Synergetic effect of multiple phases on hydrogen desorption kinetics and cycle durability in ball milled MgH2-PrF3-Al-Ni composite
Zhonghui Sun,Jia Zhou,Qingan Zhang
School of Materials Science and Engineering,Anhui University of Technology,Maanshan,Anhui 243002,China
Keywords:Magnesium hydride Hydrogen desorption kinetics Cycle durability Synergetic effect
ABSTRACT A new MgH2-PrF3-Al-Ni composite was prepared by ball milling under hydrogen atmosphere.After initial dehydrogenation and rehydrogenation,Pr3Al11,MgF2,PrH3 and Mg2NiH4 nanoparticles formed accompanying the main phase MgH2.The hydrogen absorption-desorption properties were measured by using a Sieverts-type apparatus.The results showed that the MgH2-PrF3-Al-Ni composite improved cycle stability and enhanced hydrogen desorption kinetics.The improvement of hydrogen absorption-desorption properties is ascribed to the synergetic effect of the in situ formed Pr3Al11,MgF2,PrH3 and Mg2NiH4 nanoparticles.This work provides an important inspiration for the improvement of hydrogen storage properties in Mg-based materials.
1.Introduction
As one of promising hydrogen storage materials,magnesium hydride has attracted more attention due to its high hydrogen capacity,low cost and environmental friendship[1].Nevertheless,several disadvantages of MgH2limit its on-board vehicle application.Thus great efforts have been made to overcome its disadvantages in the past decades [2].First of all,MgH2has a high enthalpy change(ΔH75 kJ/mol H2[3])for hydrogen desorption(even for nano-sized MgH2,the enthalpy change of hydrogen desorption is about ΔH71.2 kJ/mol H2[4]),leading to a high hydrogen desorption temperature under atmospheric pressure.The high enthalpy change for hydrogen desorption is related to the nature of Mg-H bond(mixed ionic/covalent bonding) [5].Alloying Mg with Ni to form intermetallics Mg2Ni can decrease the absolute value of hydrogen absorption enthalpy,but the hydrogen storage capacity decreases remarkably[6].Thus the high thermodynamic stability of MgH2is still a challenge facing its application.Secondly,Mg/MgH2show slow hydrogen sorption rates [7,8],which are generally believed to be controlled by hydrogen diffusion in MgH2for absorption and interface reaction for desorption[2].Generally speaking,adding catalytic additives into MgH2with the help of ball milling is an effective method to increase its hydrogen sorption rates.Nevertheless,different types of additives show various catalytic effects and mechanisms,and thus the combination of different catalysts is advantageous to improvement of absorption and desorption kinetics[9].Moreover,the serious degradation of hydrogen storage properties occurs upon hydrogenation-dehydrogenation cycles of MgH2due to growth of Mg crystallites as well as agglomeration and sintering of powders[10,11].In order to enhance cycle durability of MgH2,the preventions for growth of crystallites,sintering of powders and redistribution of catalysts during hydrogen absorption-desorption cycling are of great significance[12,13].For example,nanoconfinement of MgH2/Mg and microstructural stabilization of catalysts were proven to be effective pathways[14,15].
Up to now,numerous types of additives have been used for the improvement on hydrogen absorption-desorption kinetics and cycle durability of MgH2[9].Among these additives,transition metals and their derivatives[16],rare earth hydrides[17]and carbon materials[18]have been believed to be effective.Especially,in situ formed species play an important role in hydrogen absorption-desorption.For example,the in situ formed TiH2and MgF2in TiF3-added[19,20],Mg2NiH4in Ni-added[21],and YH3in Y-added MgH2[22] show positive effect on hydrogen storage properties.It is generally accepted that the dispersed TiH2,Mg2NiH4and RH3(Rrare earth elements)nanoparticles have catalytic effect on hydrogen desorption [17,19] and nanosized MgF2has grain refinement effect on Mg/MgH2crystallites[19,20].Recently it has been found that Pr3Al11nanoparticles can be in situ formed in activated Mg-Pr-Al composite and stably exist in subsequent de/hydrogenation cycles [23,24],leading to the improvement of cycle durability due to inhibition of crystallite growth [25-28].All these results demonstrate that in situ formed species are very beneficial to hydrogen storage properties.
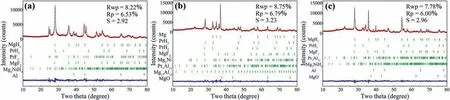
Fig.1.Rietveld refinements of observed XRD patterns for (a) ball milled,(b) initially dehydrogenated and (c) rehydrogenated MgH2-PrF3-Al-Ni samples.
Given that the combination of different catalysts is much effective in enhancing hydrogen storage properties of MgH2[9],we herein prepare a ball milled MgH2-PrF3-Al-Ni composite in which nano-sized species are in situ generated.The hydrogen absorption and desorption properties are investigated in detail.As expected,the ball milled MgH2-PrF3-Al-Ni composite shows superior hydrogen desorption kinetics and cycle durability.
