Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors
Ke Zhng ,Ze Cen ,Fng Yng,* ,Kiing Xu
a School of Mechanical and Automotive Engineering,Shanghai University of Engineering Science,Shanghai,201620,China b Research Center for Analysis and Measurement,Donghua University,Shanghai,201620,China
Keywords:Core-shell NiCo2O4 Fe2O3 Supercapacitors Synergistic effect
ABSTRACT Fe2O3 electrode materials exhibit excellent electrochemical performance in electrochemical energy storage system.However,its poor electrical conductivity limits its future practical application.The binder-free NiCo2O4@Fe2O3 composites was reasonably designed and fabricated on carbon fiber paper with NiCo2O4 nanowires as conductive scaffold in the present investigation.The three-dimensional nanostructure of the porous Fe2O3 nanorods coated the NiCo2O4 nanowire arrays showed the fascinating electrochemical performance,including high specific capacitance of 262 mF/cm2 at a current density of 1 mA/cm2,and remarkable cycle stability with~74.2% capacitance retention after 4000 cycles.The excellent pseudocapacitance performance of NiCo2O4@Fe2O3 composite materials is due to synergistic effect between NiCo2O4 and Fe2O3.The results of the present work show that NiCo2O4@Fe2O3 core-shell composite electrode is expected to exhibit excellent performance in the field of supercapacitors.
1.Introduction
Nowadays,supercapacitors with high power density,excellent cycle stability and rapid rate of charge and discharge have gradually been favored by researchers as a new type of energy storage device[1-7].As we all know,the performance of supercapacitors is seriously affected by the characteristics of electrode materials,which can limit their applications in new energy vehicle and wearable energy storage systems.Therefore,the research and design of electrode materials with high-performance will promote supercapacitors to become a new generation of efficient energy storage devices [8-14].Transition metal oxides can provide high specific capacity due to the different element valence states of their reversible reactions,thus researchers have been committed to developing transition metal oxide electrode materials with excellent pseudocapacitance properties[15-18].
Fe2O3materials have been considered to have an excellent development prospect in new electrochemical energy storage benefit from its high theoretical capacity,abundant reserves and non-toxicity [19-23].However,the poor conductivity of Fe2O3(~10-14S/cm) hinders its further development to a great extent[19,24-26].In order to resolve this problem,many measures have been conducted,such as constructing nanostructured Fe2O3,Fe2O3-based composites and oxygen defects Fe2O3[20,25,27-29].The most effective of these methods is to construct Fe2O3-based composites by using the synergistic effect among various materials.Tang et al.[24]successfully prepared Fe3O4@Fe2O3core shell nanorod arrays composites for supercapacitors,the volume specific capacitance can reach 1206 F/cm3.NiCo2O4nanomaterials can be used as high-performance support materials to improve electrode performance due to their high conductivity and electrochemical reactivity [8,10,30-32].Ouyang et al.[33] successfully synthesized NiCo2O4-N@NiO and NiCo2O4-S@NiO composites on carbon cloth,with specific capacitance of 921.9 and 852.9 mF/cm2at 2 mA/cm2,respectively.Yang et al.[11]prepared NiCo2O4@NiO composites on carbon fiber,with a specific capacitance of 1188 F/g at 2 A/g and a capacitance retention of 106.8%after 7000 cycles.Therefore,it is of great significance to build Fe2O3--based composites with NiCo2O4nanomaterials as support materials for improving the electrochemical performance of Fe2O3nanomaterials.
In this paper,NiCo2O4nanowire arrays were synthesized on carbon fiber paper,and then the porous Fe2O3nanorods were grew on NiCo2O4nanowire arrays,constructing NiCo2O4@Fe2O3composites.It was proved by various characterization methods that the porous Fe2O3nanorods grew on NiCo2O4nanowires uniformly,and had the characteristics of core-shell structure.The interlaced structure of nanowires and nanorods not only provides a channel for electrolyte ions,but also gives a full play to the high capacity of Fe2O3and the excellent conductivity of NiCo2O4,thus the whole electrode shows excellent electrochemical performance.
