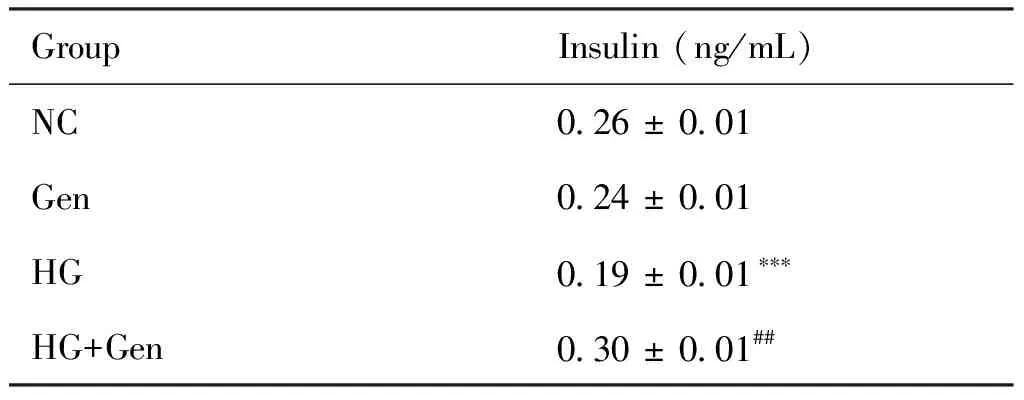
Table 1 The change of the insulin release levels (Mean ± SD, n=3 )
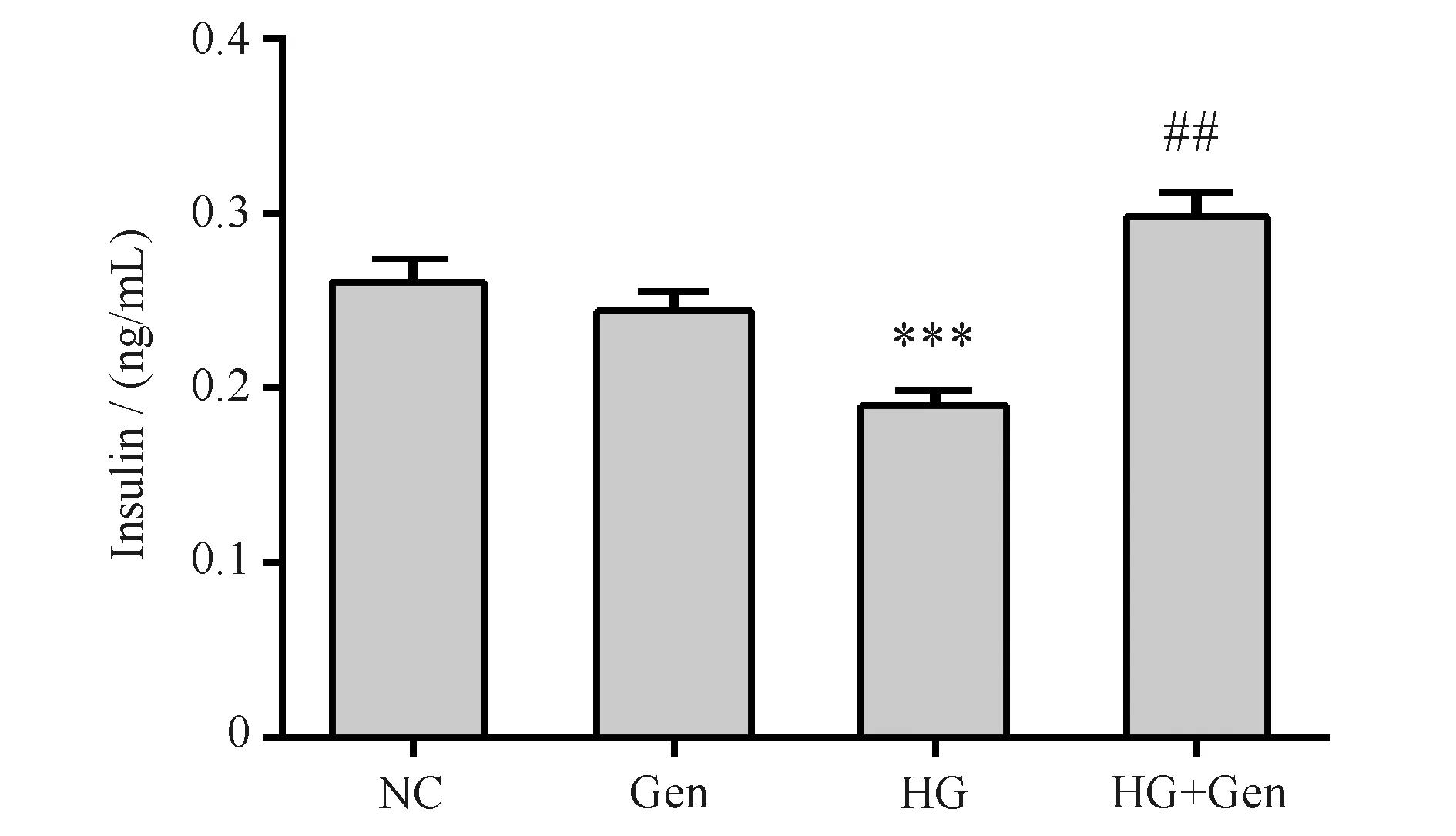
Fig.3 Insulin release assays The control group (NC) was cultured under normal conditions. The genipin group (Gen) was treated with 10 μmol/L genipin. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG + Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. Data are shown as Mean ± SD of three independent experiments. Statistical significances were calculated using Student’s t-test or one-way ANOVA, ***P <0.001 compared with NC group; ##P <0.01 compared with HG group
2.3 京尼平提高高糖诱导下MIN6细胞内ATP的含量
京尼平(10 μmol/L)分别作用于正常细胞和高糖损伤的细胞。与对照组(0.32 ± 0.01 μmol/106cell)比较,京尼平组(0.31 ± 0.01 μmol/106cell)胰岛细胞内ATP含量的差异无统计学意义(P>0.05),高糖损伤组(0.19 ± 0.01 μmol/106cell)细胞内ATP含量显著下降(P<0.001);与高糖损伤组比较,京尼平(10 μmol/L)作用于高糖损伤的胰岛细胞48 h时,细胞内ATP含量(0.25 ± 0.01 μmol/106cell)显著提高(P<0.01),结果见Fig.4。
