Rapid cooling effect during solidification on macro-and micro-segregation of as-cast Mg-Gd alloy
Xin Tong ,Guoqing You ,Jingchun Luo ,Mhmoud Erhimi ,Guohu Wu
a National Engineering Research Center of Light Alloy Net Forming and State Key Laboratory of Metal Matrix Composites,School of Materials Science and Engineering,Shanghai Jiao Tong University,Shanghai,200240,China
b College of Materials Science and Engineering,Chongqing University,Chongqing,400045,China
cNational Engineering Research Center for Magnesium Alloys,Chongqing University,Chongqing,400044,China
d Department of Mechanical Engineering,Faculty of Engineering,University of Maragheh,P.O.Box 55136-533,Maragheh,Iran
Keywords:As-cast Mg-Gd alloy Segregation Rapid cooling Solidification Sedimentation
ABSTRACT The high performance of as-cast Mg-RE alloys is always related to their high RE additions.However,RE elements can be readily segregated in Mg alloys and the segregation becomes more significant with the increasing RE content.In this research,the effect of cooling rate on the macro-and micro-segregation in the as-cast Mg-8Gd alloy was studied.The Gd content at the bottom of the fabricated ingot with the cooling rate of 4.6-6.9 °C/s was~1.7 times of that at the top and coarse eutectics as well as some non-equilibrium phases of α-Gd,MgGd,Mg2Gd were distributed along the grain boundaries.The formation mechanisms of the specific gravity segregation and grain boundary segregation were also proposed.Upon the application of the water-cooled copper mold with the cooling rate of 27-200 °C/s,only fine Mg5Gd and some nanoscale metastable β1 and β′ phases were found to disperse uniformly in the grain interior,thus the homogeneity of the composition,microstructure,and performance within the whole ingot was considerably improved.It is expected that these results will facilitate the processing design for the fabrication of the highly-homogenized Mg-RE alloy castings.
1.Introduction
As the lightest structural metallic materials,Mg alloys have attracted considerable attention in the transportation and aerospace fields because of their low density,high specific strength,superior damping characteristic,and good thermal conductivity [1-3].However,their low absolute strength and poor heat-resistance have restricted the broader engineering applications of the Mg alloys [4,5].In recent years,the addition of rare-earth(RE)elements has been reported to be a promising approach to improve the mechanical properties of Mg alloys especially at elevated temperatures[6,7].As a consequence,a large number of Mg-RE alloys with high performance have been developed and the newly designed Mg-RE alloys can mainly be classified into Mg-Gd[8,9],Mg-Y[10,11],and Mg-Nd[12,13]series alloys.
Based on the relevant literature,it can be found that the as-cast Mg alloys with the tensile strength of higher than 350 MPa are only the Mg-RE alloys with a relatively high content of Gd element[14].A lot of investigations have also demonstrated that the aging strengthening effect of the Mg-Gd binary alloy could become obvious only when the Gd content exceeds 10 wt%[15-17].Because the solute partition coefficient of Mg-Gd binary alloy is less than 1,the resulting constitutional supercooling effect during solidification can lead to grain refinement[18].The truncated lenticular-shaped β′phase which grows on triangular prismatic planes and perpendicular to the basal plane of hcp Mg,is considered to be the most effective strengthening precipitate after introducing Gd to Mg alloys [19].Moreover,the solute Gd can interact with the moving dislocations and change the deformation mechanism to viscous glide during the deformation process [20,21].Unfortunately,the Gd element can easily segregate in the Mg casting alloys,including the macro-and micro-segregations,and the degree of segregation will be more critical with the increasing Gd content [22].On the one hand,because of the considerable differences of the atomic densities between the Mg and RE elements,the sedimentation of RE elements in the prepared mold after the pouring process can result in the specific gravity segregation (macro-segregation) [23].On the other hand,the network-like eutectic structure and the coarse RE-rich second phases (micro-segregation) can be readily produced during the solidification procedure [24].Both the two types of segregation can considerably decrease the composition and microstructure homogeneity,leading to the low service reliability of the Mg-RE alloy castings.
Wang et al.[25] introduced the pulsed magnetic field during the solidification of the Mg-12Gd-3.3Y-0.4Zr alloy and found that the macro-segregation in the whole ingot was effectively restrained because of the melt vibration impact derived from the magnetic field.In our previous study[18],ultrasonic treatment with the power of 700 W was obtained useful to improve the composition homogeneity of the Mg-8Gd ingot.Although the applications of ultrasonic treatment,electromagnetic field,and mechanical stirring show some advantages in the microstructure refinement of Mg-RE alloys,they have limited influence on decreasing the volume fraction of the RE-rich phases,especially for the Mg alloys with higher RE contents.That is,the above-mentioned melt treatment technologies still cannot essentially resolve the difficulty of micro-segregation in the Mg-RE alloys.
Even worse,studies have confirmed that some of the coarse eutectic structure in the as-cast alloys cannot be completely eliminated during the subsequent solution-treatment despite increasing the temperature or extending the treated time[24,26].The residual second phases not only seriously reduce the number density of the strengthening phases precipitated in the aging process but also increase the probability of crack initiation in the casting alloys.In this regard,Xiao et al.[27]found that eutectics are remaining in the Mg-9Gd-4Y-0.6Zr cast alloy even after the solution treatment.So,the author tried to eliminate the micro-segregation by the reduction of the Gd content from 9 wt%to 7 wt%.Although there is no obvious micro-segregation in the solution-treated Mg-7Gd-4Y-0.6Zr alloy,the tensile strength was also decreased simultaneously.Thus,reducing the content of RE still cannot be a fundamental solution for the micro-segregation in the Mg-RE alloys due to some strict requirements of high mechanical properties in the actual engineering applications.
