RANS simulations on combustion and emission characteristics of a premixed NH3/H2 swirling flame with reduced chemical kinetic model
Yuze SUN, To CAI, Mohmmd SHAHSAVARI, Dkun SUN,Xiofeng SUN, Dn ZHAO,*, Bing WANG
a Department of Mechanical Engineering, University of Canterbury, Christchurch 8041, New Zealand
b School of Aerospace Engineering, Tsinghua University, Beijing 100084, China
c Collaborative Innovation Center for Advanced Aero-Engine, Beihang University, Beijing 100083, China
KEYWORDS Ammonia;Emission;Hydrogen;Premixed combustion;Reduced mechanism;Swirling flame
Abstract Ammonia(NH3)is considered as a potential alternative carbon free fuel to reduce greenhouse gas emission to meet the increasingly stringent emission requirements. Co-burning NH3 and H2 is an effective way to overcome ammonia’s relative low burning velocity.In this work,3D Reynolds Averaged Navier-Stokes (RANS) numerical simulations are conducted on a premixed NH3/H2 swirling flame with reduced chemical kinetic mechanism. The effects of (A) overall equivalence ratio φ and (B) hydrogen blended molar fraction XH2 on combustion and emission characteristics are examined.The present results show that when 100%NH3-0%H2-air are burnt,the NO emission and unburned NH3 of at the swirling combustor outlet has the opposite varying trends. With the increase of φ, NO emission is found to be decreased, while the unburnt ammonia emission is increased.NH2 →HNO,NH →HNO and HNO →NO sub-paths are found to play a critical role in NO formation. Normalized reaction rate of all these three sub-paths is shown to be decreased with increased φ.Hydrogen addition is shown to significantly increase the laminar burning velocity of the mixed fuel. However, adding H2 does not affect the critical equivalence ratio corresponding to the maximum burning velocity.The emission trend of NO and unburnt NH3 with increased φ is unchanged by blending H2. NO emission with increased XH2 is increased slightly less at a larger φ than that at a smaller φ.In addition,reaction rates of NH2 →HNO and HNO →NO sub-paths are decreased with increased XH2, when φ is larger. Under all tested cases, blending H2 with NH3 reduces the unburned NH3 emission, especially for rich combustion conditions. In summary, the present work provides research finding on supporting applying ammonia with hydrogen blended in low-emission gas turbine engines.
1. Introduction
Nowadays, developing new methods to act on the global warming is one of the main research challenges in scientific communities.1Combustion of fossil fuel plays a significant role in the global warming by producing considerable amount of greenhouse gas such as CO2.2Extensive efforts have been expanded in the past decades to reduce the CO2emission by improving efficiency of combustion devices.3Despite the concerted community efforts, improving solely the combustion efficiency is ineffective to meet increasingly stringent emission regulations.4For instance,based on the EU climate actions for 2030, greenhouse gases should be cut by 40%.5In this light,utilizing low-even non-carbon fuels is the key method to considerably reduce greenhouse gases.6Ammonia is identified as one of the best carbon free fuels thanks to its high hydrogen density and well-stablished storage and shipping.7,8Further,ammonia can be synthesized from renewable hydrogen and nitrogen from air.9Perfect combustion products of ammonia are nitrogen and water which are environmentally friendly.10Researches have been carried out over the past decades to shed light into various aspects direct combustion of NH3/air. Considerable parts of such efforts devoted to measure NH3/air laminar burning velocity under the atmospheric11and pressurized conditions,12the ignition delay time13oxidation process14and ignition characteristics.15
Unfortunately, it is still infeasible to use ammonia in conventional combustion devices,16since it has relatively low reactivity and produces considerable amount of NOxemissions.17In 1966,US army aviation material laboratories and US army engineer research and development laboratories published technical reports on the utilization and development ammonia as an alternative fuel in army aircraft engines18and gas turbine engines.19Results shows that the utilization of ammonia as fuel in engines leads lower aircraft productivity than the productivity obtained from use of hydrocarbon fuels. Since then,increasing the ammonia reactivity has been topic of growing researches in the combustion community. One of the effective methods to enhance the ammonia reactivity is to mix ammonia with high heat value fuels.20Intensive research of co-firing NH3with methane have been performed. Okafor et al.21conducted experimental study of the laminar burning velocity of premixed methane/ammonia/air flames. It is found that the burning velocity increased with an increase of methane concentration. Honzawa et al.22conducted large eddy simulation to predict NO and CO emission of NH3/CH4/air premixed combustion in a swirl burner.Results show that radiation and wall temperature has a great influence on emission. Moreover,ammonia is also tried to be applied in duel fuel system and co-fired with diesel23, gasoline24, Dimethyl Ether (DME)25,26and biodiesel.27Besides these hydrocarbon fuels, ammonia blend with hydrogen as fuel is also investigated by many researchers since hydrogen is also carbon free and renewable fuel like ammonia. Dai et al.28experimentally measured auto-ignition delay times of ammonia and hydrogen mixtures in a rapid compression machine. Pugh et al.29investigated the effect of secondary steam and operating pressure on turbulent NH3/H2flame in a gas turbine combustor.
Moreover, various experimental and numerical researchers were performed to study ammonia combustion in the conditions prevailing in gas turbine like combustors. Somarathne et al.30performed large eddy simulation on a swirl combustor to evaluate the effects of pressure on turbulent NH3/air premixed flame characteristics. Results shows that both NO and unburnt NH3emissions decrease with the augmentations of the pressure under rich combustion conditions. Hayakawa et al.31conducted experiments to study premixed ammonia flame characteristics in a swirl combustor. Their results showed that flame stability are affected by the inlet velocity,swirl intensity and the combustor geometry. Moreover, measured emission characteristics showed that the NOxemissions decrease, as the unburnt ammonia emission increases for rich conditions.Okafor et al.32developed a low NOxmicro gas turbine combustor by performing experimental and numerical investigations. Various parametric studies were carried out to evaluate effects of the fuel injection angle, combustor inlet temperature, equivalence ratio, and ambient pressure on the flame stabilization and emissions. Despite the above valuable community efforts,to the best knowledge of the authors,there are few reports in the literature simulating and discussing combustion and emission characteristics of premixed NH3/H2/air swirling flames at various hydrogen addition levels. Lack of these investigations motivated the present investigation.