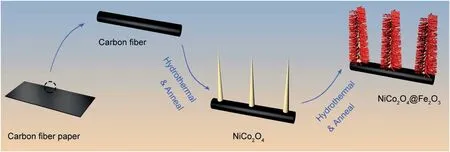
Fig.1.Schematic illustration for the formation procedure of NiCo2O4@Fe2O3 composites on carbon fiber paper.
2.Experimental
Synthesis of NiCo2O4 nanowire arrays on carbon fiber paperAll the reagents used were of analytical grade(purchased from Sinopharm)and used without further purification.First,the 1×4 cm2carbon fiber paper was ultrasonically washed with deionized(DI)water and ethanol for 20 min respectively.Ni(NO3)2·6H2O(0.29 g),Co(NO3)2·6H2O(0.58 g)and urea(0.9 g)were dissolved into 25 ml DI water and 25 ml ethanol,which were then stirred magnetically for 1 h.Then the above mixed solution and the as-washed carbon fiber paper were transferred into a 60 ml Teflon-lined stainless autoclave.The autoclave was sealed and heated at 120°C for 6 h,and then let it naturally cool to the room temperature.The carbon fiber paper supported precursor was washed with DI water and ethanol,and then dried at 60°C for 12 h.Finally,the precursor was treated at 300°C for 2 h to obtain NiCo2O4nanowire arrays.
Preparation of NiCo2O4@Fe2O3 nanowire arraysFeCl3(0.16 g)and Na2SO4(0.14 g)were dissolved into 50 ml DI water and stirred for 1 h,and then the solution was transferred into a Teflon-lined stainless autoclave with the carbon fiber paper supported NiCo2O4nanowire arrays.The autoclave was heated at 120°C for 10 h,and then let it naturally cool to the room temperature.After that,the sample was cleaned several times by DI water and ethanol,and then annealed at 350°C for 2 h to obtain NiCo2O4@Fe2O3core-shell composites.For comparison,Fe2O3nanorods were synthesized directly on carbon fiber paper by the same method.The mass loading of the Fe2O3and NiCo2O4@Fe2O3electrode was about 0.76 and 1.54 mg/cm2,respectively.
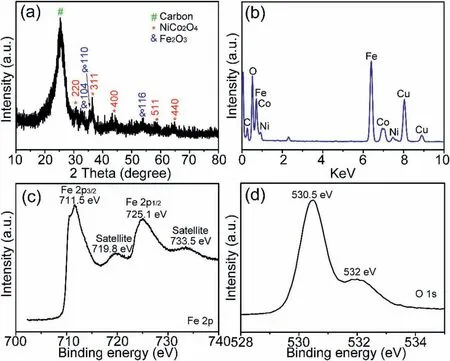
Fig.2.(a) XRD pattern,(b) EDS spectrum,(c) Fe 2p and (d) O 1s XPS spectra of NiCo2O4@Fe2O3 composites.

Fig.3.SEM images of (a) carbon fiber paper,(b,c) NiCo2O4 nanowires.(d,e) TEM images and (f) HRTEM image of NiCo2O4 nanowire.
Materials characterizationThe surface morphology and microstructure of the samples were analyzed by a scanning electron microscopy (SEM,S-4800,Hitachi) and a transmission electron microscopy(TEM,JEM-2100F,JEOL)with an energy-dispersive X-ray spectrometer(EDS,Oxford X-MAX 65T).The crystalline structure and elemental valence state were characterized using X-ray diffractometer using Cu-Kα radiation (XRD,Rigaku) and X-ray photoelectron spectroscopy (XPS,Escalab 250Xi,Thermo Fisher).
Electrochemical measurementsThe as-prepared electrode materials were used as the working electrode,a platinum plate(Pt)electrode and a saturated calomel electrode(SCE)is used as counter electrode and reference electrode,respectively.The electrolyte is 1.0 M Na2SO4aqueous solution.All the electrochemical measurements were performed with an Autolab electrochemical workstation(PGSTAT302 N).