2.Experimental details
MgH2(95 wt%MgH2+5 wt%Mg,325 mesh,Gelest),PrF3(99 wt%,325 mesh,Aladdin),Al(99 wt%,325 mesh,Alfa Aesar)and Ni(99 wt%,325 mesh,Aladdin) powders were used as starting materials;and the 80MgH2+3PrF3+15Al+2Ni(molar ratio)powders with a theoretical hydrogen storage capacity of 5 wt% were manually mixed in an argonfilled glove box.The mixture was then ball milled using a QM-3SP2 planetary mill at 400 rpm for 10 h under a 4 MPa hydrogen atmosphere.In the process of ball milling,the stainless steel pots and balls were used and the sample-to-ball weight ratio was 1:40.After ball milling,the mill pots were moved back to glove box and deflated slowly;and then the ball milled samples were taken out for subsequent experiments.
The ball milled samples were loaded into the stainless steel reactor in the glove box and the measurements were then conducted using a Sieverts-type apparatus (Suzaki Shokan Co.Ltd.,Japan).Before the measurements of kinetic curves,the samples were activated by two hydrogen absorption-desorption cycles at 300°C.For the measurement of cycle stability,however,the ball milled samples were directly de/hydrogenated at 300°C without any activation.All the tests were performed under an initial state of vacuum for dehydrogenation and 4 MPa hydrogen pressure for hydrogenation.
To evaluate phase structures and abundances of all samples,powder X-ray diffraction (XRD) measurements were carried out on a Rigaku D/Max 2500VL/PC diffractometer with Cu Kα radiation at 50 kV and 200 mA.The XRD samples were sealed in home-made holders to ensure that the measurements were performed under argon atmosphere.The XRD profiles were analyzed with the Rietveld refinement program RIETAN-2000 [29].Using the refined parameters in the Lorentzian part of a pseudo-Voigt function,the crystallite sizes were calculated as previously reported by Nakamura [30].Moreover,high-resolution transmission electron microscopy (HRTEM) images of the initially dehydrogenated MgH2-PrF3-Al-Ni sample were observed using a FEI Tecnai F20transmission electron microscope.The HRTEM sample was prepared by dispersing powders in tetrahydrofuran followed by ultrasonic vibration and then depositing the suspensions onto a copper grid.
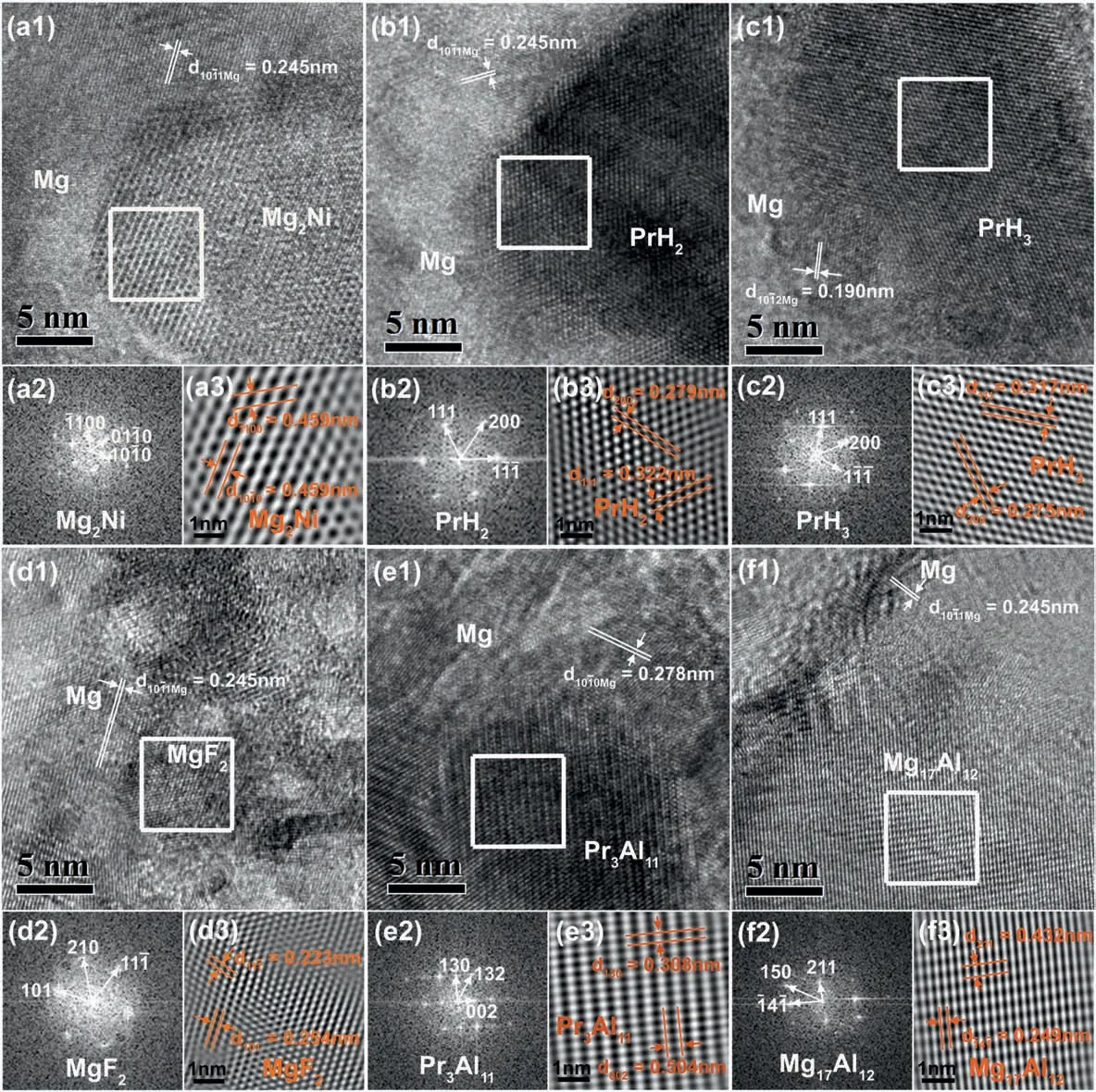
Fig.2.TEM analysis of initially dehydrogenated MgH2-PrF3-Al-Ni sample:(a)Mg2Ni,(b)PrH2,(c)PrH3,(d)MgF2,(e)Pr3Al11 and(f)Mg17Al12;(1)HRTEM image,(2) fast Fourier transform (FFT) pattern and (3) inverse fast Fourier transform (IFFT) image corresponding to the region boxed by the square in (1).