Apart from the above approaches,the rapid-cooling solidification process can significantly improve the nucleation undercooling of the alloy and reduce the residence time of the melt in the solid-liquid twophase region [28,29].Therefore,rapid cooling can effectively promote the solubility of the RE elements in the α-Mg matrix.In other words,the existence form of the RE elements will be transformed from the second phase into the solute atoms with a rapid cooling rate during the solidification procedure.Thus,the amount of the eutectic structure can be reduced and the micro-segregation of the Mg-RE alloys can be resolved without changing the alloying composition.However,most of the current literature has been mainly focused on the effect of the cooling rate on the microstructure refinement [30,31] and there is no report in accordance with restraining the segregation of the Mg-RE alloy by increasing the cooling rate during the solidification.
According to the literature survey,in the present study,the as-cast Mg-Gd binary alloy was fabricated by two different molds with different cooling rates.The segregation degree in the fabricated alloy was evaluated in terms of the microstructure observations.The corresponding hardness measurements were also performed at different sampling locations of the fabricated ingots to investigate the homogeneity performance.It is believed that the achieved results may broaden the understanding of the fabrication of high-homogeneity Mg-RE alloys and further improve their engineering applications.
2.Experimental procedure
The Mg-8Gd alloy (the compositions are in weight percent unless stated) was prepared by melting commercial pure Mg (>99.95%) and Mg-25Gd master alloys in a melting furnace protected by a mixed atmosphere of CO2(99 vol%)and SF6(1 vol%).After melting the pure Mg at 720°C,the master alloy pre-heated at 250°C was added into the melt.The melt was stirred manually for 5 min and then held at 760°C for 30 min to reach full homogenized condition.Finally,the cylindrical-shaped ingots were fabricated in steel and copper molds with different cooling conditions.Prior to the pouring process,the steel mold was pre-heated to 200°C,while the copper mold was first heated to 200°C to remove the oil and contamination and then kept at room temperature.The cooling water with a flow of~20 L/min under the cavities of the copper mold was used to further improve the cooling rate.The actual composition at different sampling locations in the fabricated ingots was determined by a fluorescence analyzer(XRF-1800 CCDE).
Commercial software Anycasting was employed to simulate the cooling rates and temperature gradients during solidification.In the simulation,the temperature of the steel and copper molds was set to 200°C and 20°C,respectively.The casting parameters used in the simulation were the same as the situation of the actual casting process.Four positions in the two molds,which corresponded to different cooling rates and temperature gradients during the solidification,were examined by the virtual thermocouples.The positions of A(top)and B(bottom)were 15 mm and 115 mm from the bottom of the steel mold,while the positions of C (top) and D (bottom) were 2 mm and 27 mm from the bottom of the copper mold,respectively.The accurate cooling rate CR can be calculated by the simulated cooling curve according to the following equation:

where the TLand tLare respectively the temperature and time point of the solidification of the α-Mg matrix,TSand tSare respectively the temperature and time point of the solidus.
Scanning electron microscopy (SEM,JEOL JSM-7800F Prime)equipped with an energy dispersive spectroscopy(EDS)was utilized for the microstructure characterization.For this aim,the samples were mechanically ground,polished,and etched in a 4 vol% natal solution.Additionally,for microstructure observations using transmission electron microscopy (TEM,FEI TECNAI G2 F20 TEM),a disc with 3 mm in diameter was cut,ground,and twin-jet electro-polished at-30°C in the combined solution of HNO3and CH3OH (1:3 in volume).Then,the prepared foil was further thinned by the low energy ion-beam milling(GATAN PIPS II 695).
X-ray diffractometer (XRD,Rigaku Ultima IV) with the Cu Ka radiation was applied for phase identification.Also,differential scanning calorimetry(DSC)in a differential scanning calorimeter(Netzsch 449F3)was employed for measuring phase transition temperature.In this regard,each sample was heated up to 700°C with a heating rate of 10°C/min under an Ar atmosphere.The DSC results were analyzed via Netzsch Proteus Thermal Analysis software.Eventually,Vickers microhardness measurements(MH-60)and the related contour were performed at a load of 49 N and a holding time of 30 s at the cross-section of these samples with the vertical and horizontal distances of 1 mm from each other.
3.Results
3.1.Simulation analysis of solidification
Four positions(A,B,C,and D)equipped with virtual thermocouples are shown in Fig.1b and e.The simulation analyses in Fig.1a and d indicate that the temperature gradient of the ingot solidified in the copper mold (20-800°C/cm) was much higher than that of the steel mold(5-200°C/cm).Meanwhile,the ingot prepared by the copper mold showed a more directional distribution of temperature gradient than that prepared by the steel mold.Also,the cooling rate in the copper mold was much higher than that in the steel mold (Fig.1b and e).Fig.1c and f shows the cooling curves recorded by the four virtual thermocouples in the molds and it is can be found that the undercooling degree of the solidification of α-Mg at different positions was quite different.The highest undercooling can be obtained at the bottom of the copper mold(position D),while the top of the steel mold (position A) exhibited the lowest one.Table 1 illustrates the accurate cooling rates of these marked areas in Fig.1 calculated through equation (1),it can be found that the simulated results were in good agreement with the measured values performed by Sun et al.[32].
