A hybrid hydrogel/textile composite as flame-resistant dress
Yuanling Nie,Innocent Tendo Mugaanire,Ying Guo,Ruili Wang,Kai Hou ,Meifang Zhu
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,College of Materials Science and Engineering,Donghua University,2999 North Renmin Road,Shanghai,201620,China
Keywords:Nanocomposite hydrogel Hydrogel/textile composite Flame resistant Silicon dioxide
ABSTRACT Currently,the functional and cost-effective flame-resistant textiles (FRTs) are on high demand.However,such FRTs based on general polymer fabrics are always expensive,readily decompose,and their temperatures quickly rise once exposed to thermal environments.Inspired by the large specific heat of water and high decomposition temperature of inorganic substances like SiO2,this study establishes a simple casting strategy for preparing flameresistant gel/textiles (FR-GTs).The findings showed that active diffusion of aqueous AAM/SiO2 pre-gel solution into the textile structure enabled the formation of a tough interfacial adhesion between the hydrogel and textiles.The interfacial toughness reached~272 J/m2 because the PAAM/SiO2 nanocomposite hydrogel was filled into textile to form the semi-interpenetrating structure at the interface.The presence of chemical crosslinker(PEGDA)and physical crosslinker (SiO2) limited the volume expansion of the hydrogel upon swelling.In addition,the PAAM/SiO2 nanocomposite hydrogel layer prevented burning in high temperature environments (over 100 °C),due to the heat dissipation of water during evaporation.This simple strategy provides a guidance towards the fabrication of hybrid hydrogel/textile composites for the applications like household fire resistant materials such as flame-resistant gloves.
1.Introduction
Every year,fire disasters from nature and human activities kill almost thousands of people globally.Most of these fire accidents occur indoors and cause severe burns towards human beings [1].In comparison to large-scale public fire-fighting equipment,portable and effective individual protective indoor gear is particularly vital.Notably,for emergent purposes,flame-resistant textiles (FRTs),such as fire resistant blankets[2],clothes[3]and heat insulation gloves[4]etc.,are most effective at saving lives in case of indoor fire outbreaks[5,6].
In most cases,typical FRTs rely on intrinsic fire-proof performances of materials,like high decomposition temperature,good thermal insulation property and char-formation after being burnt [1].Although polymer-based FRTs are widely applied,drawbacks like low decomposition temperature result in burning at high temperatures or promote flame drip of polymers leading to secondary fire disasters.In addition,some FRTs based on high performance polymers are characterized by aromatic heterocyclic and phenyl structures,such as polyaramid [7,8]and polyimide[9,10]with high decomposition temperatures,are mainly applied in special equipment rather than daily use due to their high prices.As a result,the surface modification and nanocomposite strategies are common practices employed for the preparation of polymer based FRTs.During burning processes,the thermal expansion on the surface of FRTs results in the isolation of pyrogen,oxygen and other inflammable gas to promote fire resistance[11-14].Therefore,such modified general textiles are applicable as household FRTs[15].The establishment of new types of FRTs based on modification of general textiles is an effective and cheap strategy to improve the flame-resistant behavior of household FRTs.
In this regard,hydrogels are important soft materials with inherent flexibility similar to common fabrics[16].Compared to general textiles,the large amount of water in hydrogels acts as the phase-transformation agent to promote evaporation for its large specific heat capacity[1,17].Under hot flames,heat energy is dissipated,leading to evaporation of water.Thus,hydrogels are promising candidates as flame resistant materials[18]for applications in personal daily protection[19].However,there are still challenges associated with neat polymer-based hydrogel as flame resistant materials like poor mechanical properties,inflammability and low melting point.
To overcome these drawbacks,the fabrication of organic/inorganic composite hydrogels with inorganic nano-/micro-particles as crosslinkers or fillers is highly desirable [20,21].In addition to mechanical enhancement [22] of organic/inorganic hybrid hydrogels,inorganic particles can also serve as typical flame-resistant agents.They demonstrate wide applications in fire resistant materials such as fibers and plastics [23-27] due to their high specific heat capacity,high melting point and barrier property after sintering[28].For example,a commercial organic/inorganic hybrid hydrogel seriflux consisting of hydrophilic polysaccharide and SiO2microparticles [29]enabled the fire protection from wildfires due to its inherent thixotropic property.However,such slurry hydrogels are merely applicable for spray but not suitable for FRTs.
In the present study,a simple casting strategy is developed to produce a flame-resistant gel/textile composite (FR-GT)with SiO2as both crosslinkers and fire-resistant agents.The interfacial toughness of the asprepared FR-GT reached 272 J/m2because PAAM/SiO2nanocomposite hydrogel was filled into textile to form a semi-interpenetrating structure at the interface.The presence of the chemical crosslinker (PEGDA) and physical crosslinker(SiO2)limited the volume expansion after swelling of the hydrogel.In addition,the hydrogel maintained below 100°C even in high-temperature environments till all water evaporated.Burning behavior was prohibited due to heat dissipation from the evaporation of water.This simple strategy provides a guidance towards the fabrication of hybrid hydrogel/textile composites for applications as flame-resistant gloves and other household flame-resistant equipment.
2.Material and methods
2.1.Materials
Polyethylene glycol diacrylate (PEGDA,Mn700),polyethylene glycol methacrylate (PEGMA,Mw360) and N,N,N′,N′-Tetramethylethylenediamine (TEMED) were all purchased from Sigma-Aldrich.Acrylamide (AAM) and potassium persulfate (KPS) were supplied from Sinopharm.SiO2particles with a diameter of about 1 μm,and clay(Laponite XLS,92.32 wt%[Mg5·34Li0·66Si8O20(OH)4]Na0.66and 7.68 wt%Na4P2O7)were provided by Zhejiang tongdaweipeng electric co.LTD and BYK Additives&Instruments,respectively.Viscose non-woven fabric is a commercially available product produced by Jinxu environmental protection products (Shenzhen) Co.Ltd.Ultrapure water was self-made by water purification system.