In order to simulate ammonia combustion precisely, it is mandatory to utilize accurate kinetic mechanism.33Various chemical mechanisms have been developed in the past namely34, 957-steps (128 species) mechanism proposed by Li et al.35,1397-steps(151 species)mechanism developed by Glarborg et al.36, 1231-steps (129 species) mechanism presented by Konnov37,314-steps(60 species)mechanism presented by Tian et al.38and 278-steps (55 species) mechanism proposed by Mathieu and Petersen.13However,it is highly expensive to perform numerical simulations in practical combustors using detailed mechanisms along with finite rate combustion models.39In order to overcome this problem, various reduced mechanisms have been developed in the past to minimize the numerical expenses. Miller et al.40developed a NH3oxidization mechanism comprises of 23 species and 98 reactions.Okafor et al.41reduced their detailed mechanism21to a 130-steps(42 species) mechanism. Xiao et al. also proposed several reduced NH3/H242and NH3/CH443mechanism. However,any of the above mechanisms comprise of many species and reactions. More reliable and computationally cheap reduced mechanisms are desired to perform numerical simulations using finite rate combustion models,which also partially motivated the present work.
In this work, 3D RANS numerical simulation are conducted on a swirling premixed air-NH3/H2combustor investigate its combustion and emission characteristics. Emphasis is placed on the chemical reaction pathway analysis basing on a reduced ammonia oxidation mechanism on the effect of blending hydrogen on premixed ammonia swirling flame. In Section 2, the numerical model and governing equations are described. The turbulence model and combustion reaction model are validated in Section 3. The model is then applied to evaluate the effects of (A) equivalence ratio φ and (B)hydrogen addition ratio XH2on emission and combustion performances. The results and discussion are summarized in Section 4. Finally, key findings are provided in Section 5.
2. Physical models and numerical methods
2.1. Computational domain
2.2. Governing equations
RANS method is used to solve the mass, momentum, energy,and species transport equations in compressible fluids. The governing equations are given as:
(1) Mass conservation:
The physical model of the premixed swirling combustor performed in the present study is shown in Fig. 1(a), which is a simplified model of an industrial gas turbine combustor originated from our previous experiment investigations as shown in Fig. 1(b).44The combustor radius (Rc) and length (Lc) are 50 mm and 700 mm, respectively. Moreover, the inlet (Ri)and central shaft (Rs) radiuses of the swirl burner are 4 mm and 11 mm, respectively. In order to minimize the computational expenses, details of the swirler are not included in the numerical simulations.Moreover,a 1/8th segment of the combustor with rotational periodic boundary conditions is used to perform the simulations. 3D structured grids mesh generated by using ANSYS ICEM CFD is shown in Fig.1(c). The computational domain is meshed using 5×105cells.The grids are fine the swirler outlet to appropriately resolve step gradients generated by combustion.
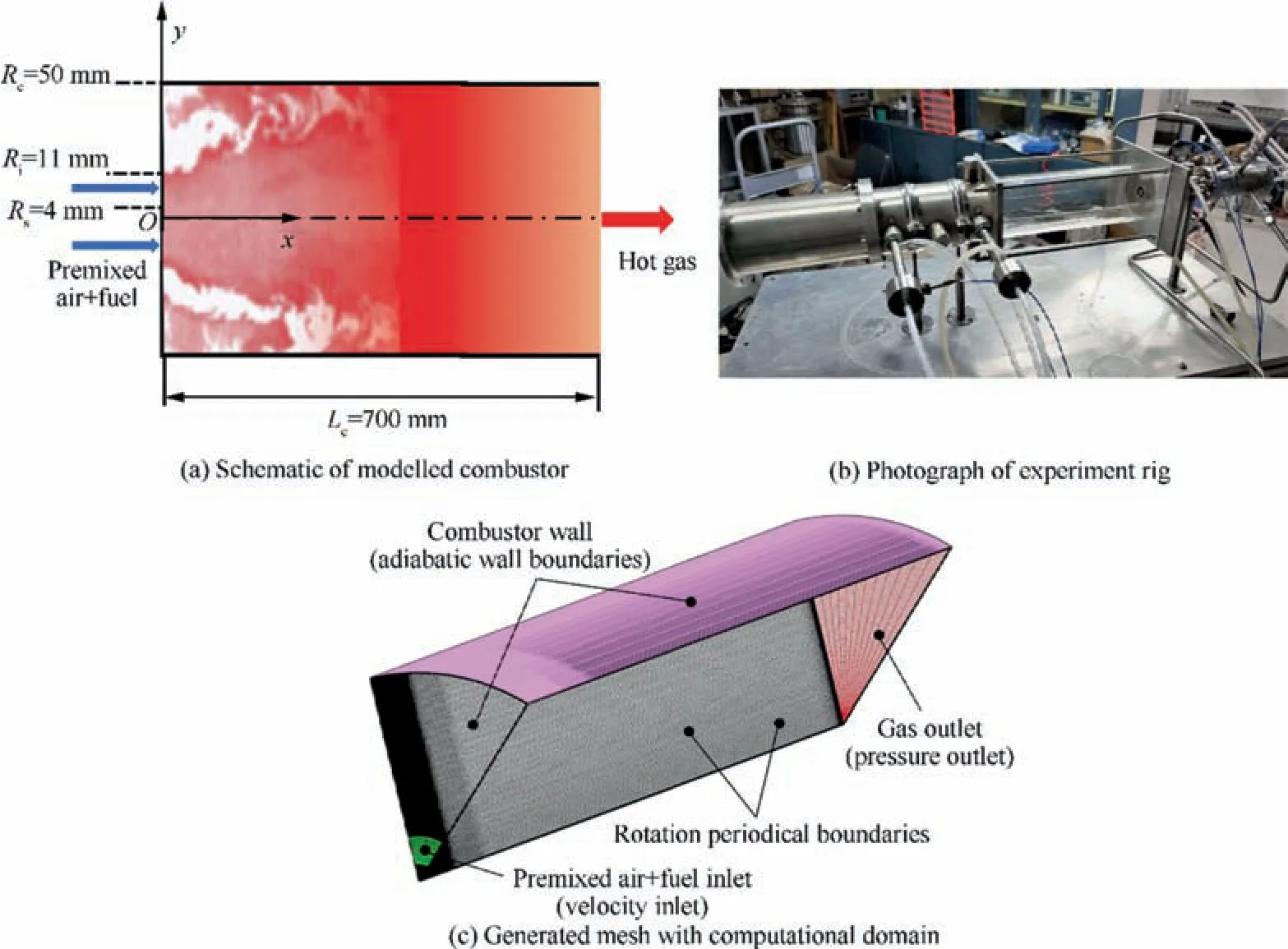
Fig. 1 Schematic of modelled combustor, photograph of experiment rig and generated mesh with computational domain.
where ρ is density,v is velocity vector,t is time,p is pressure,T is temperature, τ--is the stress tensor, τ′ijis Reynolds stress tensor,τeffis effective stress tensor,E is the total energy,keffis effective conductivity, hiis the enthalpy of species i, Jiis the diffusion flux of species i, Shis the fluid enthalpy source, Yiand ωiare the mass fraction and net rate of the production of species i, Rgis the gas constant and Mwis the molecular weight.