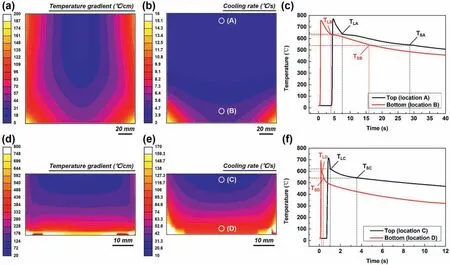
Fig.1.Simulation outputs of the(a,d)temperature gradients,(b,e)cooling rates,and (c,f) cooling curves of the ingots fabricated in the(a-c) steel mold and(d-f)copper mold.

Table 1 Cooling rates of the four labelled positions calculated via the simulated cooling curves.
3.2.Microstructure characterization
Fig.2 displays the actual Gd content at the different heights of the ascast Mg-8Gd ingots.For the ingot prepared by the steel mold,a relatively low Gd content was attained at the top section,while there is no considerable difference in the Gd content for the fabricated ingot by the copper mold.The lower slope of the fitting line of the Gd contents indicated the higher composition homogeneity of the fabricated ingot by the copper mold.
Fig.3 represents SEM images of these Mg-8Gd ingots at different heights.As can be observed in Fig.3a for the top sample,it primarily consisted of very developed primary α-Mg dendrites with the size even longer than 500 μm.With the decreasing sampling height,the volume fraction of the second phase was increased and the grain size of the α-Mg matrix was decreased (Fig.3a-c).It is worth noting that the second phases in the ingot fabricated by the steel mold mainly distributed along the dendrite boundary,and the sizes of the cubic phases were in the range of 2-4 μm.It should be mentioned that the cubic-shaped second phases are mainly single in the top section,while some of them are found to be joined to each other at the bottom section prepared by the steel mold(Fig.3f).As compared to the morphology characteristics in Fig.3a-c,the ingot fabricated by copper mold with a higher cooling rate showed the much finer microstructures.It can be seen that the second phase with a size of less than 1 μm dispersed uniformly in the α-Mg matrix.The EDS results in Table 2 proved that the plate-shaped second phases at the grain boundaries and the cubic-shape particles in the grain interior were all Mg5Gd phases.
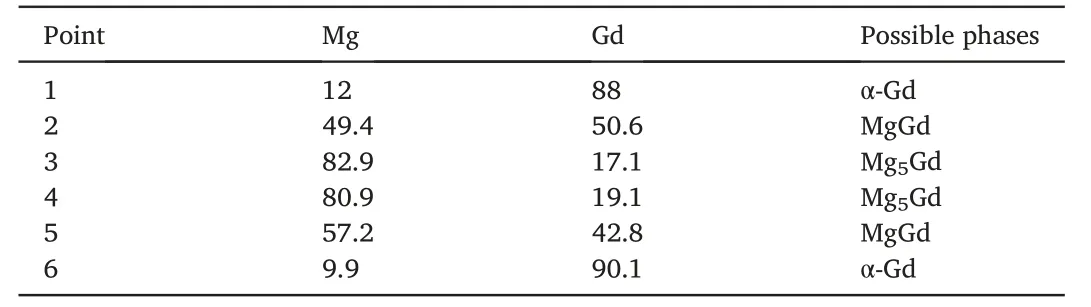
Table 2 EDS point analyses of the positions marked in Fig.3 (at.%).
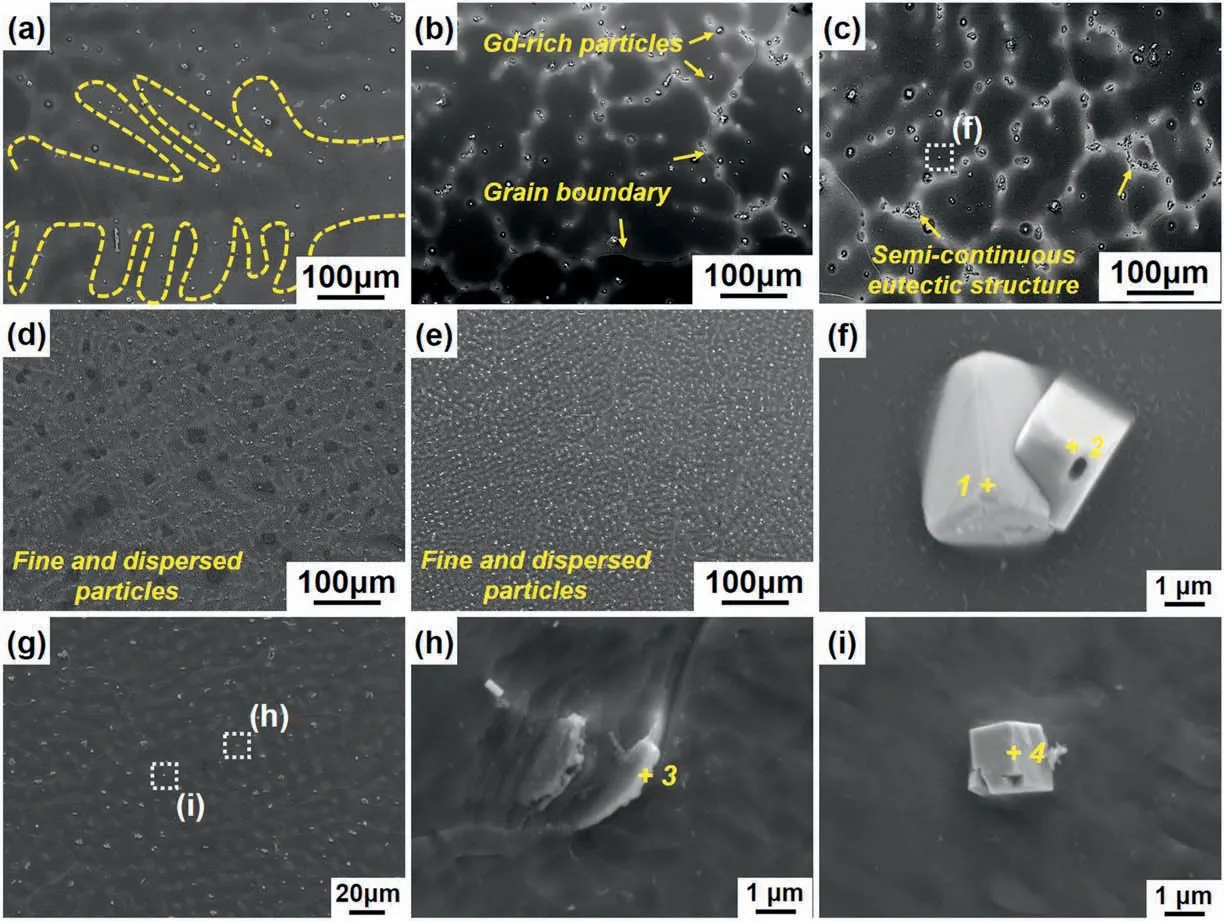
Fig.3.SEM images of the as-cast Mg-8Gd ingots at different heights:(a-c)top,middle,and bottom sections of the steel mold and(d&e)top and bottom sections of the copper mold.(f) is a magnified view of (c).(g-i) are magnified views of the bottom sections of the copper mold.
Based on the related atomic ratio of Mg and Gd in EDS analyses(Table 2) in accordance with Fig.3,it is clear that the adjacent cubic phases may respectively be α-Gd and MgGd.XRD patterns also prove that the bottom of the ingot fabricated by the steel mold contained α-Mg and α-Gd solid-solutions,Mg5Gd,and MgGd intermetallic compounds(Fig.4a).However,the top section only contained less number of diffraction peaks of Mg5Gd phase with lower intensities,indicating that the lower amount of Mg5Gd phase.In the ingot solidified in the copper mold,there were no MgGd or α-Gd phases but only some obvious peaks of Mg5Gd can be found(Fig.4b).