3.Results and discussion
3.1.In situ formation of nano-sized species
Fig.1a shows the XRD pattern of ball milled MgH2-PrF3-Al-Ni sample.It can be seen that the sample consisted of PrH3,PrF3,MgF2,Mg2NiH4and Al besides the main phase MgH2.This means that MgH2reacted with Ni to produce Mg2NiH4and with PrF3to generate PrH3and MgF2during the ball milling under hydrogen atmosphere.Table 1 lists the phase abundances calculated by Rietveld refinement of XRD pattern,which indicates that the reaction between MgH2and PrF3was incomplete.Furthermore,neither MgH2nor PrF3reacted with Al under this milling condition,which is probably due to the milling atmosphere of 4 MPa H2and low temperature[26].
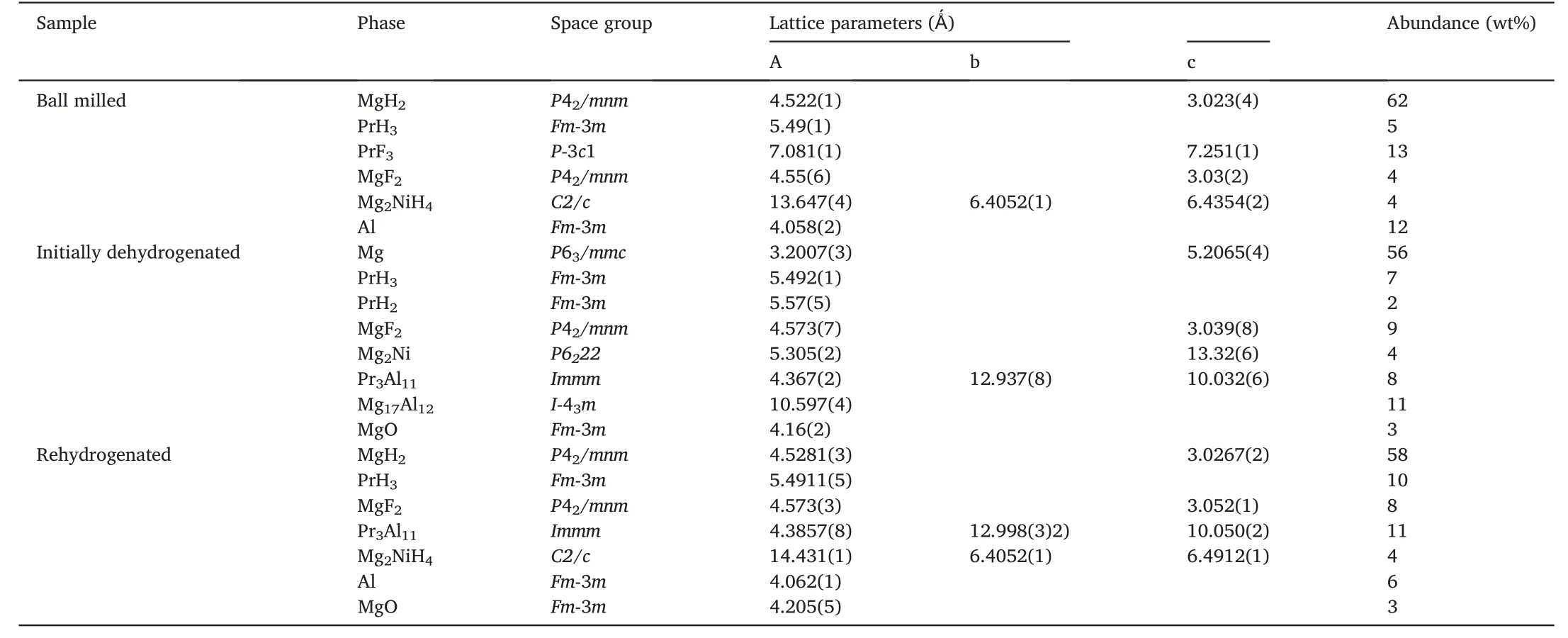
Table 1 Structural parameters and phase abundance of each phase in ball milled,initially dehydrogenated and rehydrogenated MgH2-PrF3-Al-Ni samples.