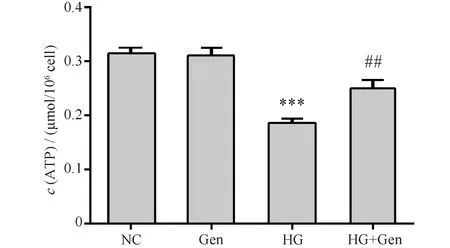
Fig.4 The effects of genipin on ATP levels The control group (NC) was cultured under normal conditions. The genipin group (Gen) was treated with 10 μmol/L genipin. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG + Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. Data are shown as Mean ± SD of three independent experiments. Statistical significances were calculated using Student’s t-test or one-way ANOVA, ***P < 0. 001 compared with NC group; ##P <0.01 compared with HG group
Data are shown as Mean ±SDof three independent experiments. Statistical significances were calculated using Student’st-test or one-way ANOVA,***P<0.001 compared with NC group;
##P<0.01 compared with HG group
2.4 京尼平抑制高糖诱导下MIN6细胞ROS的含量
与对照组(101.73 ± 8.43)比较,高糖损伤组(175.83 ± 9.51)ROS含量明显增加(P< 0. 01),而京尼平组(102.97 ± 8.21)差异无统计学意义(P>0.05);与高糖损伤组比较,京尼平组(144.27 ± 10.70)ROS含量减少(P<0.05),结果见Fig.5。
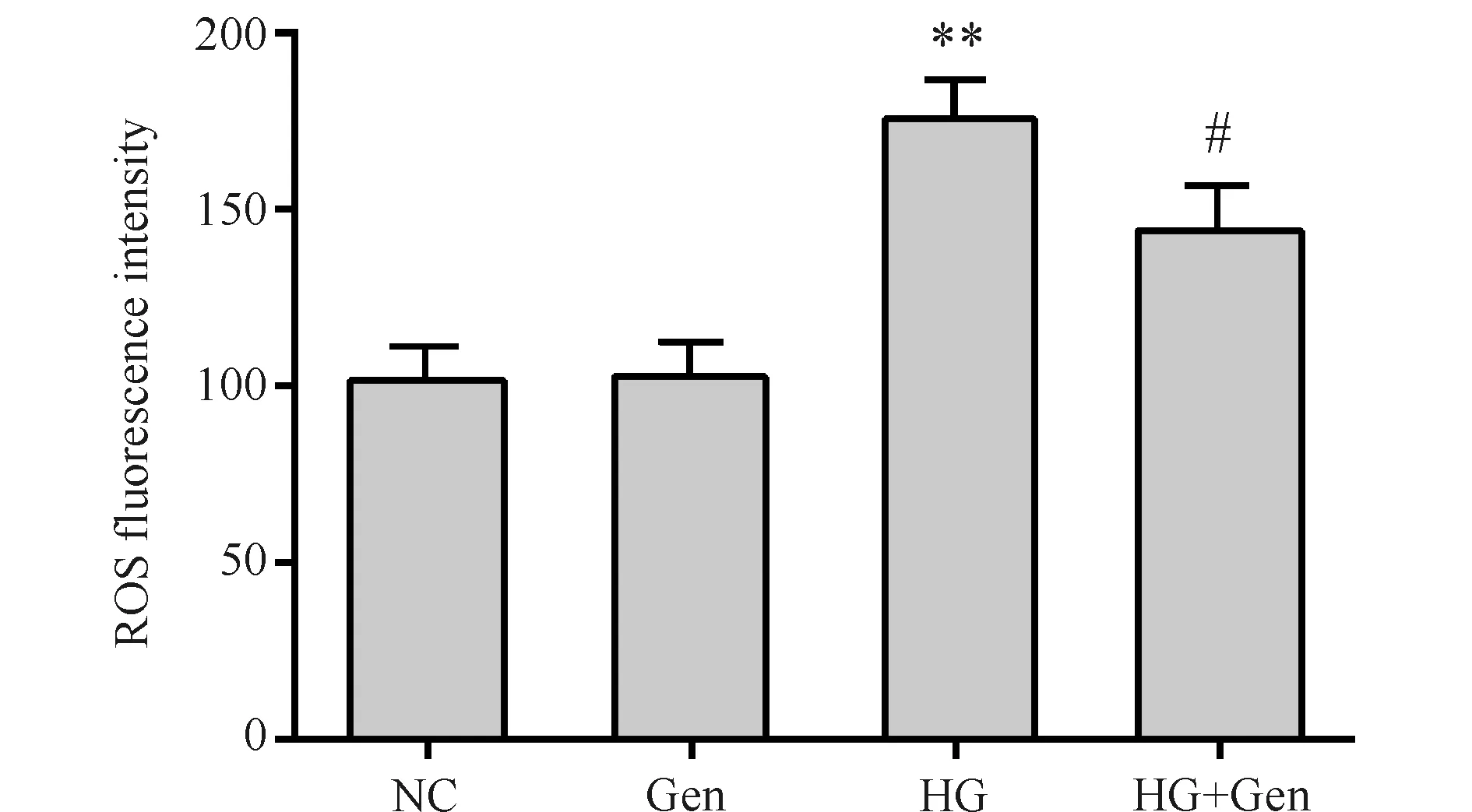
Fig.5 Effects of genipin on intracellular ROS levels The control group (NC) was cultured under normal conditions. The genipin group (Gen) was treated with 10 μmol/L genipin. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG + Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. Data are shown as Mean ± SD of three independent experiments. Statistical significances were calculated using Student’s t-test or one-way ANOVA, **P < 0. 01 compared with NC group; #P <0.05 compared with HG group
2.5 京尼平提高高糖诱导下MIN6细胞GSH/GSSH的比值
与对照组(0.66 ± 0.07)比较,京尼平组(0.66 ± 0.04)胰岛细胞GSH/GSSH比值的差异无统计学意义(P>0.05),高糖损伤组(0.48 ± 0.048)胰岛细胞GSH/GSSH比值明显下降(P<0.05);与高糖损伤组比较,京尼平(10 μmol/L)作用于高糖损伤的胰岛细胞48 h后,GSH/GSSH比值(0.68 ± 0.04)显著提高(P<0.01),结果见Fig.6。