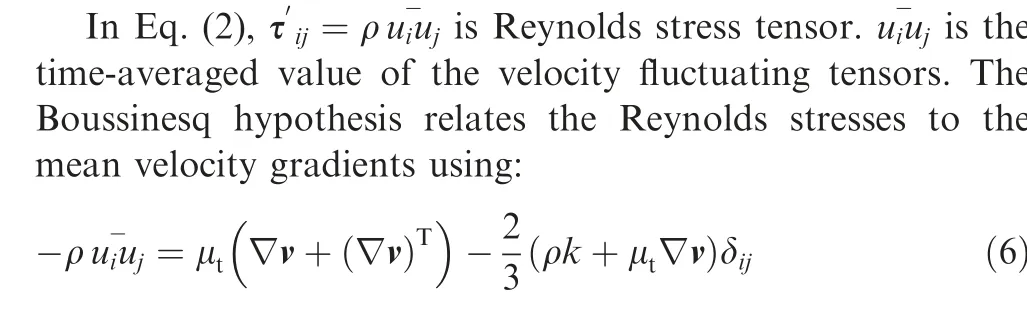
where k is the turbulent kinetic energy,μtis turbulent viscosity and δijis the Kroneker delta. Here, k-ε (RNG) turbulence model comprises of transport equations on turbulent kinetic energy and turbulent dissipation (ε) is used to evaluate turbulent viscosity.
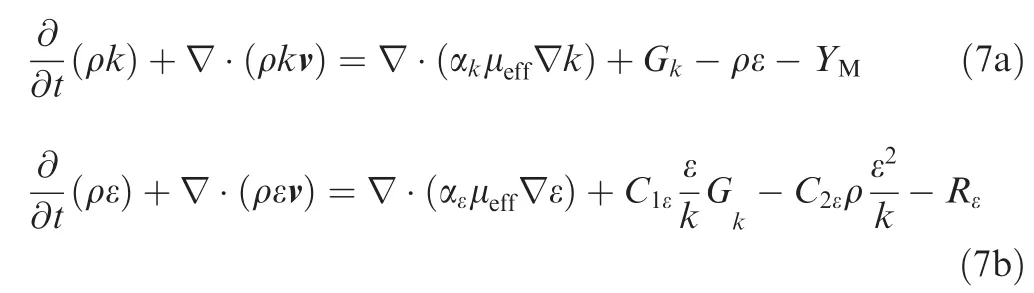
where,αkand αεare the inverse effective Prandtl numbers for k and ε, respectively. μeffis effective viscosity. Gkdenotes the generation of turbulence kinetic energy due to the mean velocity gradients. YMdenotes the contribution of the fluctuating dilatation in compressible turbulence to the overall dissipation rate.Rεis the addition term in RNG k-ε model.C1ε=1.42 andC2ε=1.68 are constant derived analytically by the RNG theory. In RNG version of k-ε model considers the effect of swirling flow on turbulence viscosity as below:

where μt0is the value of the turbulent viscosity calculated without the swirl effects. Ω is the characteristic swirl number evaluated within ANSYS FLUENT.αs=0.05 is a constant.
In this study, the pressure-velocity coupling is achieved by using COUPLE algorithm. The Eddy-dissipation-concept combustion model along with a reduced mechanism comprises of 19 species and 63 elementary reactions are used to model the turbulent combustion.The details of the reaction mechanism is provided in Supplementary Material. The least squares cellbased and second order schemes are used to discretize the spatial gradient and pressure terms. The third-order MUSCL scheme is also used to discretize other term, except the turbulence dissipation which discretized using the second-order upwind scheme. The inlet boundary condition is velocity inlet corresponding an air flow rate of Va=180 L/min at a temperature of 300 K with an 8% turbulence intensity. The equivalence ratio φ vary from 0.8 to 1.4. In order to generate a swirling flow at the inlet boundary condition,the swirl number SNdefinition is utilized in the computation. Swirl number is defined as the ratio of the axial flux of the tangential momentum to the product of the axial flux of the axial momentum and the combustor inlet diameter:
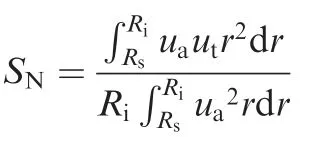
where Rsand Riare the radius of the central shaft and inlet;uaand utare axial and tangential velocity. In the present work,inlet swirling number is fixed at SN=0.6. The pressure outlet of condition of 0 Pa relative pressure is applied at the domain exit. Combustor wall boundaries defined as adiabatic non-slip walls. All the boundary condition and numerical schemes are summarized in Table 1.
3.Turbulence model and chemical reaction validations and mesh independence study
Fig. 2 presents the normalized mean velocity components at various axial locations obtained by the presented numerical simulations and experimental investigations of Taamallah et al.45,where uaur,utand uinletare axial,radial and tangential and inlet velocity respectively. Obtained results show that, the overall agreement between the present numerical results and the experimental data is reasonable. Present 19 species, 63 reactions mechanism is reduced from the Okafor reduced mechanism. The mechanism utilized in present study is validated against Okafor reduced mechanism41and several experimental results from Hayakawa12, Takizawa11, Pfahl46,Zakaznov47and Han16et al. Fig. 3 plots the laminar flame speed(SL)of NH3/air premixed flame as a function of φ under atmospheric pressure condition and the laminar flame speed of NH3/H2/air premixed flame at φ=1.0 as a function of XH2.The results show that the present reduced mechanism can well predict the laminar flame speed the present reduced mechanism is precise enough for the present investigations.

Table 1 Boundary conditions and numerical schemes used by present simulation.