3.Results and discussion
The synthesis process for the NiCo2O4@Fe2O3composites is displayed in Fig.1.The entire fabricating process can be divided into two parts.Firstly,porous NiCo2O4nanowire arrays as a supporting material were synthesized on carbon fiber paper via hydrothermalcalcination processes;Subsequently,Fe2O3nanorods were fabricated on the NiCo2O4nanowire arrays by hydrothermal and calcination treatment uniformly,forming NiCo2O4@Fe2O3core-shell composite.The as-prepared NiCo2O4@Fe2O3composites were directly grown on carbon fiber paper can effectively reduce interface resistance and improve electron transfer rate by avoiding the use of additive and binder [34].In addition,the porous structure enables the material to have a relatively large specific surface area and generates much reactive sites [35].
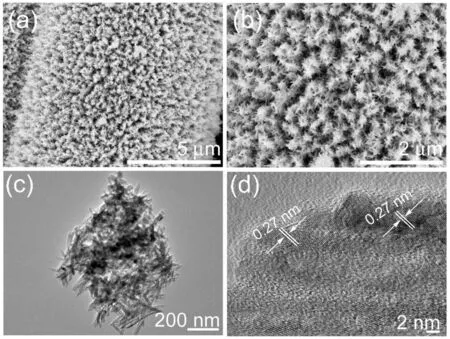
Fig.4.(a,b) SEM images,(c) TEM image and (d) HRTEM image of NiCo2O4@Fe2O3 composites.
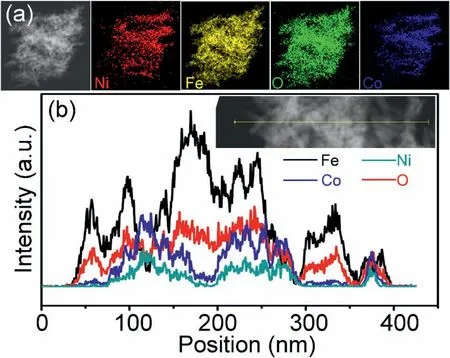
Fig.5.(a) EDS elemental mapping images and (b) EDS line scan curves of NiCo2O4@Fe2O3 composites.
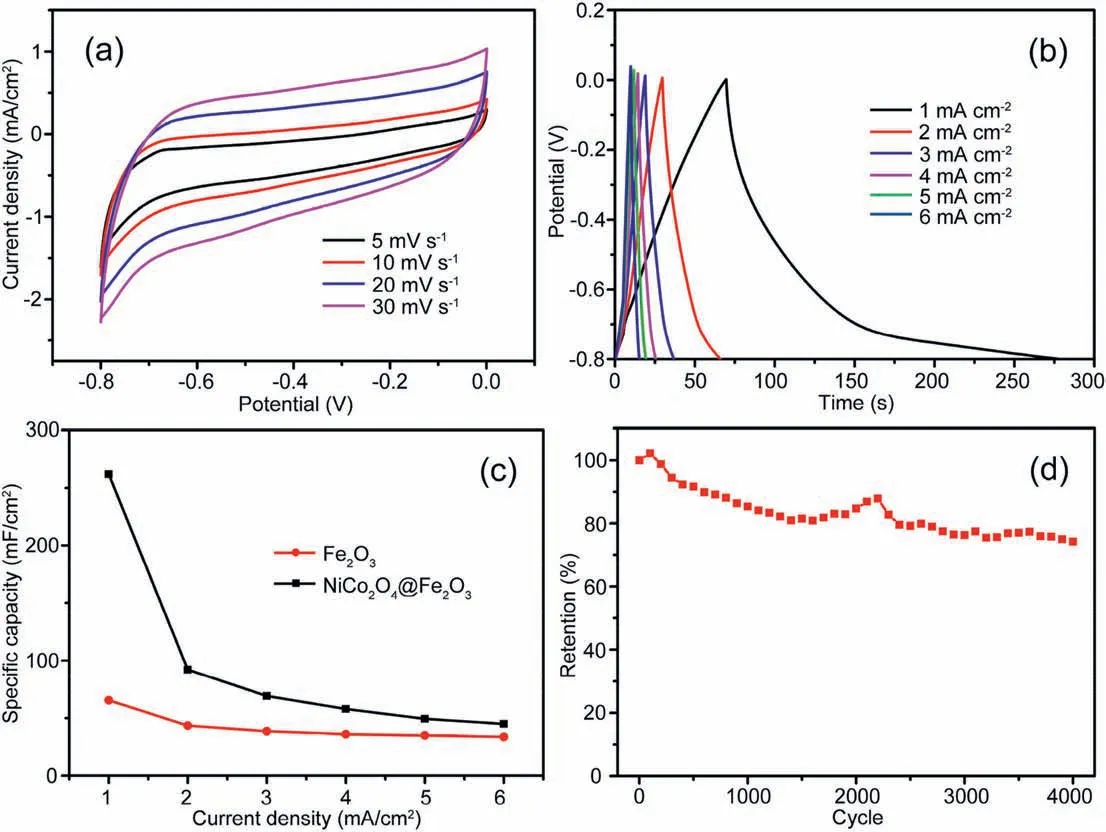
Fig.6.(a) CV curve,(b) CD curve and (d) cycle performance of the NiCo2O4@Fe2O3 electrode.(c) Comparative of specific capacitance for the NiCo2O4@Fe2O3 and pure Fe2O3 electrode.