Fig.4c and d shows the DSC curves of those samples fabricated in different conditions.Two endothermic reactions can be observed at the temperature range of 540-550°C (labelled as A) and 600-640°C(labelled as B),individually.According to the Mg-Gd binary phase diagram,it can be speculated that the reaction A may be ascribed to the eutectic decomposition while the reaction B may be the melting of the α-Mg matrix.Table 3 shows the calculated heat flow value of the peak A as well as the melting temperature of the α-Mg matrix.It is worth noting that the heat flow of the reaction A increased and the melting point decreased gradually with the increasing sampling height of the ingot prepared by the steel mold.However,upon the utilization of the copper mold,not only the melting temperature of α-Mg of the two samples all tended to be around 620°C but also the two samples showed similar heat flow value during the decomposition of the eutectic structure.
Fig.5 exhibits the TEM bright-field images and corresponding selected area electron diffraction(SAED)patterns of the Mg-8Gd alloys.These two inlaid cubic particles in Fig.5a were obtained in our previous research[18]containing the same experimental condition as this work.It can be found from the EDS line(Fig.5b)and point(Table 2)analyses that they are α-Gd and MgGd phases.It is worth noting that,in the bottom of the ingot fabricated by copper mold,a large number of ellipsoidal-shaped precipitates with the size of~100 nm dispersed unevenly in the matrix(Fig.5c).It can be speculated from their directional distribution and nanometer-size that they cannot be formed during the solidification procedure.The SAED pattern with the incident electron beam parallel to[2110]αconfirmed that the elliptical precipitate was the metastable β′phase which always precipitates during the aging process after the solution treatment[15,33].The diffraction points of β′phase can be readily observed at 1/4 {0110}α,1/2 {0110}α,and 3/4 {0110}αpositions.However,as presented in Fig.5d,the β1phase was also found at the junction of β′phase,indicating that the β1phase was nucleated via an in-situ transformation from the decomposed β’ phase.Additionally,although OM and SEM observations of the bottom section prepared by copper mold showed that the grain size was~200 μm,there were some subgrains with the size of only~1 μm in the TEM bright-field image,as shown in Fig.5f.
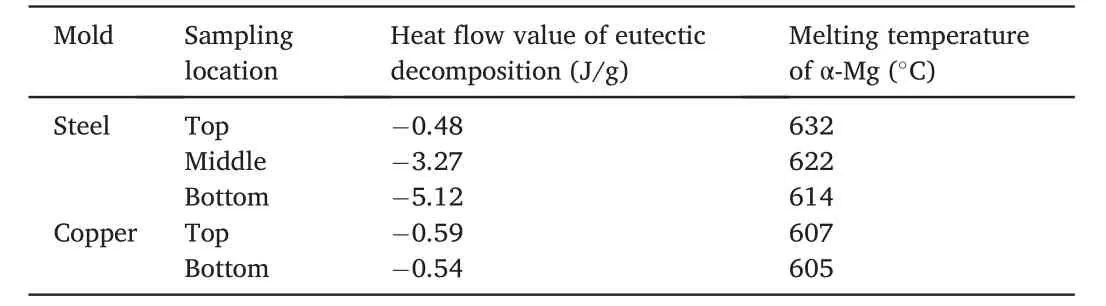
Table 3 Calculated heat flow value of the eutectic decomposition and the melting temperature of α-Mg.