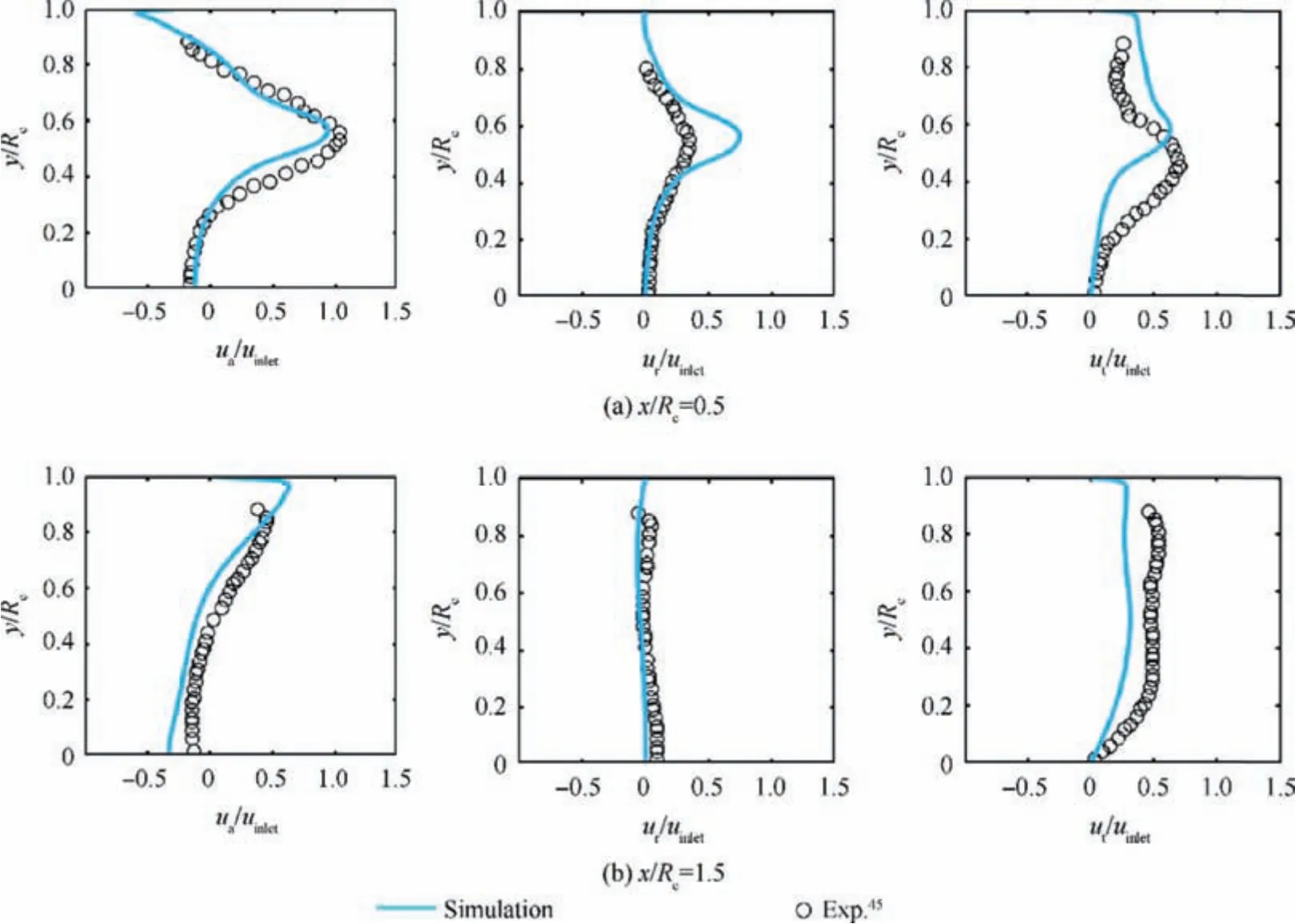
Fig.2 Validation results:mean axial(left),radial(center)and tangential(right)velocity profiles at different cross-sections of combustor.
In order to make sure that the numerical simulation results are independent of the grid number, three different meshes of combustor computational domain with 1×105cells, 5×105cells and 1×106cells are used to do the mesh independence study. Fig. 4 plots the normalized axial velocity and temperature profile at x/Rc=0.5. The results show that grid with 1×105cells is too coarse, while the profiles of grid with 5×105and 1×106cells are almost identical, which indicates that 5×105cells are enough for present study.
4. Results and discussion
4.1. Effect of equivalence ratio on premixed NH3-air swirling flame

Fig. 3 Chemical combustion reaction mechanism validation.
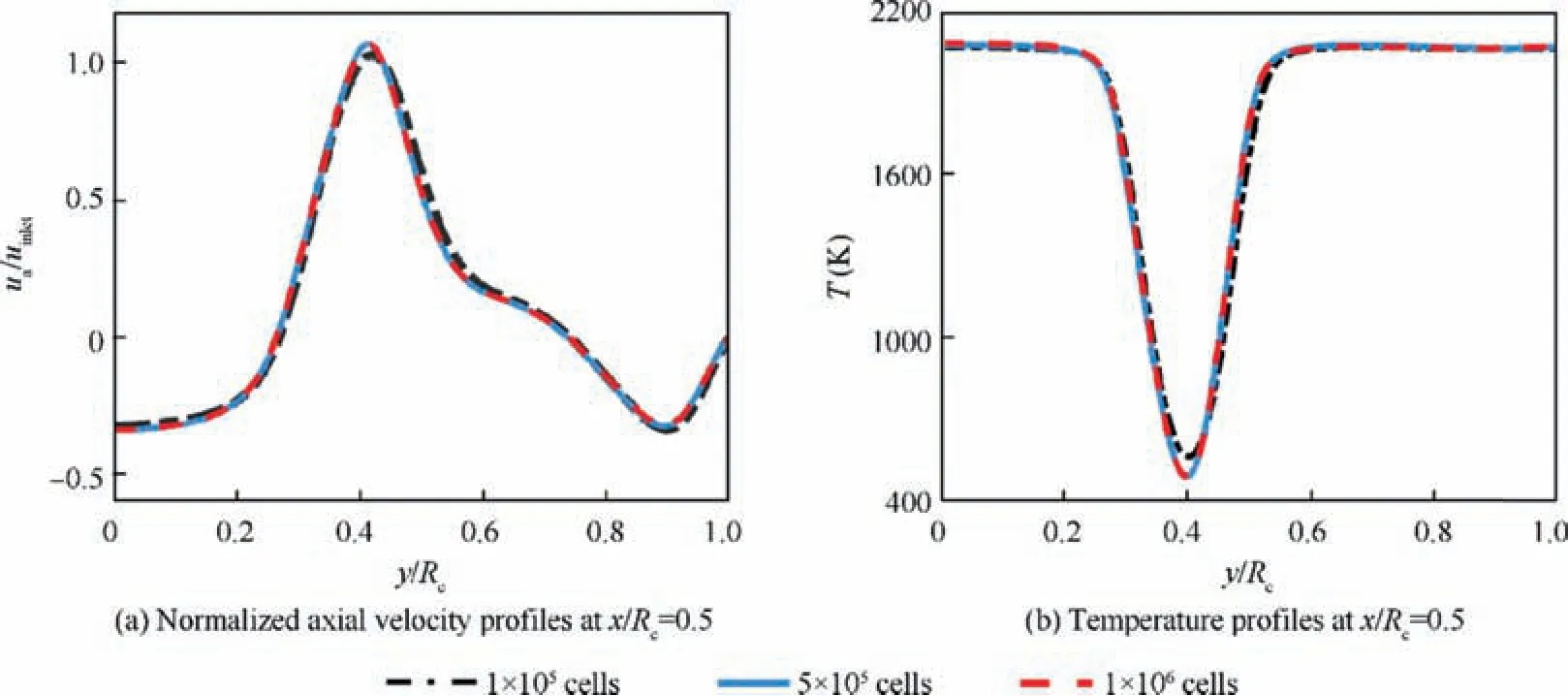
Fig. 4 Mesh independence study.