Fig.1b presents the XRD pattern of initially dehydrogenated MgH2-PrF3-Al-Ni sample.Obviously,both Pr3Al11and Mg17Al12formed accompanying the hydrogen desorption of MgH2,Mg2NiH4and PrH3during the initial dehydrogenation process at 300°C.Although the dehydrogenation reaction of PrH3into PrH2was incomplete due to its sluggish kinetics,the reaction between MgH2and PrF3occurred completely(see Table 1).To confirm the in situ formed species,HRTEM images are acquired as shown in Fig.2.It can be seen that Mg2Ni,PrH2/PrH3,MgF2,Pr3Al11and Mg17Al12particles in the initially dehydrogenated MgH2-PrF3-Al-Ni sample were present with the sizes ranging from several to 20 nm.
Fig.1c shows the Rietveld refinement of observed XRD pattern for rehydrogenated MgH2-PrF3-Al-Ni sample.It can be seen that the rehydrogenated sample was composed of MgH2,PrH3,Mg2NiH4,MgF2,Pr3Al11and Al.This indicates that Mg17Al12was hydrogenated into MgH2and Al,but MgF2and Pr3Al11kept unchanged during the hydrogen absorption process (see Table 1).Hence,the in situ formed PrH3,Mg2NiH4,MgF2,and Pr3Al11nanoparticles can play important roles in subsequent hydrogen absorption and desorption cycles.
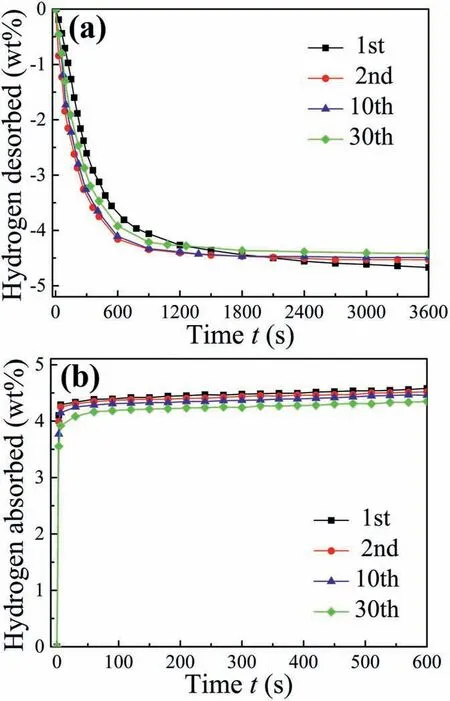
Fig.3.Isothermal (a) dehydrogenation and (b) hydrogenation curves of MgH2-PrF3-Al-Ni sample at 300 °C for different cycles.
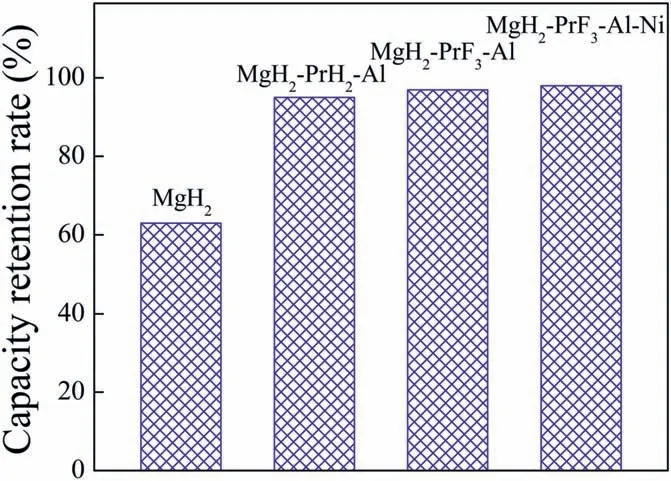
Fig.4.Comparison of hydrogen capacity retention rates of pure MgH2 [24],MgH2-PrH2-Al [24],MgH2-PrF3-Al (see Supplementary data) and MgH2-PrF3-Al-Ni samples after 30 cycles.