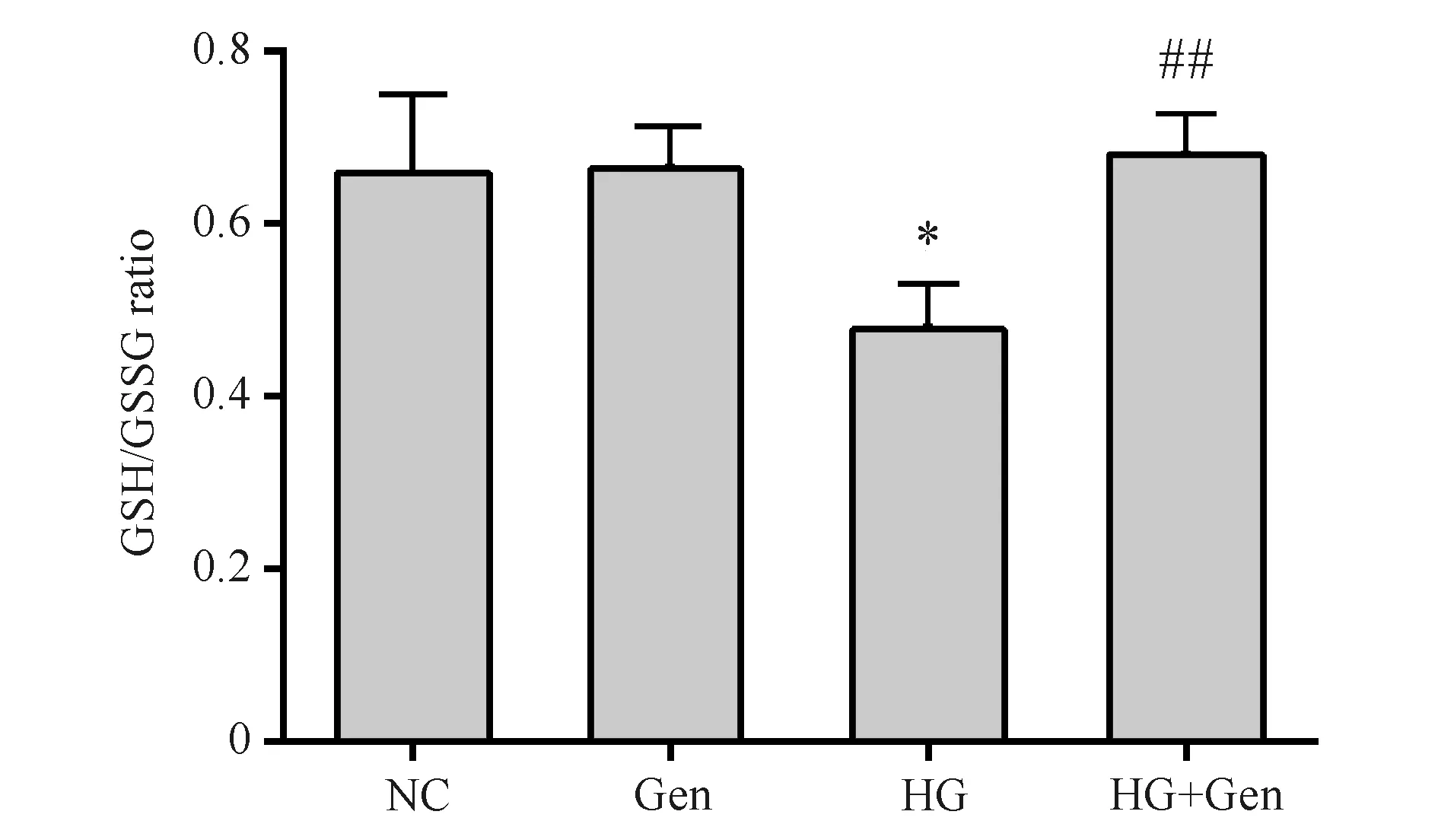
Fig.6 Effects of Genipin on the GSH/GSSG ratio The control group (NC) was cultured under normal conditions. The genipin group (Gen) was treated with 10 μmol/L genipin. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG + Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. Data are shown as Mean ± SD of three independent experiments. Statistical significances were calculated using Student’s t-test or one-way ANOVA, *P < 0. 05 compared with NC group; ##P <0.01 compared with HG group
2.6 京尼平调节细胞内氧化应激水平
与对照组比较,京尼平组胰岛细胞内MDA、LDH、SOD和CAT水平的差异无统计学意义(P>0.05),高糖损伤组胰岛细胞内MDA(2.80±0.14 nmol/mg)和LDH(295.98±6.23 U/g)水平显著升高(P<0.001),SOD(3.73±0.40 U/mg)和CAT(33.33±1.78 U/mg)水平明显降低(P<0.001);与高糖损伤组比较,京尼平(10 μmol/L)作用于高糖损伤的胰岛细胞48 h时,MDA(2.09±0.14 nmol/mg)和LDH(177.64±6.83 U/g)水平显著降低(P<0.01),SOD(7.24±0.45 U/mg)和CAT(63.29±3.55 U/mg)水平明显升高(P<0.01),结果见Table 2。