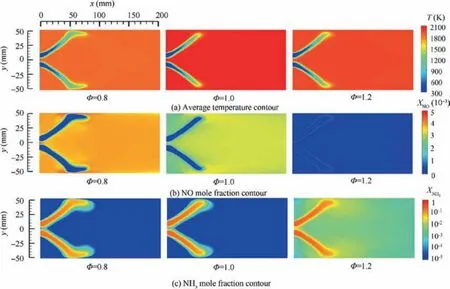
Fig. 5 Numerical predicted average temperature, NO mole fraction contour and NH3 mole fraction contour of NH3/air premixed combustion at different equivalence ratio φ.
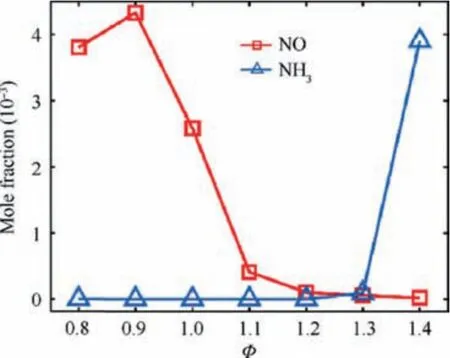
Fig. 6 Variation of average NO and unburnt NH3 emissions at combustor outlet with equivalence ratio φ.
Fig. 5 presents spatial distribution of temperature, NO and NH3mole fractions at various equivalence ratio.Results show that the premixed NH3/air swirling flame shape is V-type under different equivalence ratios. The length of flame is approximate 60 mm under the stoichiometric condition. However, the flame length increases as the equivalence ratio deviates from the stoichiometric condition. Moreover, at lower or higher equivalence ratio condition,flame length is increased due to the low burning velocity. It is observed that the flame tips touch the combustor wall. Fig. 5(b) shows that the NO concentration is high close to the flame front and decreases toward the combustor outlet. The NO emission is quite high at lean condition, while there is relatively low NO emission under the rich condition.Unburnt NH3distributions presented in Fig. 5(c) show that there is almost no unburnt NH3in the downstream of combustor under the lean condition, while a large amount of ammonia is not burned under the rich condition.NH3and NO emissions measured at the combustor outlet are presented in Fig.6.As it is expected from the literature,the NO and NH3emissions have opposite trends as functions of the equivalence ratio. As the equivalence ratio is increased,NO decreases, while unburnt NH3increases. In the following part of this section,discussions are made to address the details of the NO formation pathways as well as the effects of equivalence ratio on them.
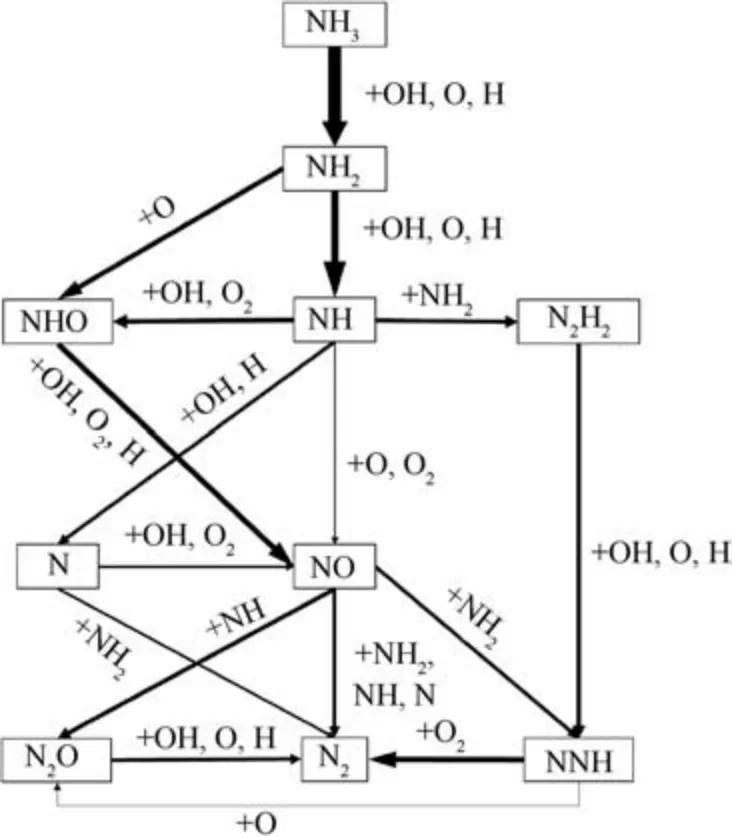
Fig. 7 NH3 oxidation reaction path analysis.