2.2.Fabrication of flame-resistant gel/tex composite (FR-GT)
The fabrication process of FR-GT is shown as Scheme 1 illustrated.AAM and SiO2were added into aqueous clay dispersion (obtained by exfoliation of clay in water after magnetic string) under vigorous string(15,000 rmp) for 10 min to obtain homogeneous AAM/SiO2/clay dispersion.PEGDA and PEGMA were mixed via high speed stirring(15,000 rpm) for 10 min until the pre-gel solution was obtained.After adding KPS (5 wt%) and TEMED into the pre-gel solution,the solution was immediately transferred into a mold with viscose non-woven fabric at the base.After polymerization at 30°C for 1 h,the Gel/Tex composite(FR-GT) was successfully obtained.Since the parameters of viscose nonwoven fabric were constant,the flame-resistant hydrogel was denoted as FR-GTX(abbreviated as GTX),where X is the weight percentage of SiO2in the pre-gel solution.As a control group,the neat hydrogels without non-woven fabric layer (FR-G) were also fabricated for the morphology,tensile properties,swelling behavior,heat insulation and thermogravimetry tests.The neat hydrogel was denoted as FR-GX(abbreviated as GX),where X is the weight percentage of SiO2in the pregel solution.The compositions of the samples are summarized in Table 1.
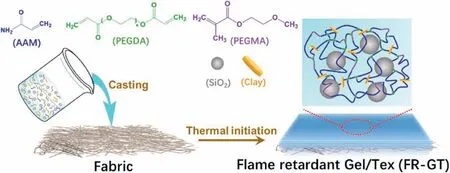
Scheme 1.Schematic illustration of the preparation of flame-resistant Gel/Tex composite (FR-GT).
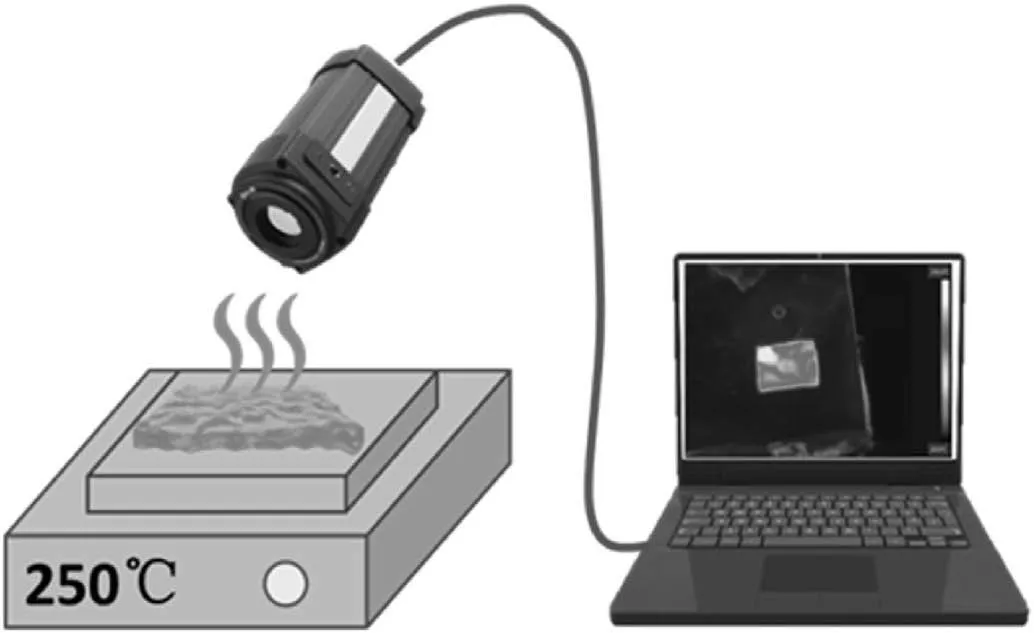
Scheme 2.Heat insulation experiment of hydrogel.
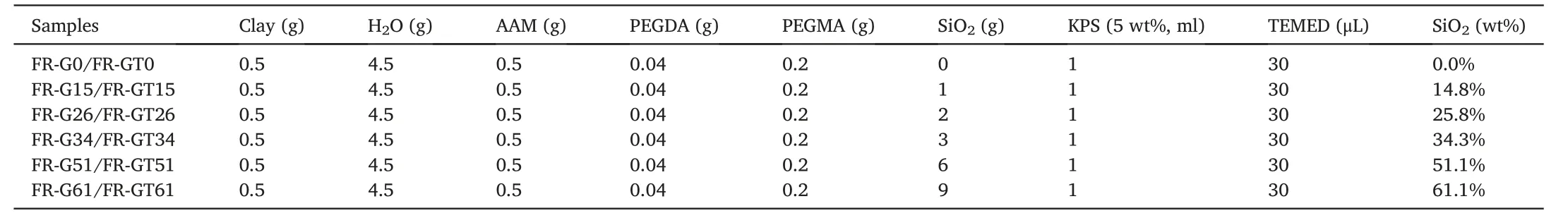
Table 1 Components of the samples.