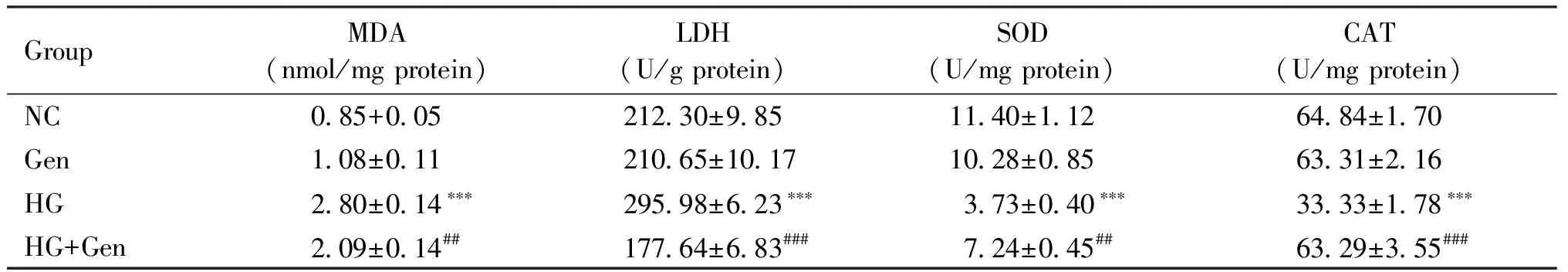
Table 2 The change of the MDA,SOD,CAT and LDH levels (Mean ± SD. n=3 )
2.7 京尼平提高高糖诱导下MIN6细胞线粒体膜电位水平
激光共聚焦检测线粒体膜电位,结果显示与对照组(1.82 ± 0.07)比较,高糖损伤组(1.15 ± 0.10)胰岛细胞中JC-1聚集体红色荧光与JC-1单体绿色荧光比值显著下降(P<0.01);与高糖损伤组比较,HG+Gen(1.40 ± 0.04)细胞中JC-1聚集体红色荧光与JC-1单体绿色荧光比值显著上升(P<0.05),结果见Fig.7。
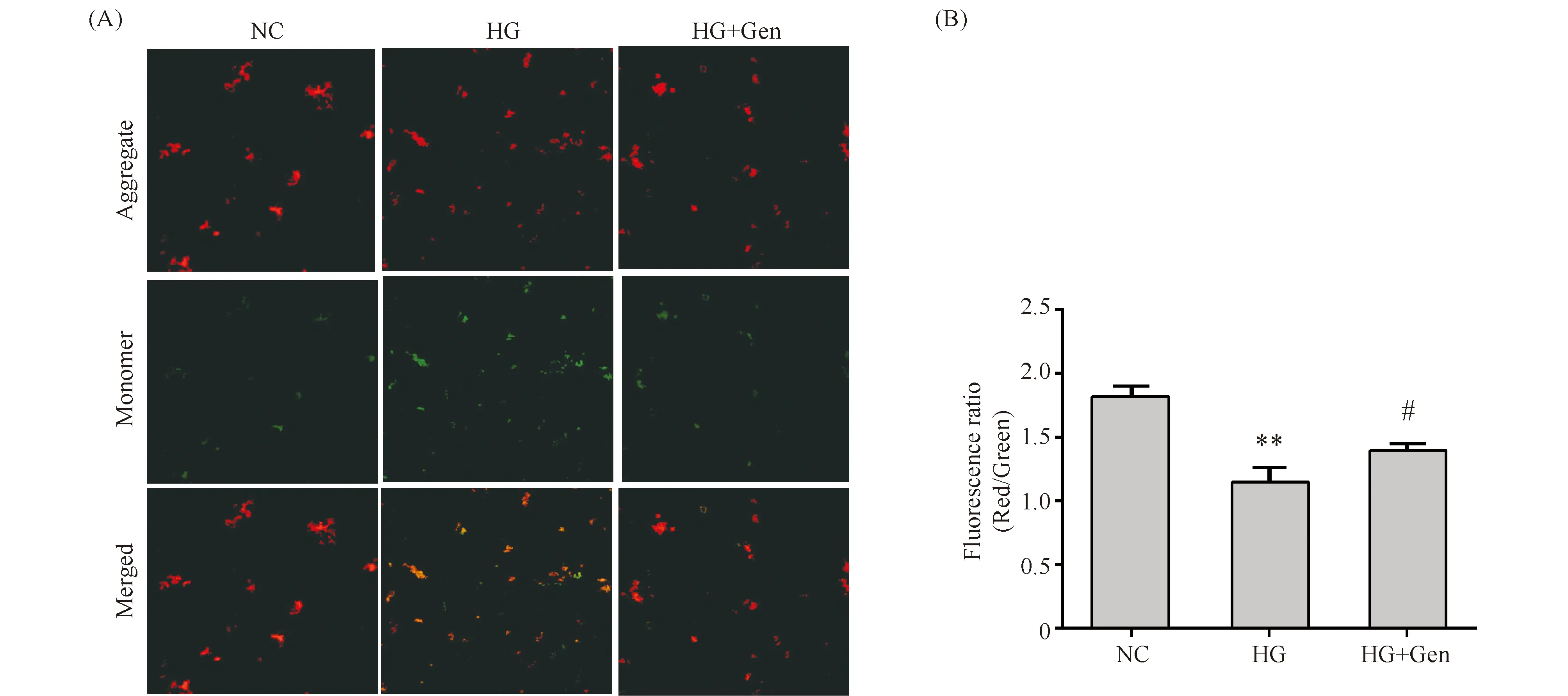
Fig.7 Effects of genipin on intracellular MMP levels (A) MMP assays. The control group (NC) was cultured under normal conditions. The high glucose group (HG) was treated with 33 mmol/L glucose. The high glucose and genipin group (HG+Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. (B) Quantification of MMP wherein relative MMP levels in the experimental group were normalized to the control