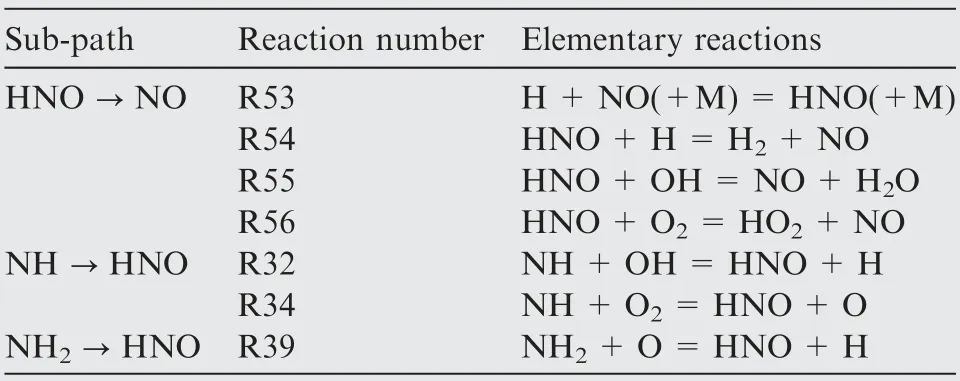
Table 2 Sub-paths and elementary reactions involving with NO formation.
Pathway analysis are performed to further understand the effect of equivalence ratio on NH3oxidation and NO production.Fig.7 shows the diagram of the ammonia oxidation reaction paths under stoichiometric condition determined by 1D premixed flame using CHEMKIN-PRO.Note that,in the diagram the thickness of each arrow denotes the relative magnitude of the rate of reaction and the reaction rates are normalized by the reaction rate of NH3→NH2.It can be seen that the major source of the NO formation comes from oxidation of HNO. The rates of NO formation are mainly affected by NH2→HNO, NH →HNO and HNO →NO. Involved elementary reactions are listed in Table 2.
Fig. 8 shows normalized sub-paths rate of reaction for various equivalence ratio. It can be seen that all of subpaths normalized reaction rates are decreased with the increase of equivalence ratio, which caused NO formation is decreased.
4.2. Effect of XH2 on premixed NH3/H2/air swirling flame
Co-firing NH3with H2is an effective method to enhance the reactivity of ammonia combustion. This section presents effects of hydrogen addition on combustion and emission characteristic of NH3/H2/air swirling flame. Fig. 9 compares the laminar flame speed of NH3/H2/air flame under various equivalence ratios and hydrogen blended ratios. As it is expected,the flame laminar burning velocity increases significantly as the hydrogen content of the fuel increases. However, the hydrogen addition does not change the equivalence ratios corresponding to the maximum burning velocity, which are φ=1.1 for all the hydrogen blended ratios. Fig. 10 and Fig. 11 present spatial distribution of temperature, NO and NH3mole fractions for various XH2at φ=1.1 and φ=1.4. Fig. 10(a), Fig. 10(c), Fig. 11(a) and Fig. 11(c) show that the length of flame and unburnt NH3mole fraction decrease as the XH2increases at both equivalence ratios.Fig. 10(b) shows that the NO formation is dramatically increased with the increase of XH2, especially at the edge and the tip of the swirling flame. However, at φ=1.4, as XH2is increased, there is no significant increases in the NO formation.

Fig. 8 Normalized rate of chemical reaction sub-paths as combustion condition is changed from lean to rich (0.8 ≤φ ≤1.4).
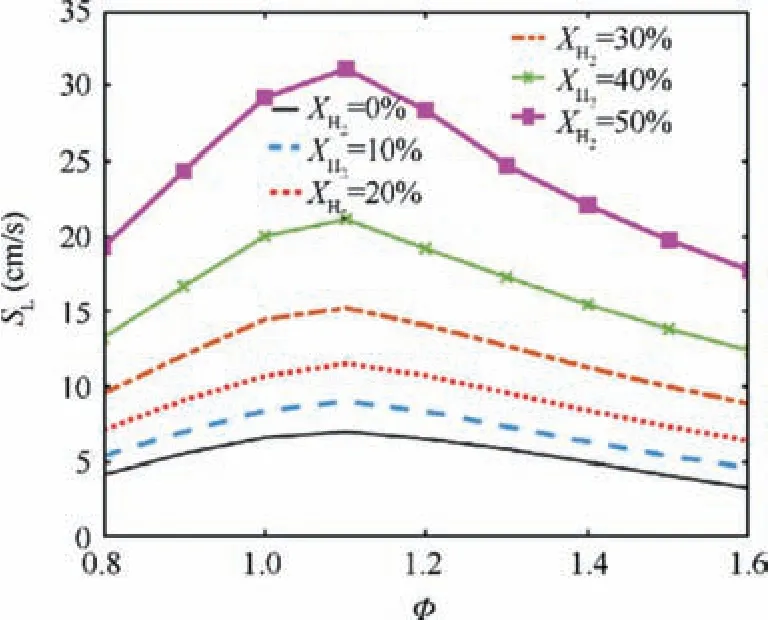
Fig.9 Variation of burning velocity SL with φ as XH2 is set to 6 different values.
Furthermore, the NO emissions at combustor outlet are summarized in Fig. 12. At lean conditions, NO emissions increase remarkably with the increase of XH2.For example,when φ=0.8, NO mole fraction at the combustor outlet is 3.8×10-3for XH2=0, whereas it is 7.6×10-3for XH2=0.5. However, results show that NO emission is less sensitive to the XH2under the rich condition as compared to the lean condition. For example, when φ=1.3, NO mole fraction at outlet is 5.7×10-5at XH2=0, while it is 7.6×10-5for XH2=0.5. The Variation of unburnt NH3emission at combustor outlet with XH2are shown in Fig. 13. Since there is negligible unburnt NH3for φ<1.2, only the results of φ=1.3 and 1.4 are presented here.
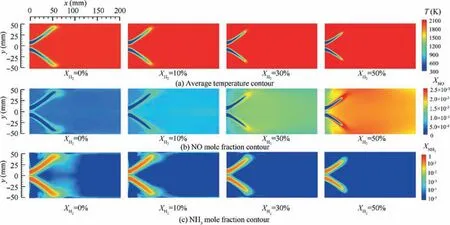
Fig.10 Numerically predicted average temperature,NO emission and NH3 mole fraction contour at φ=1.1 as XH2 is set to 4 different values.

Fig.11 Numerically predicted average temperature,NO emission and NH3 mole fraction contour at φ=1.4 as XH2 is set to 4 different values.