2.3.3 characterization
2.3.1.Morphology and structure
The morphologies of FR-G and FR-GT were observed by environmental scanning electron microscope (ESEM,Quanta-250,FEI,Czech).The samples were lyophilized and gold sprayed on the surface before observation.The elements distribution of silicon (Si),magnesium (Mg)and nitrogen (N) belonging to SiO2,clay and PAAM respectively was analyzed by energy dispersive spectroscopy(EDS,AZtec X-Max,Oxford,UK).
2.3.2.Swelling behavior of hydrogel
The swelling behavior of FR-G was characterized by both weight and dimension analysis.The as-prepared uniform cylindric sample was 2 cmin diameter and 0.5 cm in thickness.For weight analysis,the frozen-dried samples were immersed in water at 25°C for some time,and then the residual water on the surface was wiped off.The swelling ratio(SRw)was calculated according to equation(1)below:

where Wt,and Wdrepresent weight of sample at real-time swelling and frozen-dried state,respectively.
For dimension analysis,the as-prepared samples were immersed in deionized water for 72 h and then the residual water on the surface was wiped off.The equilibrium dimension swelling ratio (ESRD) was calculated based on equation(2)below[30]:

where DEand D0are the diameters of the sample at equilibrium swelling and as-prepared state,respectively.
In each case,five samples were measured for the mean value and standard deviation.
2.3.3.Mechanical and interfacial adhesion properties
The tensile properties of hydrogel were measured by tensile and peeling mode on a testing machine (Cell scale,Canada).The FR-G for tensile test was 5 cm in length,1 cm in width and 1 mm in thickness with the elongation rate of 4 cm/min at room temperature (20°C).For recovery test,the tensile and recovery rate were 4 cm/min and 2 cm/min,respectively.Each sample was tested under three different strains,including 10%,30%and 50%for 4 cycles.
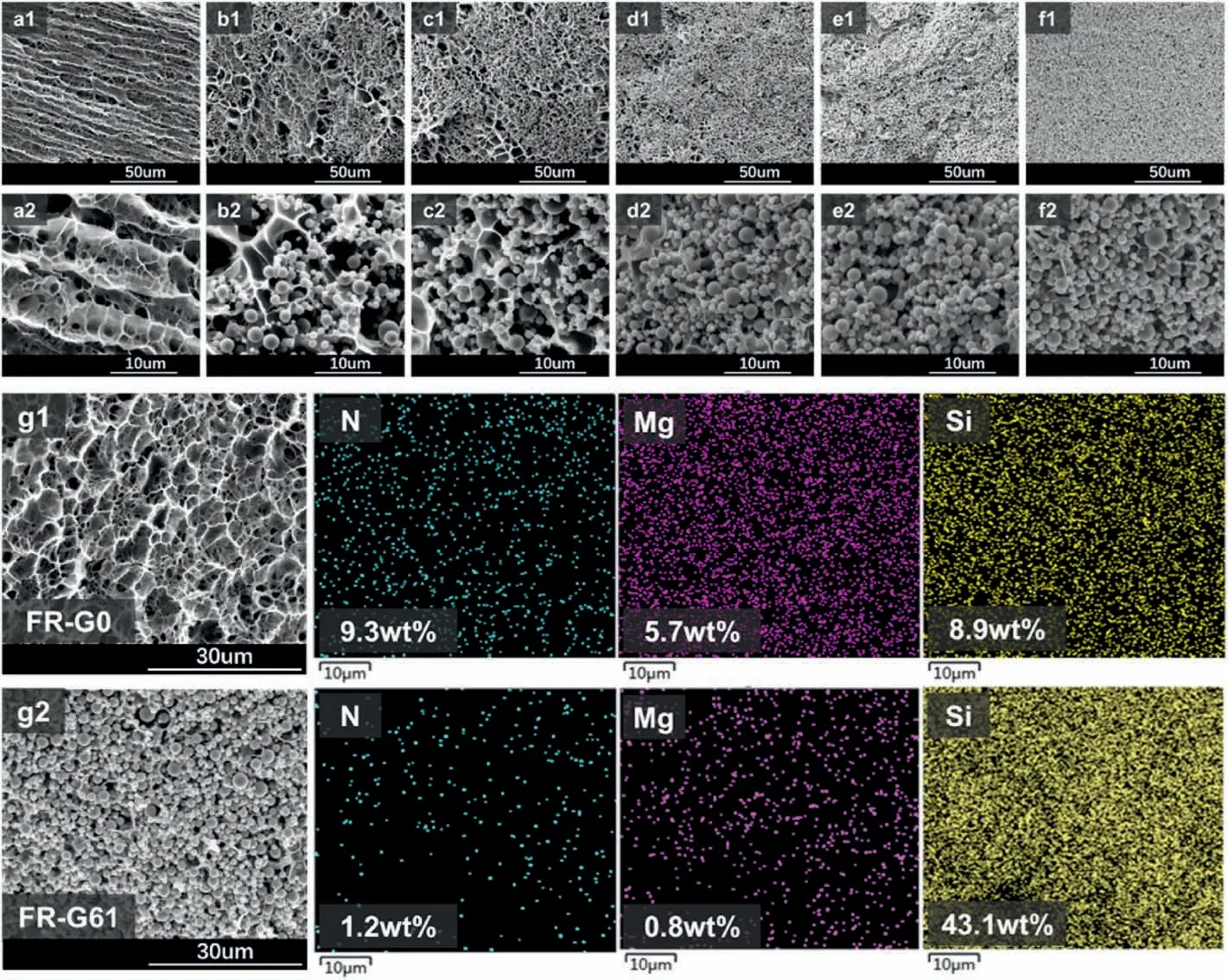
Fig.1.Morphologies of the FR-G.(a-f) SEM illustration of the hydrogel with different SiO2 content in different magnification.(a:FR-G0,b:FR-G15,c:FR-G26,d:FRG34,e:FR-G51,f:FR-G61);(g) element distribution analysis of hydrogel without (g1) and with (g2) SiO2.