3.3.Micro-hardness homogeneity
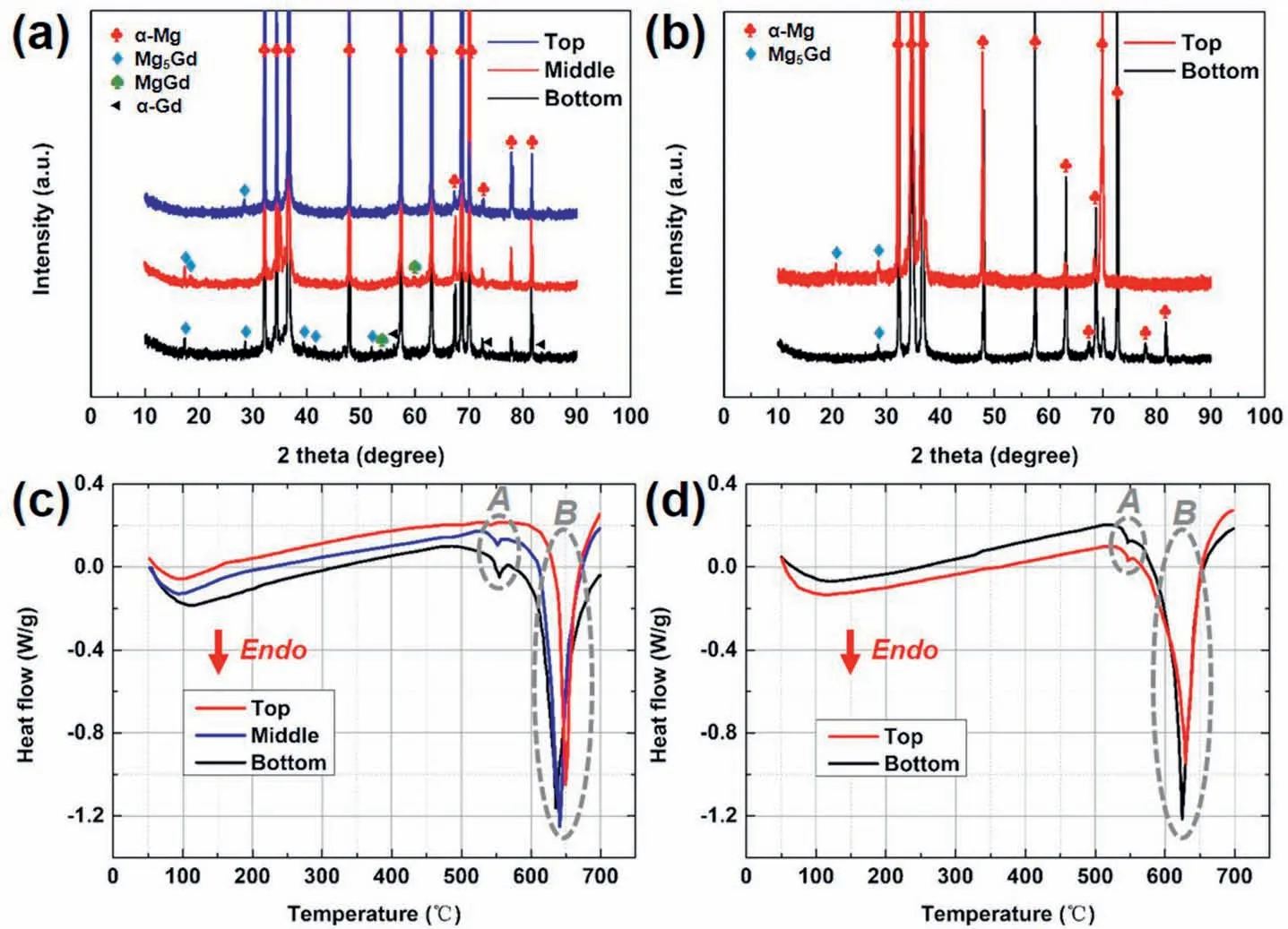
Fig.4.XRD (a,b) and DSC (c,d) analyses of the as-cast Mg-8Gd ingots prepared by (a,c) steel and (b,d) copper molds.
As the distribution of microstructure segregation can be reflected by the profile of the local microhardness,the hardness test was performed in a macro-region with a size of 10 mm×10 mm,as shown in Fig.6.In the ingot produced by the steel mold,the average hardness was improved constantly with the reducing height in which it reached the maximum 66.2 HV at the bottom section.This indicates that almost 14%improvement at the average hardness magnitude was obtained as compared to the top section.By fabrication of the as-cast ingot by the copper mold,there is no significant difference in the hardness magnitude of the top and bottom sections of the sample.The average hardness magnitude of the produced ingot by the copper mold is 63.4 and 63.6 HV for the top and bottom sections,respectively.Their two hardness surfaces with much lower variations also indicated their higher microstructure homogeneities as compared to the ingot solidified in the steel mold.