3.2.Improvement of cycle stability
Fig.3 shows the isothermal dehydrogenation and hydrogenation curves of MgH2-PrF3-Al-Ni sample at 300°C for different cycles.It can be seen that the ball milled sample was activated after one de/hydrogenation cycle.At the second cycle,the activated sample desorbed 4.16 wt%H2within 600 s and 4.53 wt%H2within 3600 s.For the 30th cycle,the sample still released 3.92 wt% H2within 600 s and 4.42 wt% H2within 3600 s.This means that the MgH2-PrF3-Al-Ni sample has good cycle durability.Fig.4 compares the capacity retention rates of pure MgH2[24],MgH2-PrH2-Al [24],MgH2-PrF3-Al (see Supplementary data) and MgH2-PrF3-Al-Ni samples after 30 cycles.Evidently,the MgH2-PrH2-Al sample had a higher retention rate as compared with pure MgH2due to the inhibition effect of in situ formed Pr3Al11nanoparticles on crystallite growth[24].It should be noted that the capacity retention rates of MgH2-PrF3-Al and MgH2-PrF3-Al-Ni samples increased further as compared with the MgH2-PrH2-Al sample.
Fig.5 shows the Rietveld refinements of XRD patterns for the dehydrogenated MgH2-PrF3-Al-Ni samples after different cycles.It can be seen that the phases in these samples kept unchanged,which were the same as those in the initially dehydrogenated sample.However,the phase abundance of Pr3Al11increased gradually and the phase abundances of Mg17Al12and PrH2/PrH3decreased with cycling number(see Table 2).Similarly,the XRD patterns for the hydrogenated MgH2-PrF3-Al-Ni samples after different cycles (see Figs.1c and 6)indicate that all the hydrogenated samples were composed of MgH2,PrH3,Mg2NiH4,MgF2,Pr3Al11and Al.The Rietveld refinement results of XRD patterns show that de/hydrogenation cycling led to the increase in phase amount of Pr3Al11and decrease in those of Al and PrH3(see Table 3).From these results the following features can be extracted:(i)The hydrogen-induced decomposition of Mg17Al12into MgH2and Al occurs during hydrogenation process and the reverse reaction occurs during dehydrogenation process.(ii) With the increase of cycling number,PrH3,gradually instead of MgH2,reacts with Al to produce Pr3Al11during dehydrogenation process.(iii)Pr3Al11and MgF2are stable during de/hydrogenation cycling.
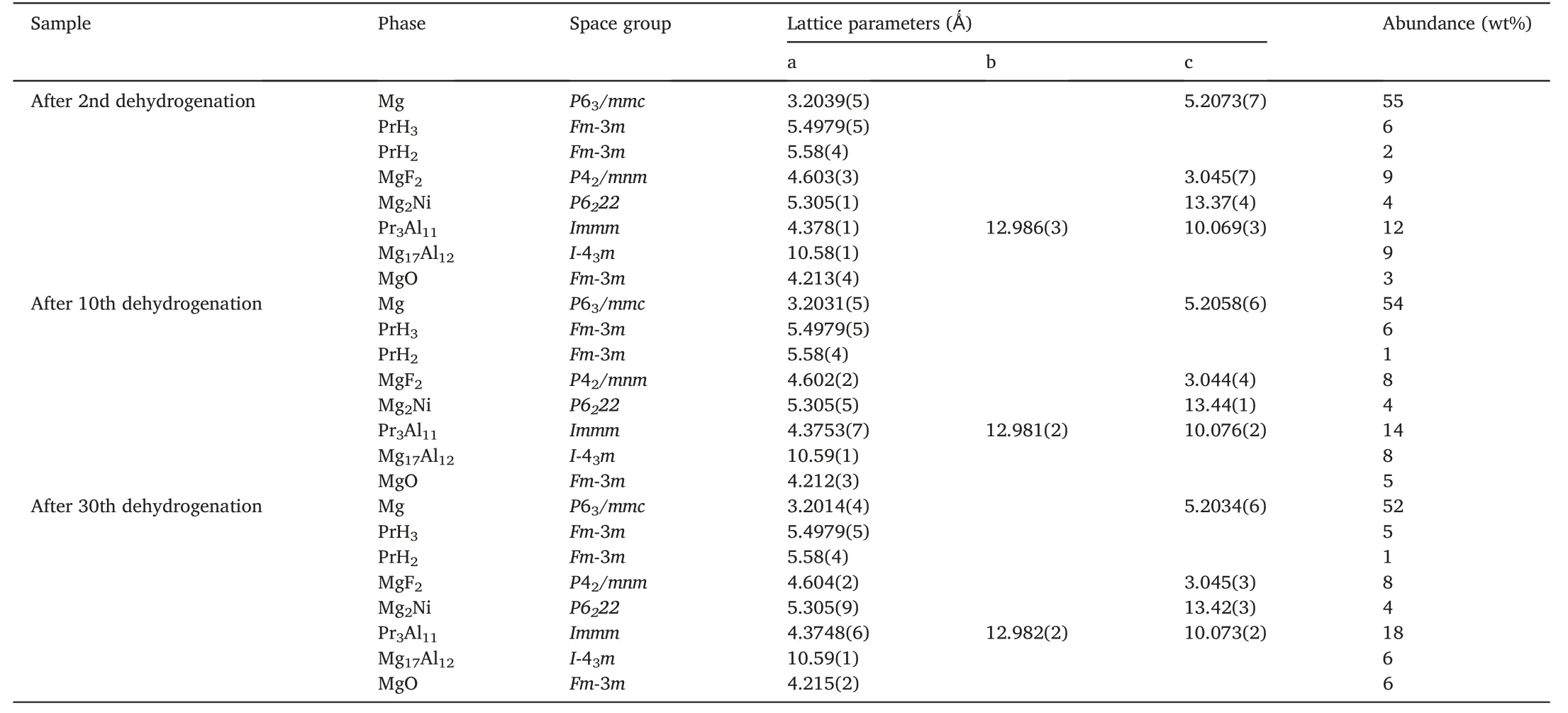
Table 2 Structural parameters and phase abundance of each phase in the MgH2-PrF3-Al-Ni samples after dehydrogenations at 300 °C.