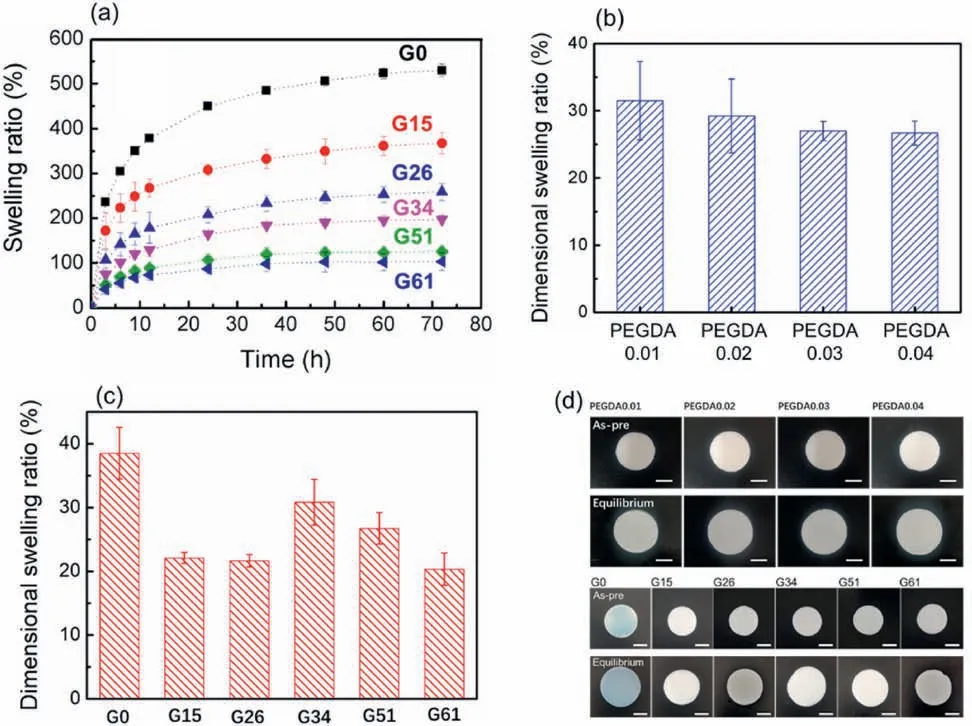
Fig.2.Swelling behavior of the hydrogel.(a)Swelling behavior of FR-G with different SiO2 content;Equilibrium dimensional swelling ratio of FR-G with different(b)PEGDA and (c) SiO2 content;(d) Digital images of hydrogel in as-prepared and equilibrium swelling state (scale bar is 1 cm).
The interfacial adhesion property of FR-GT was carried out by 180°peeling of the hydrogel from nonwoven fabric.In this measurement,the hydrogel layer was adhered to polyethylene terephthalate backing with cyanoacrylate adhesive to prevent the elongation during the test.The hydrogel layer for peeling measurement was 1 cm in width and 1 mm in thickness.The gauge length and peeling rates were 5 cm and 0.5 mm/s,respectively.Interfacial toughness (Γ) was calculated according to equation(3)below[31]:

where Γaveis the average force in the peeling process,and w is the width of the sample.
Compression cyclic performance was carried out on a universal test machine(Instron 5969).Cylindric sample(Φ 2 cm×3 cm)was placed at the center of the compression plate under different compression strains including 10%,30%and 50%for 3 cycles.
In either case,five samples were measured to calculate the mean value and standard deviation.
2.3.4.Heat insulation experiment
First,the as-prepared cylindrical FR-G(Φ2cm×4 cm,storage in 25°C before testing)was placed on the heating stage(250°C).Heat insulation experiment was carried out by the recording of temperature variation and infrared images of the sample in real-time on heating stage by a thermal infrared imager (FLIR A300-Series,Sweden),as shown in Scheme 2.
2.3.5.Thermogravimetry (TG) experiment
The thermal stability of FR-G was evaluated by thermogravimetric analysis(Libra 209F1).The weight of samples for TG analysis was about 5 mg-8 mg.The measured temperature was from 30°C to 310°C at a heat rate of 10 k/min in air conditions.The differential thermogravimetry analysis (DTG) was obtained by the derivative of TG curve with NETZSCH-Proteus software.
2.3.6.Fire resistance experiment of FR-GT
A blowtorch with alcohol (>95% w/w) as fuel was used as a fire source to evaluate the fire resistance property of FR-G.The flame temperature was about 1000°C.The sample for fire resistance experiment was 4 cm×4 cm×5 mm in size and clamped at 5 cm above the blowtorch.The tests were carried out for 5 min and the digital images of the samples were recorded with a camera after 1 min,3 min and 5 min.