4.Discussion
Fig.7 shows the schematic diagram of the formation and inhibition mechanism of macro-segregation under different cooling rates.The simulated cooling rate of the melt in the steel mold is only 4.6-6.9°C/s because of the low thermal conductivity and relatively large size of the steel mold,and thus the residence time of the liquid phase in the solidliquid two-phase region was longer.As shown in Fig.7a,sedimentation of the Gd elements could appear within the melt due to the great difference in atomic density between Gd and Mg elements,which resulted in the Gd content at the bottom of the crucible being higher than that of the top [23].Because the solute partition coefficient of Mg-Gd binary alloy k0<1 [34],the upper part of the liquid phase with a lower Gd content in the crucible possessed a higher melting point,and α-Mg was first nucleated in the upper of the mold.Meanwhile,with the growth of α-Mg dendrites,some Gd atoms were continuously discharged from the solid phase into the melt (shown as the red balls) and then continue to sink.Finally,the formed α-Mg solid phase with lower Gd content and lower density proceeded to float up to the melt surface,which could aggravate the degree of specific gravity segregation,as shown in Fig.7c.This also can explain why the Gd content of the bottom section of the ingot fabricated by the steel mold was much higher than that of the top.
Fig.7d-f shows the solidification process of the alloy melt in the copper mold.Since the cooling water can keep the temperature of the copper mold low,the temperature gradient of the solidified melt in the copper mold increased.A higher temperature gradient led to better heat conduction,resulting in a higher cooling rate.As the cooling rate of the water-cooled copper mold reached up to 26.5-260°C/s,the kinetic undercooling of the melt in the copper mold is much higher than that in the steel mold [28,35].The relationship between the degree of undercooling ΔT and the critical nucleus radius R can be expressed as[36]:
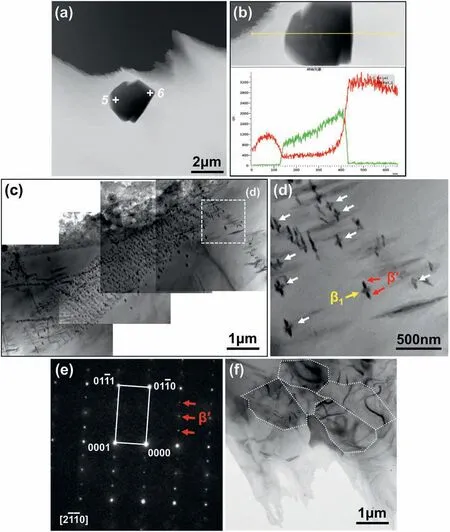
Fig.5.TEM bright-field images,SAED patterns,and EDS analysis of Mg-8Gd alloys fabricated in different conditions:(a,b)the bottom section of the fabricated ingot by steel mold,(c-f) the bottom section of the fabricated ingot by copper mold.

where σ is the surface energy per unit area of the ball-shaped nucleus and Tmis the theoretical crystallization temperature,ΔHfis the latent heat of fusion.This indicates that the critical nucleus radius of α-Mg in the copper mold is lower,and more crystal nuclei in the melt can stably exist and develop gradually into the dendrites.Under the same solidification time of t1~t3,the volume fraction of the solid phase in the copper mold is much higher than that of the steel mold.As a result,the solid dendrites rapidly generated in the melt can effectively prevent Gd elements from settlement and migration for a long distance,inhibiting the specific gravity segregation of the ingot.Furthermore,the higher cooling rate promotes non-equilibrium solidification of the alloy[28,37],and a large amount of Gd elements are directly retained in the form of solution atoms in the α-Mg matrix,which further improved the overall homogeneity of the ingot.All these factors led to the better Gd distribution uniformity of the ingot solidified in the copper mold,and it has been proved by XRF results in Fig.2.
Based on the Mg-Gd phase diagram [38],the top section with Gd content near 6 wt%in the ingot fabricated by the steel mold will almost be transformed into the α-Mg phase above the eutectic temperature.The amount of the generated eutectics was quite limited,so the XRD intensity of the diffraction peak related to the second phase was low,and also the heat flow of the eutectic decomposition in DSC was not obvious.Besides,the lower Gd content in the top section also resulted in the restrictive constitutional supercooling for grain refinement [4].As a result,the coarse α-Mg dendrites with large dendrite arm spacing can be easily observed in the SEM image.On the contrary,the severe specific gravity segregation of the Gd element caused a much higher Gd content(>10 wt%) of the bottom section,which led to the quite different solidification processes as compared to the top one.SEM microstructure of the bottom section contained a large number of continuous eutectic structures,and the decomposition peak of the eutectics at~550°C in the DSC curve also reconfirmed the presence of these eutectics.There were also some non-equilibrium phases with high Gd contents at the bottom of the ingot,such as α-Gd,MgGd,and Mg2Gd.
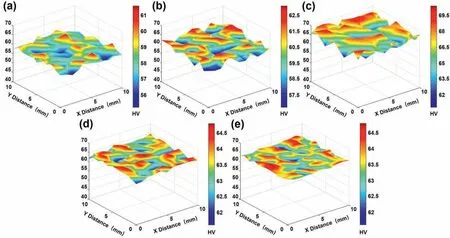
Fig.6.Vickers microhardness profiles of the as-cast Mg-8Gd alloys produced by(a-c)steel and(d&e)copper molds at the different sampling heights:(a&d)top,(b)middle,and (c &e) bottom sections.
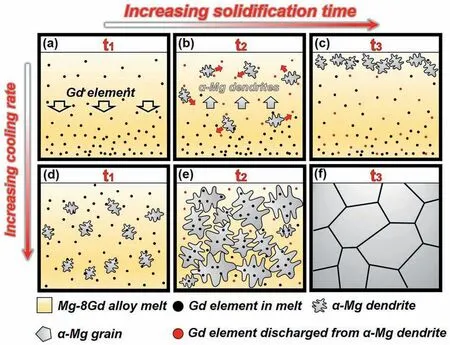
Fig.7.Schematic diagram of the formation and inhibition mechanism of macro-segregation under different cooling rates:(a-c) steel mold,(d-f) copper mold.