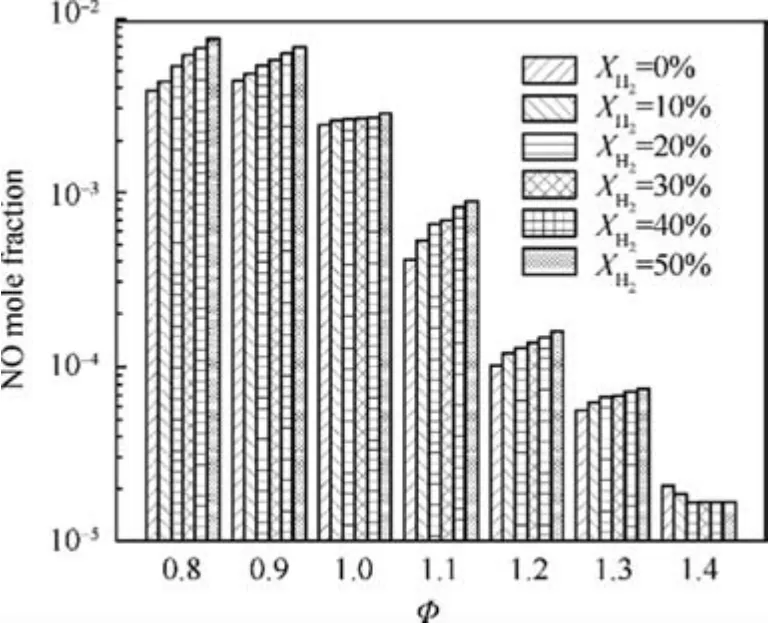
Fig. 12 Variation of NO emission at combustor outlet with equivalence ratio φ and hydrogen blended ratio XH2.
To further examine the effects of hydrogen blended ratio on NO formation,Fig.14 presents the rate of the elementary reactions contributing to NO formation calculated by using the 1D premixed flame model over the XH2range of 0-50%. Results show that the normalized reaction rate of NH →HNO increases with the increase of XH2for all equivalence ratios.As XH2is increased, normalized rate of the HNO →NO elementary reaction increases for φ ≤1.2 but decrease for φ ≥1.3. Moreover, the normalized rate of the NH2→HNO elementary reaction increases at φ ≤1.0 with augmentation of XH2, while the rate of this reaction remains unchanged at φ=1.1,and decreases at φ ≥1.2,with the increase of hydrogen addition.
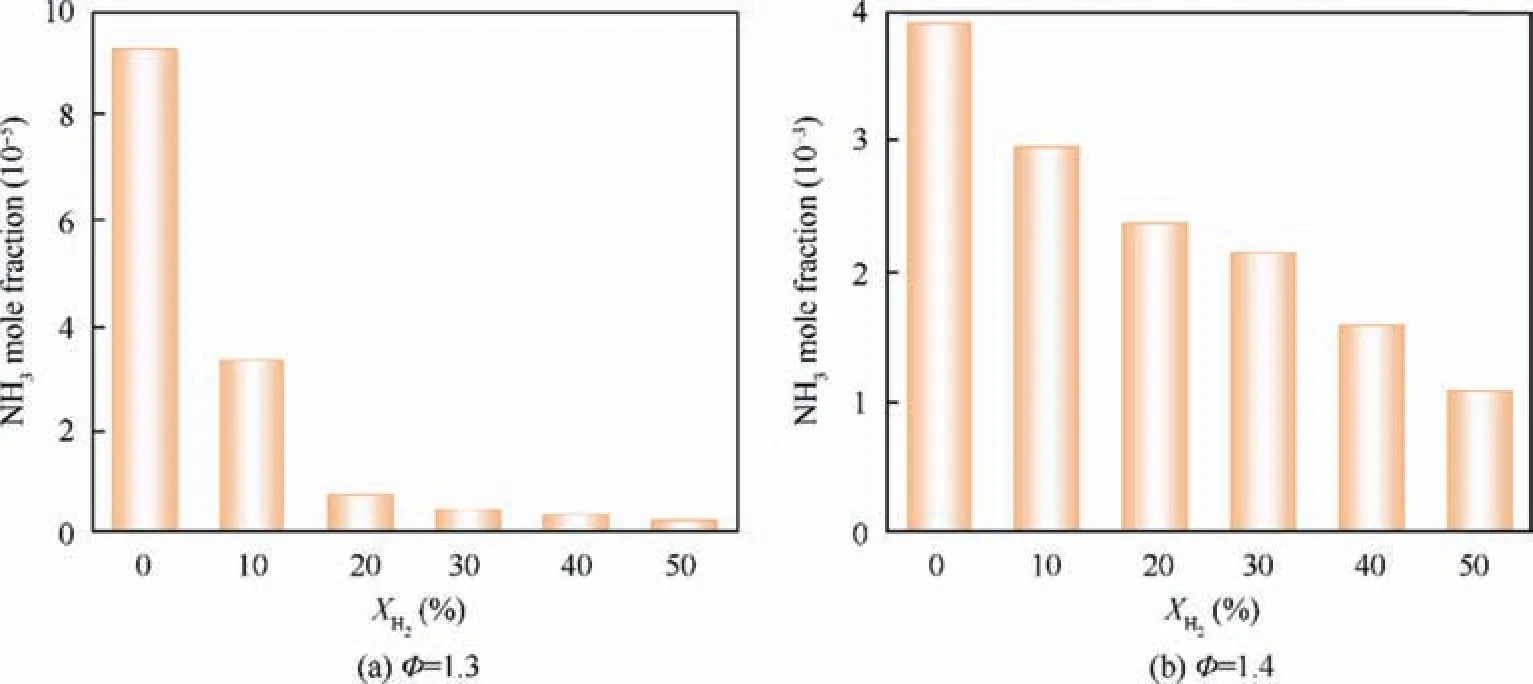
Fig. 13 Variation of unburnt NH3 emission at combustor outlet as a function of XH2 at φ=1.3 and φ=1.4.