3.Results and discussion
The flame-resistant Gel/Tex composite(FR-GT)was prepared via insitu polymerization based on a simple casting strategy.In order to enhance the mechanical property of hydrogel,clay acted as the physical crosslinker.Based on previous reports,nanocomposite hydrogels formed by large amounts of organic components and clay via physical crosslinking,like hydrogen bond,van der Waals force etc.,always possess enhanced mechanical property and stimuli-responsiveness [32-35].In order to confine volume expansion and enhance the mechanical behavior of the hydrogel in its swollen state,PEGDA with diacrylate terminal group was introduced into the hydrogel as a chemical crosslinker[36]to form a physi-chemical dual crosslinking structure.Moreover,PEGMA in this research worked in two ways.Firstly,PEG-type macromolecule served as a surfactant to disperse the nanoparticle without aggregation,which adsorbed on the surface of nanoparticles to reduce surface energy and enhance the steric hindrance [37,38].Secondly,PEGMA with relative longer chain length than AAMenlarged chain length between chemical crosslinker,preventing the fragile/brittle inherent property of chemical crosslinked structure,and improved the mechanical property of hydrogel [39,40].After thermal-initiation of the casting precursor solution onto viscose nonwoven fabric,the FR-GT was obtained(Scheme 1).The fluidic precursor solution easily permeated the fabric to form a fiber reinforcement structure at the interface.Due to the hydrogen bonding interaction and fiber reinforced structure between fabric and hydrogel,FR-G displayed excellent flexibility.Based on the casting strategy,the hydrogel was compatible with textile substrates daily used such as gloves as Gel/Tex composites.
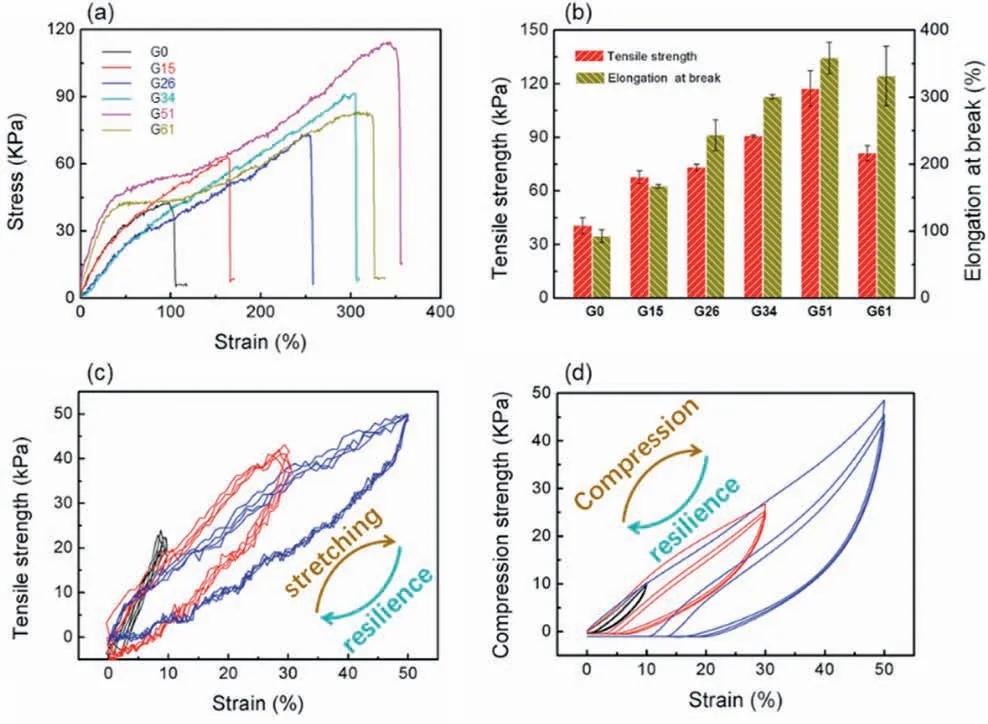
Fig.3.Tensile property of the FR-G.(a) strain-stress curve for FR-G with different SiO2 content;(b)Statistics of tensile stress and elongation at break of FR-G with different SiO2 content;(c) tensile and (d) compression cyclic curve of FR-G51.
Morphology analysis of FR-G with different SiO2contents was carried with SEM,and the results are shown in Fig.1.The hydrogel without SiO2(FR-G0) as filler exhibited a typical porous structure like traditional hydrogels(Fig.1a).However,FR-G with SiO2particles exhibited closely packed structures.In addition,SiO2was homogeneously dispersed in FRG even when the content of SiO2particles reached 61 wt% (FR-G61).From Fig.1b and c,low content of SiO2in gel network(FR-G15 and FRG26) was beneficial for the enhancement of mechanical properties.However,as SiO2content further increased (Fig.1d-f),the gaps of neighboring SiO2particles were filled with the polymer to form a compact structure.The closely packed structure implied that the rigid microparticles could function well as fillers,which is crucial for improving the mechanical stiffness of hydrogels[41],and promoting the formation of flame-resistant layer upon inflaming.
The EDS technique was used to verify the distribution of SiO2in hydrogel.According to Fig.1g,N and Mg belonging to PAAM andclay,respectively,were well distributed in FR-G0 and FR-G61.Thus,polymer and clay were well dispersed in the hydrogel irrespective of SiO2contents.Notably,Si from both clay and SiO2was higher in FR-G61 compared to FR-G0.The denser distribution of Si and sporadic distribution ofN and Mg implied homogeneous dispersion of SiO2in the hydrogel,contributing to the dense structure in FR-G61.The content of Si element in FR-G61 increased from 8.9%to 43.1%compared to FR-G0,while Mg and N contents decreased from 5.7% to 0.8% and from 9.3% to 1.3%,respectively.