Fig.2a reflects the XRD pattern of NiCo2O4@Fe2O3composite grown on the carbon fiber paper.As shown in the pattern,all the peaks can be perfectly correlated with the diffraction peaks of the cubic phase NiCo2O4(JCPDS,No.20-0781)and Fe2O3(JCPDS,No.33-0664),except for the diffraction peak of the carbon fiber paper substrate.There are no other residues or contaminants peaks,proving that NiCo2O4@Fe2O3composite had high purity.Fig.2b demonstrates EDS spectrum of the NiCo2O4@Fe2O3composite.There were only Fe,Co,Ni and O elements in the composite,among which Cu and C elements corresponds to the carbon-coated copper grid.Fig.2c shows the XPS spectra of Fe 2p.It can be seen that the binding energy at 725.1 eV and 711.5 eV are assigned to the Fe 2p1/2and Fe 2p3/2peaks,respectively,and there are two“satellite”peaks,which illustrates the existence of Fe2O3in the prepared materials [19,24,29].Fig.2d gives the XPS spectra of O 1s.The binding energy at 532 eV is assigned to the defects with low oxygen,and the binding energy at 530.5 eV is assigned to the metal-oxide bond [24,36,37].The above results indicate that NiCo2O4@Fe2O3composite material was successfully prepared on carbon fiber paper.
As shown in Fig.3a(SEM image of pure carbon fiber paper),carbon fiber paper is a three-dimensional network composed of carbon nanofibers,which is widely used in supercapacitors,fuel cells and Li-ion batteries due to its high specific surface area and excellent conductivity.Fig.3b and c shows the SEM image of NiCo2O4nanowire arrays,it can be seen that the needle -like NiCo2O4nanowire arrays were synthesized uniformly on carbon fiber paper with large pores between each other.Fig.3d shows the TEM image of NiCo2O4nanowire with an average length of~1.8 μm and a porous nanostructure.It can be seen from Fig.3e that the pores diameter in NiCo2O4nanowires was only a few nanometers.HRTEM image of NiCo2O4nanowires(Fig.3f)show that the samples have excellent crystallinity and clear lattice fringes.The lattice spacings of 0.244 nm and 0.287 nm correspond to(311)and(220)crystal planes of NiCo2O4phase,respectively.
Fig.4a and b shows the SEM images of NiCo2O4@Fe2O3composite material.It can be illustrated that Fe2O3nanorods uniformly grew on NiCo2O4nanowire arrays.Importantly,the original NiCo2O4nanowire arrays were not damaged by the composite Fe2O3nanorods.It can be illustrated from Fig.4c that the prepared NiCo2O4@Fe2O3composite material was a core-shell structure and the Fe2O3nanorods showed a porous nanostructure.Fig.4d shows the HRTEM images of porous Fe2O3nanorods,where the lattice spacings of 0.27 nm are assigned to the(104)crystal plane of Fe2O3.
The microstructure of NiCo2O4@Fe2O3composite was further proved by EDS element mapping and line scan curves.The Fe,Co,Ni and O elements uniformly distributed through the whole material as shown in the EDS elemental mapping(Fig.5a).Fig.5b shows EDS line scan curves of NiCo2O4@Fe2O3,which further prove that Fe,Co,Ni,and O elements existed in NiCo2O4@Fe2O3composite material,and they had core-shell structure characteristics.