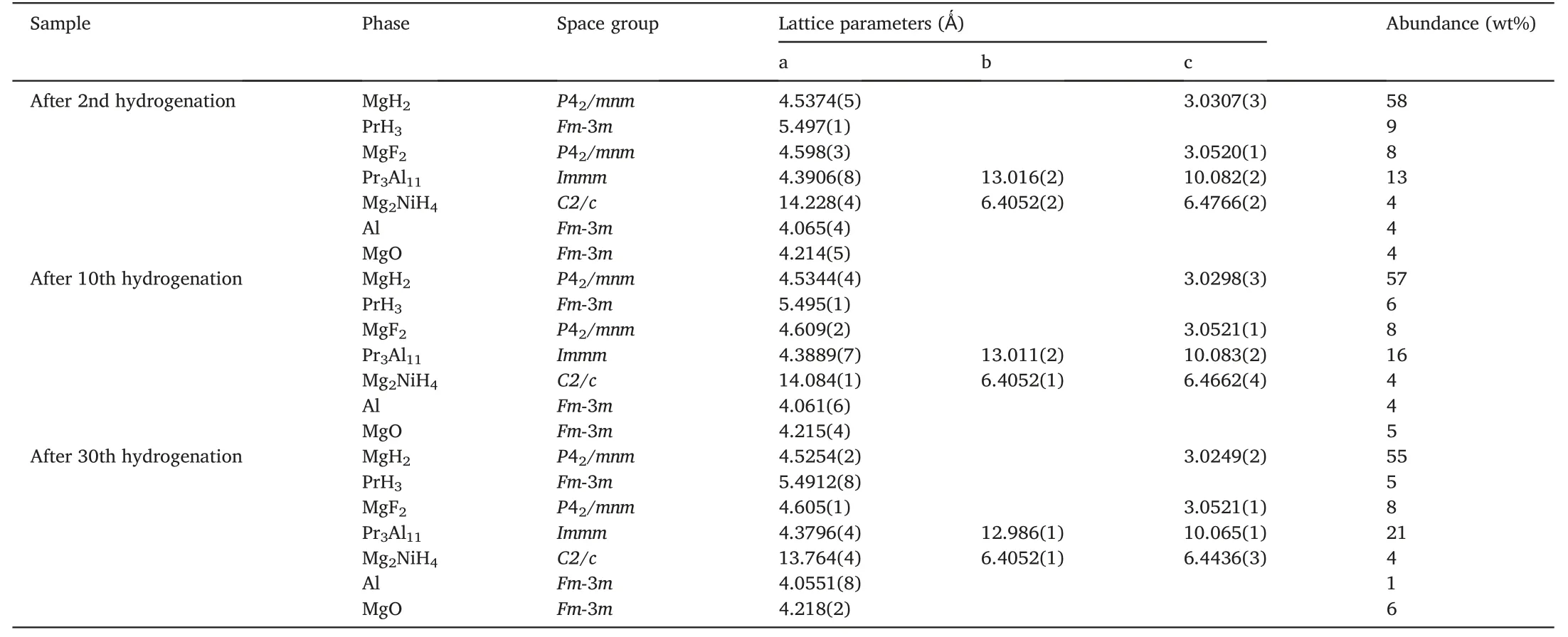
Table 3 Structural parameters and phase abundance of each phase in the MgH2-PrF3-Al-Ni samples after hydrogenations at 300 °C.
As reported previously,both Pr3Al11and MgF2nanoparticles can inhibit the growth of Mg crystallites,leading to the enhancement of cycle durability[20,24].To understand the inhibition effect of mixed Pr3Al11and MgF2nanoparticles on the growth of Mg crystallites,the Mg crystallite sizes of dehydrogenated MgH2-PrF3-Al-Ni samples were calculated using the refined parameters based on the Rietveld refinements of XRD patterns.Fig.7 compares the Mg crystallite sizes in dehydrogenated MgH2-PrF3-Al-Ni samples with those in dehydrogenated MgH2[24],MgH2-PrH2-Al [24] and MgH2-PrF3-Al (see Supplementary data) samples.Clearly the Pr3Al11and MgF2nanoparticlles in MgH2-PrF3-Al and MgH2-PrF3-Al-Ni samples have synergetic inhibition effect on growth of Mg crystallites.Hence the improvement of cycle durability in MgH2-PrF3-Al-Ni sample is attributed to the coexisted Pr3Al11and MgF2nanoparticles.