Water in hydrogel is indispensable for fire resistant application due to the heat dissipation during its evaporation.In this section,the swelling behavior of FR-G is discussed and the results are shown in Fig.2.As SiO2content increased,both the swelling rate and equilibrium swollen ratio(ESR) decreased.As for FR-G0,the ESR reached 529.9 ± 14%.On the contrary,FR-G61 showed poor swollen property with ESR reaching only 103.0 ± 20%.The reason would be that the increase of SiO2acted as crosslinking points in the hydrogel and fully filled its porous structure,which inhibited the chain extension of hydrogel upon swelling [29].Besides,dimensional swelling ratio(DSR)was also important during the fabrication process via casting strategy.In this case,the large volume expansion of hydrogel layer induced interfacial tension,which macroscopically changed the shape of FR-GT.Then the effect of SiO2and PEGDA content on dimensional swelling behavior of FR-G was investigated in details.From Fig.2b,equilibrium DSR of FR-G61 decreased with the increase of PEGDA content,due to the enhanced chemical crosslinking structure from PEGDA as crosslinking points.Such hydrogel showed less DSR than that of the corresponding as-prepared hydrogel.On the contrary,their equilibrium DSR demonstrated a“decrease-increase-decrease”tendency with increasing SiO2content.During the first decreasing stage(G0 to G26),SiO2filled into the pores of hydrogel and served as a physical crosslinking point to confine volume expansion upon swelling.Then further increasing the content of SiO2(G26 to G34)blocked the crosslinking interaction between PEGDA and other monomers,leading to the increase of volume expansion upon swelling [29].Lastly,above 34 wt%SiO2content (G34 to G61),the acquired hydrogel filled the gaps among SiO2,contributing to confined volume expansion behavior.
To verify the prominent the mechanical property of hydrogel,the cyclic tensile and compression measurements were carried out.As shown in Fig.3a and b,the tensile property of hydrogel was enhanced with the increase of SiO2.In detail,the tensile strength and elongation at break of hydrogel without SiO2(FR-G0) were 40.6 ± 4.7 kPa and 92.7 ± 9.2%,respectively.In comparison,the hydrogel with high SiO2content (FRG51)showed improved tensile strength and elongation of 117.3±10.0 kPa and 358.7 ± 23.6%,respectively,which were 2.9 and 3.9 times higher than those of FR-G0.The reason could be ascribed to nanoparticle reinforced mechanism in the nanocomposite hydrogel.However,for FRG61,with higher concentrations of SiO2,its tensile strength and elongation at break both decreased because excessive SiO2in the polymer matrix leads to the formation of stress-defect points.In addition,cyclic mechanical measurement in tensile mode was performed to investigate the recovery of nanocomposite hydrogels from deformation like the bending and twisting behaviors encountered under normal usage.However,in the compression mode,the predominant SiO2network in hydrogel collapsed at higher strain and exhibited undesirable recovery from strain.
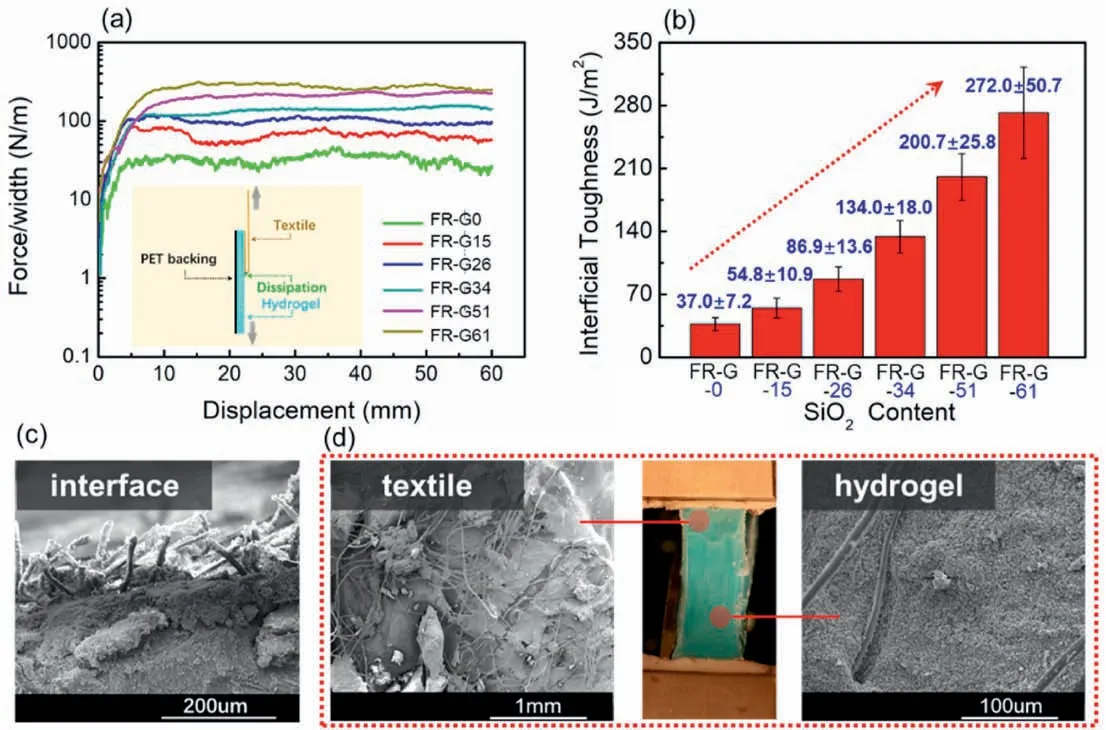
Fig.4.Interfacial adhesion property of the FR-GT.(a)Peeling curves of force per width of the FR-GT versus the displacement;(b)Relationship of interfacial toughness of the FR-GT with different SiO2 content;(c) SEM images of cross section morphology,(d) peeled hydrogel and textile layer of FR-GT.