Furthermore,the electrochemical properties of as-prepared electrode material were evaluated by a typical three-electrode system using 1.0 M Na2SO4aqueous solution.The CV curves of NiCo2O4@Fe2O3electrode at a scanning rate ranging from 5 to 30 mV/s are shown in Fig.6a.With increasing the scanning rate,the shape of the curve remained basically unchanged,indicating the excellent pseudocapacitance and good reversibility of the NiCo2O4@Fe2O3electrode.The galvanostatic chargedischarge (CD) curves of NiCo2O4@Fe2O3electrode at the current density ranging from 1 to 6 mA/cm2are shown in Fig.6b,and the nearly symmetric CD curve illustrates the excellent coulombic efficiency of the electrode materials.According to the discharge time (Fig.6b),the area specific capacitance of the electrode was calculated through the following formula:Cwhere I is discharge current (mA),t is discharge time(s),S is the geometric area of the electrode(cm2),and △V is voltage interval(V)of the discharge.As shown in Fig.6c,the specific capacitance of NiCo2O4@Fe2O3electrode was 262 mF/cm2at a current density of 1 mA/cm2,which is much higher than that of pure Fe2O3electrode(65.6 mF/cm2at 1 mA/cm2).The high specific capacitance of NiCo2O4@Fe2O3composite is also superior to some recently reported electrodes,such as Co3O4nanowires@Fe2O3nanorods electrode (245 mF/cm2at 1 mA/cm2) [38],Fe2O3/Vertical graphene nanosheets electrode (151 mF/cm2at 10 mV/s) [39],α-Fe2O3/polyaniline hybrid nanostructured hydrogels electrode (236.8 mF/cm2at 1 A/g) [40].The NiCo2O4@Fe2O3electrode also showed excellent cycling stability(Fig.6d),and retained 74.2%of the initial specific capacitance after 4000 cycles at 60 mV/s.

Fig.7.Schematic illustration electron paths of the NiCo2O4@Fe2O3 composites on the carbon fiber paper.
Fig.7 shows the ion/electron transfer mechanism of NiCo2O4@Fe2O3composite electrode.Compared with other reported electrode materials,the main reasons for the excellent electrochemical performance of NiCo2O4@Fe2O3electrode are:(1) The carbon fiber substrate with strong conductivity as the support of the active material can promote rapid electrons transfer;(2)The open and free interspaces among these Fe2O3nanorods can enhances the specific surface area of the electrode and provides more active sites for the reaction,thus making the material more fully utilized[41,42];(3)The direct growth of NiCo2O4@Fe2O3on carbon fiber can avoid using binders and conductive additives,reducing the interface resistance and improves electron transmission[43];(4)The porous NiCo2O4nanowire arrays provide electron“superhighways”for ion transfer,overcoming the limited conductivity of Fe2O3nanorods.
4.Conclusions
The NiCo2O4nanowire arrays have been prepared on carbon fiber paper,and then Fe2O3nanorods grown on NiCo2O4nanowires by hydrothermal-calcination method,constructing NiCo2O4@Fe2O3composites.The NiCo2O4@Fe2O3composite materials show the excellent electrochemical properties,including a high specific capacitance of 262 mF/cm2at 1 mA/cm2,and excellent cycling stability of 74.2% capacitance retention after 4000 cycles.The excellent capacitive behavior benefits from the special core-shell hierarchical structure.Meanwhile,the direct growth of the active material on the support material is conducive to improving the overall conductivity of the electrode.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript,and manuscript is approved by all authors for publication.I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously,and not under consideration for publication elsewhere,in whole or in part.All the authors listed have approved the manuscript that is enclosed.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(Grant No.51602049).
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Surface study of the reconstructed anatase TiO2 (001) surface
- Effect of Na+ in situ doping on LiFePO4/C cathode material for lithium-ion batteries
- Electric transmission behavior of self-assembled Cu-W nano multilayers
- A hybrid hydrogel/textile composite as flame-resistant dress
- Improvement on hydrogen generation properties of Zr(BH4)4·8NH3
- A TEM study on the microstructure of spark plasma sintered ZrB2-based composite with nano-sized SiC dopant