group. Data are shown as Mean ± SD of three independent experiments. Statistical significances were calculated using Student’s t-test or one-way ANOVA, **P < 0. 01 compared with NC group; ##P <0.01 compared with HG group
2.8 京尼平促进高糖诱导下MIN6细胞 GR/Grx1蛋白质水平的表达
Western 印迹结果显示,与对照组(UCP2 1.33 ± 0.11,GR 1.62 ± 0.08,Grx1 2.87 ± 0.11)比较,高糖损伤组(GR 1.06 ± 0.08,Grx1 1.25 ± 0.10)胰岛细胞中GR、Grx1的蛋白质表达水平显著降低(P<0.001), UCP2(1.86 ± 0.10)表达显著升高(P<0.01);与高糖损伤组比较,高糖和京尼平组细胞内GR、Grx1的蛋白质(GR 1.19 ± 0.059,Grx1 2.20 ±0.15)表达水平明显提高(P<0.05),UCP2 (1.54 ± 0.07)表达减低(P<0.05),结果见Fig.8。
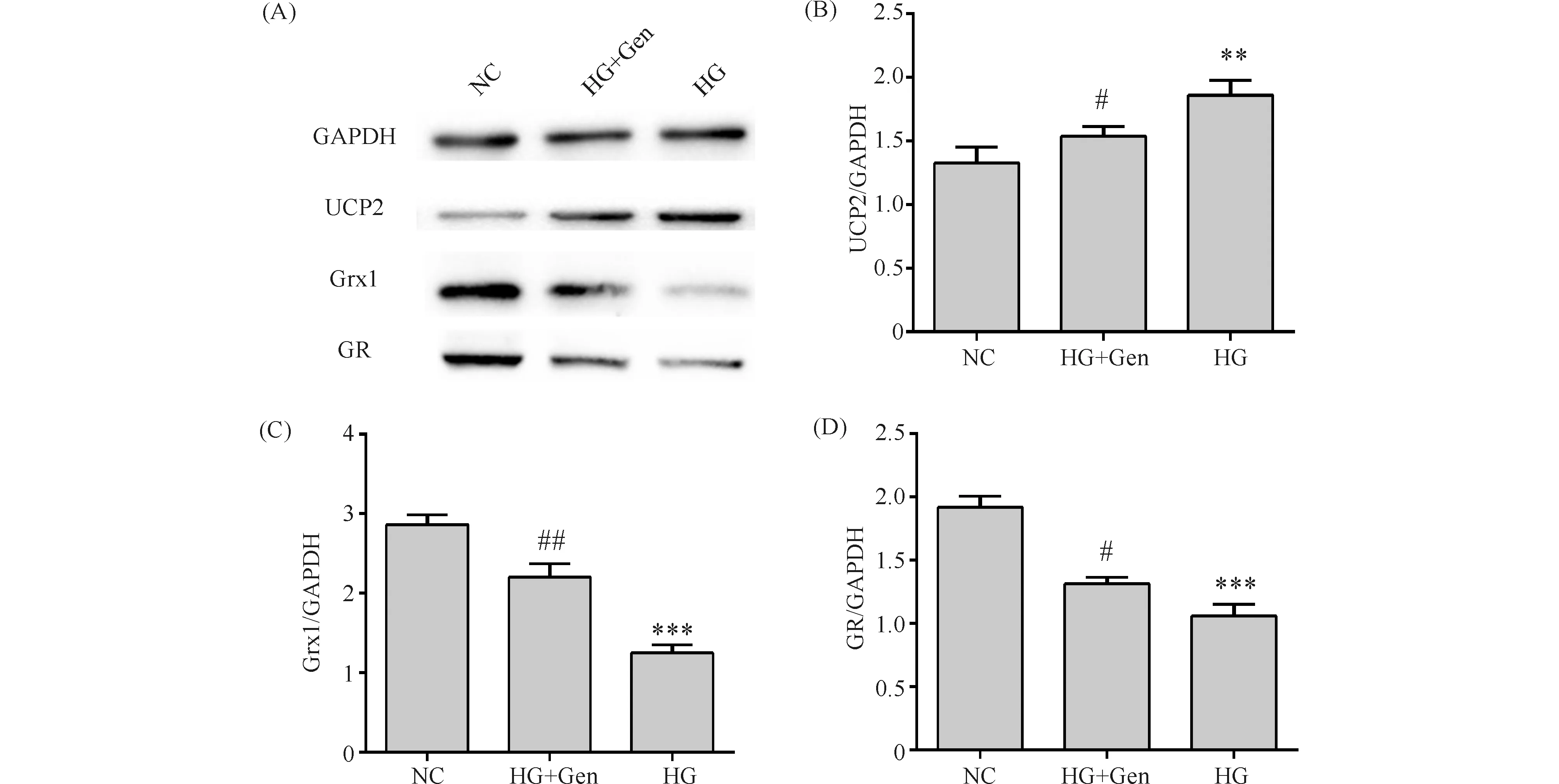
Fig.8 The protein expression levels by Western blotting analysis The control group (NC) was cultured under normal conditions. The high glucose group (HG) was treated with 33 mmol/L glucose.The high glucose and genipin group (HG+Gen) was treated with 33 mmol/L