3.3.Enhancement of hydrogen desorption kinetics
Fig.8a shows the isothermal dehydrogenation curves of activated MgH2-PrF3-Al-Ni sample.It can be seen that this sample had fast hydrogen desorption rate at 300°C.According to Johnson-Mehl-Avrami-Kolmogorov(JMAK)model[31,32],hydrogen desorption fraction α can be expressed as

where k is hydrogen desorption kinetic parameter,n is Avrami index related to growth dimensionality and t is time.The apparent activation energy Eafor dehydrogenation can be described as

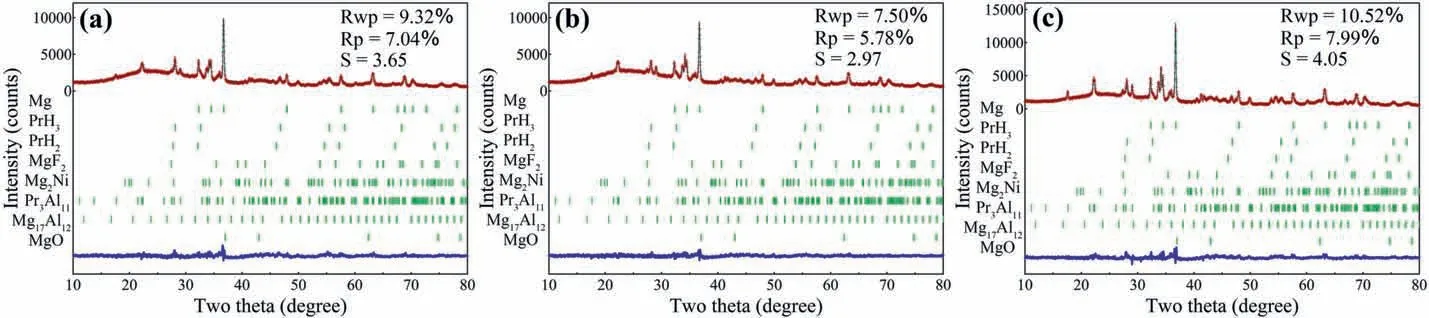
Fig.5.Rietveld refinements of observed XRD patterns for the (a) 2nd,(b) 10th and (c) 30th dehydrogenated MgH2-PrF3-Al-Ni samples.
where R is the gas constant,k0is the pre-factor and T is temperature.Fig.8b and c show the JMAK plots of ln(-ln(1-α)) vs.lnt and Arrhenius plots of activated MgH2-PrF3-Al-Ni sample,respectively.Therefore the activation energy Eafor dehydrogenation of activated MgH2-PrF3-Al-Ni sample can be determined to be 109.1 kJ mol-1,which is much lower than 160 kJ mol-1for ball milled MgH2[33].
Fig.9 compares the activation energies for hydrogen desorption in activated MgH2[33],MgH2-PrH2-Al [24],MgH2-PrF3-Al (see Supplementary data) and MgH2-PrF3-Al-Ni samples.It can be seen that MgH2-PrH2-Al and MgH2-PrF3-Al have lower activation energies(121.6 kJ mol-1and 116.4 kJ mol-1,respectively) as compared with pure MgH2due to the catalytic effect of PrH3nanoparticles [24].More importantly,the synergetic effect of PrH3and Mg2NiH4nanoparticles leads to further decrease of activation energy in MgH2-PrF3-Al-Ni sample,similar to the situations in other Mg-based hydrogen storage materials [34,35].This may be related to the enhanced reactivity of surfaces caused by the coexisted PrH3and Mg2NiH4nanoparticles[36].
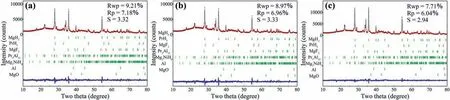
Fig.6.Rietveld refinements of observed XRD patterns for the (a) 2nd,(b) 10th and (c) 30th hydrogenated MgH2-PrF3-Al-Ni samples.
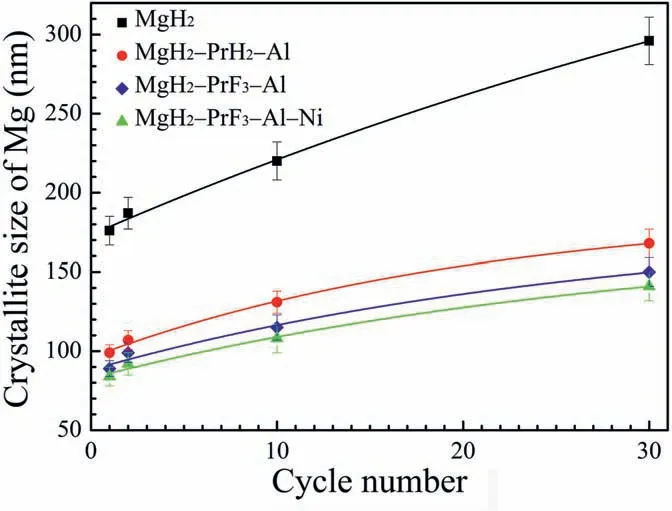
Fig.7.Comparison of Mg crystallite sizes in dehydrogenated MgH2 [24],MgH2-PrH2-Al [24],MgH2-PrF3-Al (see Supplementary data) and MgH2-PrF3-Al-Ni samples.