Fig.8a-f discusses comprehensively the microstructure evolution during the solidification process of the melt at the bottom of the steel mold according to its alloying composition and cooling condition.According to the literature [39],owing to the sedimentation of the Gd elements,the aggregation of the Gd at the bottom of the steel mold can result in a higher Gd content in the local micro-zone in the melt.According to the phase diagram,some primary phase such as β-Gd,MgGd may be generated from the local liquid with high Gd content,as shown in Fig.8b.Subsequently,α-Gd and MgGd phases can be produced simultaneously by the eutectoid reaction of β-Gd (Fig.8c),while the MgGd can also react with the surrounding liquid to produce Mg2Gd.Since α-Gd,MgGd,and Mg2Gd are all equilibrium phases of Mg-Gd alloys with high Gd contents,they can be kept stably at room temperature.As a result,the observations by SEM and TEM exhibited the cubic-shaped particles joined together which were proved to be α-Gd and MgGd or MgGd and Mg2Gd phases.As the melt temperature dropped below the solidus,the primary α-Mg phase nucleated from the liquid and grew gradually.Because of the low cooling rate as well as the mismatched crystal structure between α-Mg and those Gd-rich second phases,second phases can only be slowly pushed into the intercrystalline residual liquid by the development of α-Mg dendrites[18],as shown in Fig.8d and e.At the same time,the Gd element discharged from the α-Mg to the liquid further increases the Gd content of the intergranular liquid phase.When the temperature was reduced below the eutectic temperature,the residual liquid phase transformed into the eutectic structure.Therefore,in the OM and SEM microstructures related to the bottom of the ingot,a large number of cubic particles as well as the eutectic structures were distributed along the dendrite boundaries.From the above analysis,it can be found that the Gd atoms have enough time to sink to the bottom of the steel mold because of the slow cooling rate,which was the main reason for the specific gravity segregation.However,the specific gravity segregation caused the higher Gd content at the bottom,continuously facilitating the formation of grain boundary segregation(micro-segregation).
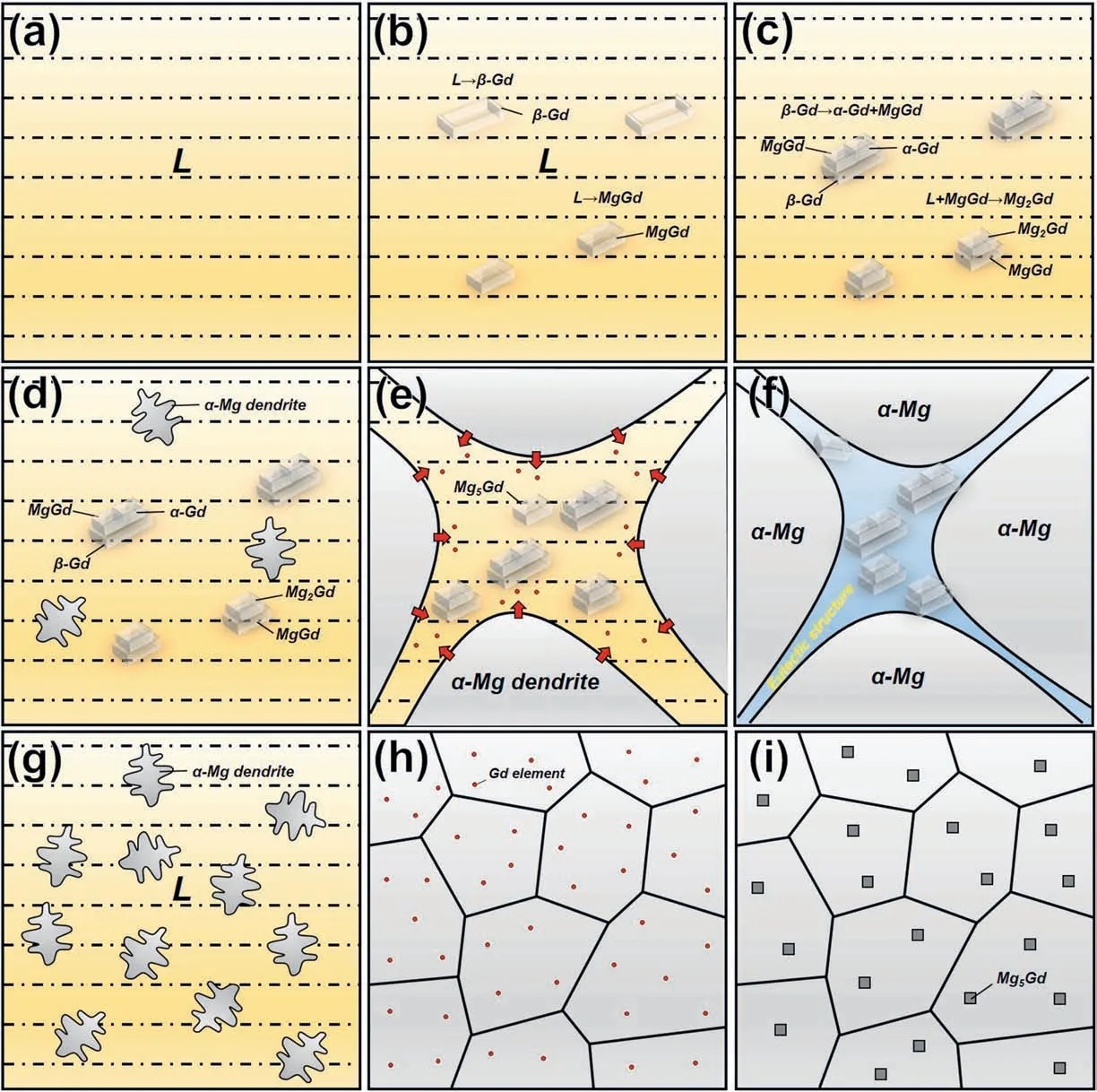
Fig.8.Schematic diagram of the formation of micro-segregation under different cooling rates:(a-i) steel mold and (g-h) copper mold.