glucose and 10 μmol/L genipin. Data are shown as Mean ± SD of three independent experiments. Statistical significances were calculated using Student’s t-test or one-way ANOVA, **P < 0. 01 and ***P < 0. 001 compared with NC group ; #P <0.05 compared with HG group;##P <0.01 compared with HG group
Data are shown as Mean ±SDof three independent experiments. Statistical significances were calculated using Student’st-test or one-way ANOVA,***P<0.001 compared with NC group;###P<0.001 and##P<0.01 compared with HG group
3 讨论
2 型糖尿病(T2DM)患者的健康管理主要是血糖检测达标,降低血糖是治疗糖尿病及其并发症的关键[9-12]。胰腺β细胞合成的胰岛素在降低血糖浓度方面发挥重要的作用。正常生理情况下,胰岛素可以维持血液中葡萄糖在正常范围内,但长期处于高糖环境中的胰岛细胞将出现氧化应激,其是胰腺β细胞凋亡的主要原因,这使得胰岛素分泌量降低[13-15]。体外高葡萄糖环境培养小鼠胰岛MIN6细胞,模拟高葡萄糖下胰岛细胞相关指标的改变,同时探讨抗氧化药物京尼平对高糖诱导损伤的MIN6细胞的作用机制。我们在开展本研究的前期,摸索了不同葡萄糖浓度和不同浓度京尼平及作用时间对MIN6细胞各项指标的影响,建立了高糖诱导的MIN6细胞损伤模型。高糖浓度为33 mmol/L,京尼平浓度为10 μmol/L。