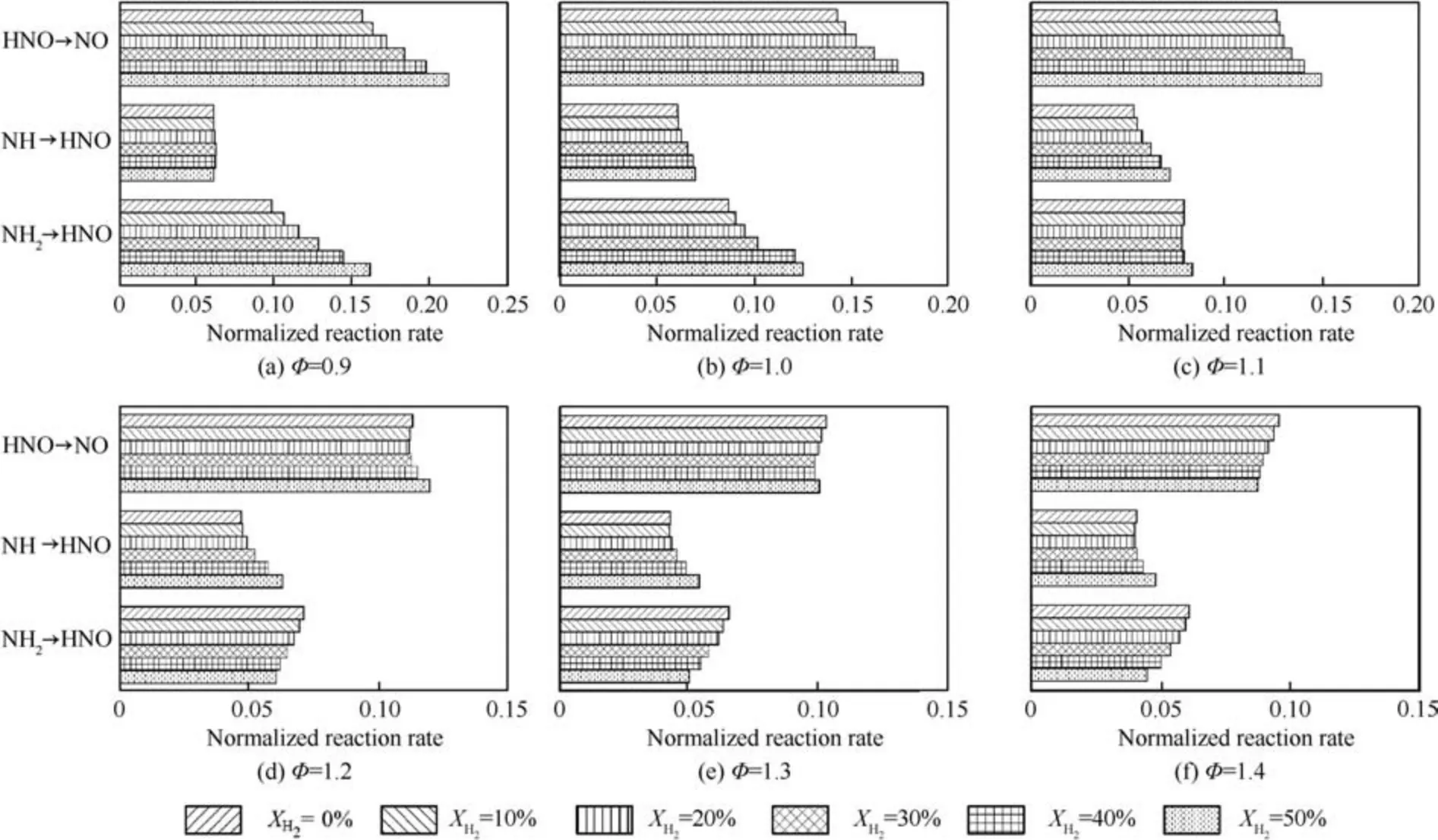
Fig. 14 Variation of normalized rate of reaction sub-paths with XH2.
5. Conclusions
In this work, we numerically investigate the combustion and NOxemission characteristics of a premixed NH3/H2/air swirling flame. For this, 3D RANS numerical model with a reduced combustion chemical kinetic mechanism is developed and applied. The effects of (A) equivalence ratio φ and (B)blended hydrogen molar ratio XH2are examined one at a time.It is found that the combustion and NO emission characteristics are significantly affected by the equivalence ratio. The swirling premixed NH3/H2/air flame are V-shaped at all tested equivalence ratios. Comparing with that at the stoichiometric condition, the flame axial length is shorter, and the flame tip moves towards the combustor inner wall at either lean or rich combustion conditions. It is shown that NO and NH3emissions at the combustor outlet present a ‘‘trade off” relationship. That is to say, NO mole fraction is decreased, while unburnt NH3is increased with increased φ. Further study on NH3oxidation reaction paths reveals that the rates of NO formation are mainly affected by NH2→HNO, NH →HNO and HNO →NO sub-paths. Normalized reaction rates of all these three sub-reactions are decreased with increased φ.
Blending H2with NH3is found to enhance the burning velocity of NH3, benefiting NH3as an alternative carbonfree fuel in industrial engines. Laminar burning velocities are shown to increase with XH2, but the maximum burning velocities still occur at φ=1.1,no matter what XH2is set to.As the hydrogen addition ratio XH2is increased, the axial length of the premixed swirling flame and unburnt NH3are decreased dramatically, while NO generation is increased especially at the flame edges and tips. Blending hydrogen with NH3does not change the "trade off" relationship between NO and NH3emission at the combustor outlet. As XH2is increased,NO emission is increased significantly at a lower φ, while increased slightly or even remaining unchanged at a higher φ. The variation of the normalized reaction rates of NH2→HNO and HNO →NO sub-paths are found to be at the same trend. Moreover, at rich condition i.e. φ>1.0, hydrogen addition benefits on reducing unburnt NH3emission. In general, the present work reveals the combustion and emission characteristics of premixed NH3/H2/air swirling flame. It provides research basis for applying ammonia with hydrogen blended in low emission gas turbine engines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the University of Canterbury, New Zealand (No.452STUPDZ),the National Research Foundation, Prime Minister’s Office, Singapore(No.NRF2016NRF-NSFC001-102)and the National Natural Science Foundation of China(No.11661141020).
 CHINESE JOURNAL OF AERONAUTICS2021年12期
CHINESE JOURNAL OF AERONAUTICS2021年12期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Entropy based inverse design of aircraft mission success space in system-of-systems confrontation
- Nonlinear resonance characteristics of a dual-rotor system with a local defect on the inner ring of the inter-shaft bearing
- Failure mechanisms of bolted flanges in aero-engine casings subjected to impact loading
- Synchronized perturbation elimination and DOA estimation via signal selection mechanism and parallel deep capsule networks in multipath environment
- Improving seeking precision by utilizing ghost imaging in a semi-active quadrant detection seeker
- A high dynamics algorithm based on steepest ascent method for GNSS receiver