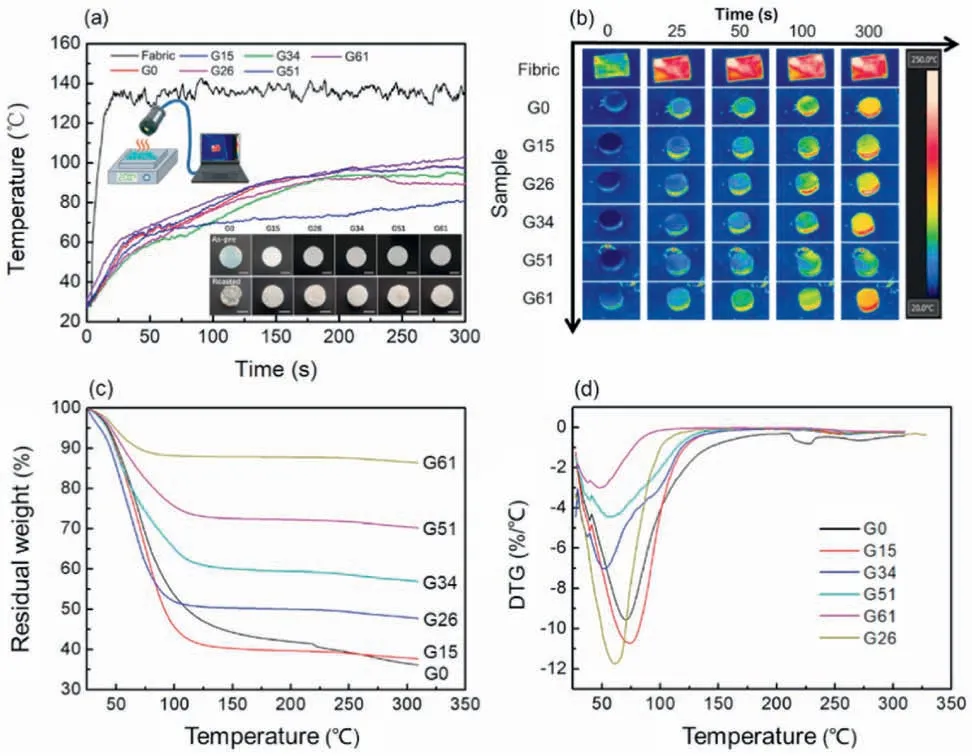
Fig.5.Thermal insulation property of hydrogels.(a)Heating curve of hydrogels with different SiO2 content.(b) Near infrared imaging of hydrogels.(c) TG and (d)DTG curves of hydrogels with different SiO2 contents.
Tough interfacial adhesion of each layer is essential for realizing the composition of hydrogel and textile.Herein,the 180°peeling experiment of hydrogel from viscose nonwoven fabric was carried out to evaluate the interfacial adhesion property of FR-GT.As shown in Fig.4a,stable peeling curves for FR-GT reflected proper adhesion between textile and hydrogel,demonstrating the effectiveness of the casting method.Moreover,the influence of SiO2content on the interfacial toughness was investigated.As shown in Fig.4b,the interfacial toughness was enhanced by SiO2,which was~37.0±7.2 J/m2for FR-G0 and 272.0±50.7 J/m2for FR-G61.It indicated that SiO2particles could indeed improve the adhesion between the hydrogel and the viscose nonwoven fabric.Moreover,after peeling off the hydrogel layer,the residual surface of the fabric layer was rough with hydrogel residues.Similarly,the fiber residues remained on the hydrogel surface.This demonstrated the enhanced interfacial adhesion of FR-GT due to the formation of a fiber reinforcement structure at the interface among hydrogel and fabric (Fig.4c).Therefore,the peeling process of FR-GT resulted in two types of fracture including separation of the hydrogel from the textile and self-fracture of the hydrogel.For FR-GT,hydrogel adhered to the textile via non-covalent interlinks,forming a semi-interpenetrating structure.Therefore,the interfacial toughness of FR-GT could be mainly attributed to the inelastic energy dissipation from molecular chain breaking.
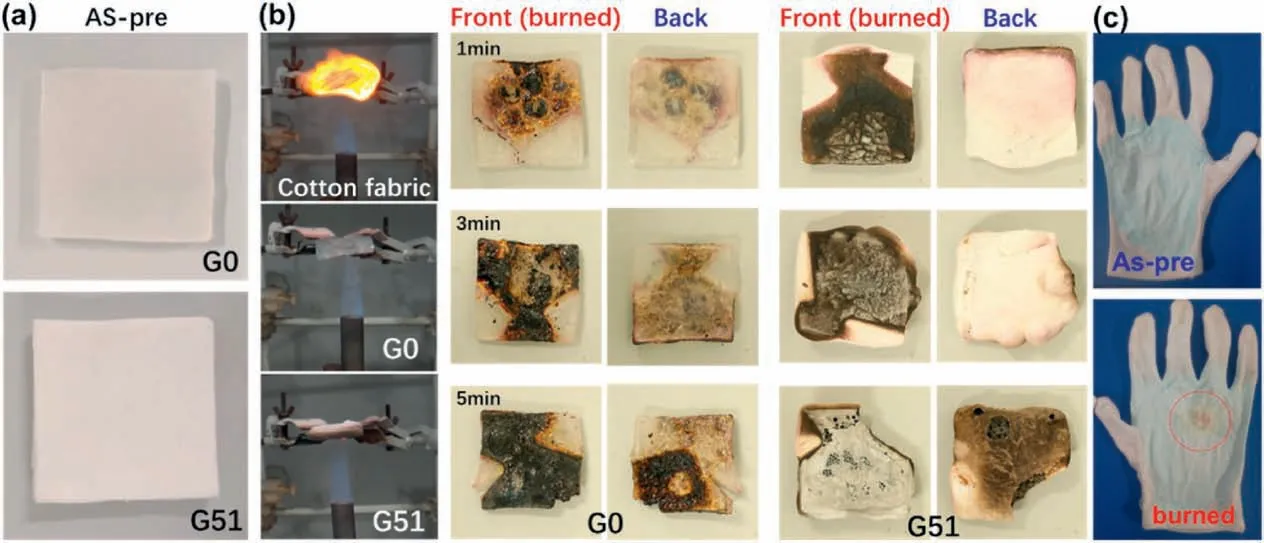
Fig.6.Combustion test of hydrogel.(a)Digital images of the hydrogel before combustion;(b)Digital images of the combustion test process for front/back images of samples under different burning times;(c) Practical application of hybrid hydrogel/textile composites as flame retardant gloves.