CCK-8检测高糖损伤组和京尼平组。结果显示,高糖损伤组降低MIN6细胞的存活率,而抗氧化剂京尼平10 μmol/L对MIN6细胞的存活率未见影响,京尼平作用于高糖组能提高损伤组的细胞存活率,提示京尼平对高糖损伤的小鼠胰岛MIN6细胞有一定的保护作用。
Eizirik等[16]早期发现,人体长时间处于高葡萄糖浓度下会损伤β细胞。本文中发现,京尼平不仅有效保护胰岛细胞的活性还增强了GSIS急性刺激,胰岛素释放量增加,促进葡萄糖的摄取和MIN6细胞内ATP含量的增加,改善了小鼠胰岛细胞长期高糖诱导下的细胞毒性,GSIS的改善可能与细胞内ATP水平调节相关的AMPK系统有关[17-19]。因此,京尼平可以调节胰腺β细胞对胰岛素的分泌,促使细胞对葡萄糖的吸收和代谢,京尼平又是解偶联蛋白2(Uncoupling protein 2,UCP2)的抑制剂,进而提高细胞内ATP含量[20]。
高血糖将导致机体的氧化应激,使体内抗氧化防御系统与活性氧(ROS)调节失衡[21-22]。高糖培养的细胞ROS的生成增加,抗氧化蛋白质GR和Grx1的水平降低,而高糖和京尼平组GSH/GSSG值和抗氧化蛋白质GR、Grx1的表达水平较高糖损伤组明显提高,提示京尼平可有效提高小鼠胰岛MIN6细胞在高糖环境中的抗氧化性,京尼平提高细胞内Grx1水平,Grx1可以改善细胞内胰岛素水平[23]。线粒体是细胞能量代谢的工厂,高糖培养的胰岛细胞将出现细胞氧化应激的损伤,线粒体功能异常,高糖损伤后较正常组细胞线粒体膜去极化,膜电位发生改变,JC-1法检测发现红绿荧光的比值降低,而京尼平有效改善线粒体的去极化现象,提高红绿荧光的比值。由此说明,京尼平可以抑制线粒体的去极化和凋亡,改善线粒体功能。
综上所述,通过本研究发现,京尼平对高糖损伤的小鼠胰岛细胞功能的保护有一定作用,提高胰岛素的释放量,作用的机制与减少因高糖培养产生的ROS,提高细胞内抗氧化物,改善线粒体功能有关,这将为糖尿病血糖的调节提供实验依据。