On the contrary,the cooling rate of the water-cooled copper mold is much higher than that of the steel mold,which not only suppressed the macro-segregation but also effectively reduced the degree of microsegregation.On the one hand,α-Mg rapidly nucleated to form a“scaffold”in the melt with a faster cooling rate as mentioned earlier,which prevented the sedimentation of Gd thus suppressed the specific gravity segregation.On the other hand,a large number of Gd atoms were dissolved into the matrix under a rapid cooling rate[28,35],which showed the same effect of“solution treatment”,greatly reducing the number of eutectics along the grain boundaries,and thereby the micro-segregation was also restrained (Fig.8h).As the temperature of the matrix was continuously decreased to room temperature,the Mg5Gd equilibrium phases with the size of only~1 μm were precipitated from the matrix due to the decreasing Gd solid solubility in α-Mg[18].Also,the precipitation of the nanoscale metastable β1and β′phases can precipitate in the matrix,which was observed in the TEM images.
The distribution profiles of microhardness values can indirectly reflect the microstructure uniformity of the prepared ingots.The average hardness of the ingot solidified in the steel mold gradually increased as the sampling height decreased.As mentioned above,there were both macro-and micro-segregation in the ingot solidified in the steel mold with a low cooling rate.Higher Gd content at the bottom section not only caused the finer dendrite spacing because of the stronger effect of constitutional supercooling but also resulted in a higher amount of hard intermetallic compounds which can act as strong obstacles for the dislocation motion[4,40].These factors contributed to the strong grain boundary strengthening and second phase strengthening effects,resulting in the higher hardness of the bottom section.At the same time,it can be found that even at the same sampling height,the hardness value at the different positions also varied considerably.The maximum difference in the detected value of the top section was~5 HV(Fig.6a),while that of the bottom was closed to 8 HV,which reconfirmed that the micro-segregation at the bottom was more serious than that of the top.
The average hardness of the top and bottom parts of the ingot prepared by water-cooled copper mold was very close,and also the variety of the hardness at the same height was small (2.5 HV).This indicates that the high cooling rate was favorable for the fabrication of as-cast Mg-Gd alloy with high homogeneity.The ingot prepared at a high cooling rate contained the α-Mg matrix with finer grains and higher solid solubility,as well as dispersed strengthening phases [35].Therefore,grain boundary strengthening,solid solution strengthening,and precipitation strengthening should be the three main strengthening mechanisms of the alloy prepared by water-cooled copper mold.
5.Conclusions
1.Serious macro-and micro-segregation exist in the Mg-8Gd alloy ingot solidified with the cooling rate of 4.6-6.9°C/s,which leads to an extremely nonuniform distribution of the microstructure and hardness in the whole ingot.The Gd content at the top section of the ingot is~60% of that at the top,thus the average hardness of the bottom reaches~114%of the top.
2.Two main formation mechanisms have been proposed for specific gravity segregation.Firstly,the Gd element can sink in the melt due to the great difference in atomic density between Gd and Mg.Secondly,the primary α-Mg dendrites with lower Gd contents and densities can float to the melt surface,deteriorating further the specific gravity segregation.
3.The combined effect of specific gravity segregation and low cooling rate leads to severe micro-segregation at the bottom of the Mg-8Gd ingot solidified in the steel mold.Some non-equilibrium phases of α-Gd,MgGd,Mg2Gd,and coarse eutectics distributing along the grain boundaries results in a significant variation (~8 HV) of hardness at the tested horizontal plane.
4.Upon increasing the cooling rate by the usage of water-cooled copper mold,the solid phases with a large volume fraction generate rapidly in the melt and acted as the“scaffolds”,so the sedimentation of the Gd element was effectively suppressed.Therefore,the difference in the Gd content between the top and bottom of the Mg-8Gd ingot is limited to 1.5%.
5.During the solidification with a rapid cooling rate of 260°C/s,the Gd element retains in the matrix as solid solution atoms.Therefore,no eutectics exist in the alloy,and the micro-segregation significantly reduces.Only some fine Mg5Gd and nanoscale metastable precipitates β1and β′phases have been found to disperse uniformly in the grain interior.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Key Project of the National Key Research&Development Program of China(No.2016YFB0301100),the Key Research &Development Project of Guangdong Province (No.2020B010186002),and the National Natural Science Foundation of China(No.51775334).
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Surface study of the reconstructed anatase TiO2 (001) surface
- Effect of Na+ in situ doping on LiFePO4/C cathode material for lithium-ion batteries
- Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors
- Electric transmission behavior of self-assembled Cu-W nano multilayers
- A hybrid hydrogel/textile composite as flame-resistant dress
- Improvement on hydrogen generation properties of Zr(BH4)4·8NH3