In order to evaluate the thermal insulation property of the hydrogel,its surface temperature and corresponding near infrared images were recorded in real-time (Fig.5a).The neat textile reached equilibrium temperature at about 135°C within 25 s,indicating poor thermal insulation of neat textiles.However,hydrogel showed notable improvement in thermal insulation due to the large amount of water,which possessed high specific heat capacity and evaporation.The surface temperature of such hydrogel with SiO2reached about 50°C in 25 s and then slowly increased to equilibrium temperature at about 90°C within 300 s(Fig.5a and b).Moreover,with the increase of SiO2content,the heating rate and equilibrium temperature of FR-GT both decreased.Such effect was obviously observed in FR-G51,which possessed equilibrium temperature at about 75°C,merely 50%of neat fabric.The results demonstrate that higher SiO2content contributed to the higher thermal insulation property of hydrogel,forming a compact and thick residual layer during the heating process.However,when SiO2content was high to 61 wt%,thermal insulation of FR-G61 was reversed due to the dominant effect of inorganic components,which normally possess lower thermal insulation properties than organic components with the large amount of water.This result implies that SiO2layer was required for thermal insulation,but the high content of SiO2accelerated the thermal conduction rate than the organic component with water.Consequently,FR-G51 was chosen as the sample for fire resistant application.Thermogravimetric (TG) and derivative thermogravimetric (DTG) analyses were carried out to investigate their thermal degradation (Fig.5c and d).From TG curves,dramatical weight loss at the first stage(<100°C)was attributed to the evaporation of water of hydrogel.Moreover,decomposition of G0 occurred at~200°C-225°C.In comparison,the decomposition temperature of G15 to G26 increased to~250°C due to existing physical interactions between nanoparticles and polymer,which is beneficial for the improvement of thermal stability in nanocomposites [42,43].With SiO2content increasing,the residue was enhanced[44].The degradation temperature of hydrogel with higher SiO2content started earlier due to its low water content and weak binding forces among water and SiO2[45].
The fire resistance of neat fabric,FR-G and FR-GT was performed(Fig.6).The neat fabric immediately burnt after exposure to the hot flame.However,both FR-G0 and FR-G51 withstood the flame for a much longer time(Fig.6b).Even though the frontages(burnt surface)of FR-G0 and FR-G51 burnt in the first 1 min,the back remained relatively unchanged after exposure to the hot flame.Moreover,the hydrogel remained flexible after burnt due to the residual water.With continuous exposure to the flame (until 3 min),the back surface of FR-G0 became char due to the complete evaporation of water.In comparison,the back of FR-G51 remained almost the same due to the presence of a thermal insulation layer containing charred SiO2.After 5 min of flame exposure,the back surface of FR-G51 charred with destructive hydrogel layer.The fire-resistant FR-G could cast on general cotton gloves (Fig.6c).The gloves with hydrogel layer (2 mm in thickness) could not be burnt on alcohol lamp for almost 10 s without degradation,demonstrating possible applications as fire blanket and fire protection household gloves.
4.Conclusion
A simple and effective casting method has been established for fabricating hydrogel/fabric composite flame-retardant materials with a silica-filled hydrogel as a flame retardant layer.The hydrogel/fabric composites possess excellent interfacial bonding due to the semiinterpenetrating polymer networks and fiber reinforcement at the interface.Silica (physical crosslinker) and PEGDA (chemical crosslinking)limit the volume expansion of the hydrogel.Moreover,silica acts both as physical cross-linker and inorganic filler to improve the mechanical properties and the interfacial bonding properties of the hydrogel/fabric composites.Acting as the coating layer,the hydrogel improves the thermal insulation performance of non-woven fabrics,which is applicable for flame-retardant gloves construction.This simple,continuous,and costing effective strategy provides a guidance on the fabrication of hybrid hydrogel/textile for potential applications in fields of firefighting and personal protection.
Declaration of competing interest
There are no conflicts of interest regarding this manuscript,which has been approved by all authors.
Acknowledgments
This work is financially supported by National Natural Science Foundation of China(NO.51803022,51733002);National Key Research and Development Program of China (2016YFA0201702/2016YFA0201700);The Fundamental Research Funds for the Central Universities (Grant No.2232018A3-01);Program for Changjiang Scholars and Innovative Research Team in University (IRT16R13) and China Postdoctoral Science Foundation(2018M631980).
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Dynamic response characteristic of 7N01/7A01/7050 aluminium multilayer plate at high strain rate
- Synergetic effect of multiple phases on hydrogen desorption kinetics and cycle durability in ball milled MgH2-PrF3-Al-Ni composite
- Growth mechanisms of Ag and Cu nanodendrites via Galvanic replacement reactions
- Highly mechanical and high-temperature properties of Cu-Cu joints using citrate-coated nanosized Ag paste in air
- Ab-initio investigation for the microscopic thermodynamics and kinetics of martensitic transformation
- Rapid directionally solidified microstructure characteristic and fracture behaviour of laser melting deposited Nb-Si-Ti alloy