4.Conclusions
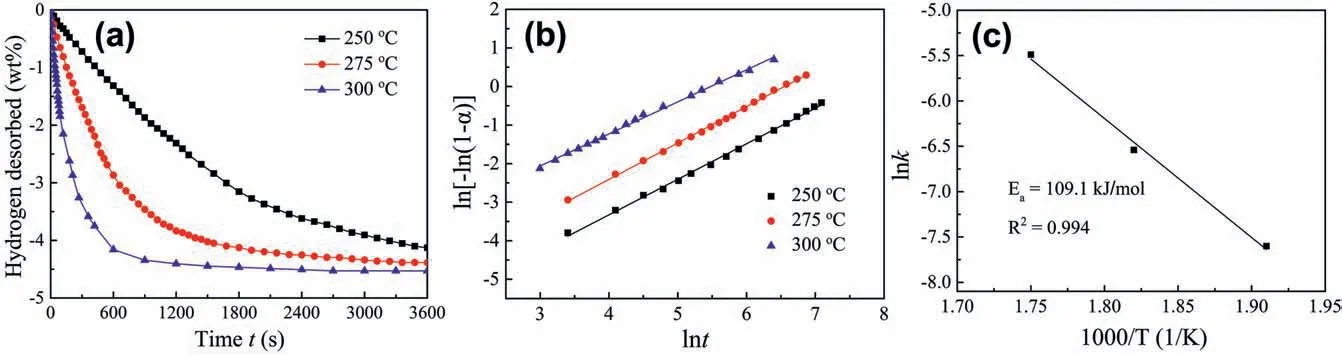
Fig.8.(a) Isothermal dehydrogenation curves at different temperatures,(b) corresponding JMAK plots and (c) Arrhenius plot of activated MgH2-PrF3-Al-Ni composite.
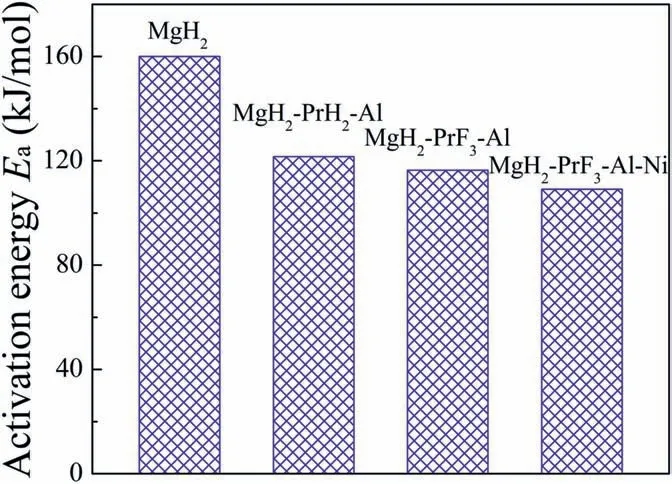
Fig.9.Comparison of activation energies for hydrogen desorption in activated MgH2 [33],MgH2-PrH2-Al [24],MgH2-PrF3-Al (see Supplementary data) and MgH2-PrF3-Al-Ni samples.
The MgH2-PrF3-Al-Ni sample ball milled under hydrogen atmosphere is composed of MgH2,PrH3,PrF3,MgF2,Mg2NiH4and Al.After initial dehydrogenation and rehydrogenation,Pr3Al11,MgF2,PrH3and Mg2NiH4nanoparticles are in situ formed together with MgH2in the activated sample.The Pr3Al11and MgF2nanoparticles synergistically inhibit growth of crystallites during subsequent hydrogen de/hydrogenation cycles,leading to the improvement of cycle stability.Meanwhile,the in situ formed PrH3and Mg2NiH4nanoparticles have synergistically catalytic effect on hydrogen desorption of MgH2,enhancing hydrogen desorption kinetics.This synergetic effect of multiple phases in MgH2-PrF3-Al-Ni composite provides an important inspiration for the improvement of hydrogen storage properties in Mg-based materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China(No.51871002).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.pnsc.2021.01.001.
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Dynamic response characteristic of 7N01/7A01/7050 aluminium multilayer plate at high strain rate
- Growth mechanisms of Ag and Cu nanodendrites via Galvanic replacement reactions
- Highly mechanical and high-temperature properties of Cu-Cu joints using citrate-coated nanosized Ag paste in air
- Ab-initio investigation for the microscopic thermodynamics and kinetics of martensitic transformation
- Rapid directionally solidified microstructure characteristic and fracture behaviour of laser melting deposited Nb-Si-Ti alloy
- Microstructure evolution and mechanical properties of a hot-rolled Ti alloy
