Improvement on hydrogen generation properties of Zr(BH4)4·8NH3
D.F.Wu ,L.Z.Ouyng ,J.M.Hung ,J.W.Liu ,H.Wng ,X.S.Yng ,H.Sho,M.Zhu
a School of Materials Science and Engineering,Guangdong Provincial Key Laboratory of Advanced Energy Storage Materials,South China University of Technology,Guangzhou,510641,China
b Guangdong Research Institute of Rare Metals,Guangdong Key Laboratory of Rare Earth,Development and Application,Guangzhou,510651,China
c China-Australia Joint Laboratory for Energy&Environmental Materials,Key Laboratory of Fuel Cell Technology of Guangdong Province,Guangzhou,510641,China
d Joint Key Laboratory of the Ministry of Education,Institute of Applied Physics and Materials Engineering (IAPME),University of Macau,Macau SAR,China
e Advanced Manufacturing Technology Research Centre,Department of Industrial and,Systems Engineering,The Hong Kong Polytechnic University,Hung Hom,Kowloon,Hong Kong,China
Keywords:Hydrogen production Zr(BH4)4·8NH3 Hydrolysis properties Ammonia coordination number
ABSTRACT Hydrolysis of Zr(BH4)4·8NH3 in deionized water can generate high purity hydrogen at room temperature.However,the sluggish hydrolysis kinetics of Zr(BH4)4·8NH3 hinders its practical use.To improve its hydrogen generation properties,the effects of magnetic stirring,changing hydrolysis solution and tuning the ammonia coordination number on the hydrolysis properties of Zr(BH4)4·8NH3 were investigated.Results show that both changing hydrolysis solution and tuning the ammonia coordination number can enhance the hydrolysis kinetics.The hydrolysis kinetics properties of Zr(BH4)4·8NH3 were significantly improved in MgCl2 and CoCl2 solutions.The Zr(BH4)4·xNH3(x ≤8)samples were synthesized by a ball-milling method with different ammonization time(10,60 and 180 min).Both the hydrolysis kinetics and hydrogen yield of Zr(BH4)4·xNH3(x ≤8)were enhanced as the ammonia coordination number (x)decreased.Thus,tuning ammonia coordination number is an effective way to control the hydrolysis properties of Zr(BH4)4·xNH3 (x ≤8).
1.Introduction
Hydrogen is regarded as one of the most promising energy carrier in future energy systems[1,2],due to its high energy density,lightweight and green products.To realize large-scale utilization of hydrogen energy,one of the most critical challenges is to develop suitable ways to store hydrogen.In the past decades,researchers have devoted tremendous efforts to develop a safe,lightweight,and economical hydrogen storage material with moderate operating temperatures.Metal based hydrides,complex hydrides,and carbon materials have been widely investigated as potential hydrogen storage materials [3-5],but unfortunately none of them can meet all the requirements for hydrogen storage system.
In recent years,borohydrides have been extensively studied as promising hydrogen storage candidates due to the high hydrogen capacity and adjustable dehydrogenation conditions [6,7].The main drawbacks of borohydrides are the high dehydrogenation temperature and the release of toxic boranes.Studies show that forming borohydride ammoniates is a useful way to regulate the dehydrogenation properties of borohydrides[8-10].
A new borohydride ammoniate,Zr(BH4)4·8NH3was reported and considered as a potential hydrogen storage material [11].With the highest ammonia coordination number(x=8),Zr(BH4)4·8NH3has high hydrogen capacity (14.1 wt%) and low dehydrogenation temperature(around 130°C).However,Zr(BH4)4·8NH3suffers from releasing ammonia during the dehydrogenation process,which might be due to its high ammonia coordination number.To suppress the release of ammonia,many additives including NH3BH3,LiBH4and Mg(BH4)2have been studied[12,13].Recently,Wu et al.reported that hydrolysis of Zr(BH4)4·8NH3was also an effective way to thoroughly suppress the release of ammonia [14].Zr(BH4)4·8NH3could react with deionized water at room temperature and release about 1067 mL/g H2in 240 min,without releasing any toxic diborane or ammonia impurity gases.Furthermore,the hydrolysis mechanism of Zr(BH4)4·8NH3was clarified as a three-step process:1) Zr(BH4)4·8NH3reacted with H2O to form Zr(BH4)4and NH4OH;and then 2) Zr(BH4)4reacted with H2O to form Zr(OH)4,B(OH)3and H2;finally,3)B(OH)3reacted with NH4OH to form NH4B(OH)4.The hydrolysis equation could be summarized as:

Fig.1.Hydrogen evolution curves of Zr(BH4)4·8NH3 with or without magnetic stirring.
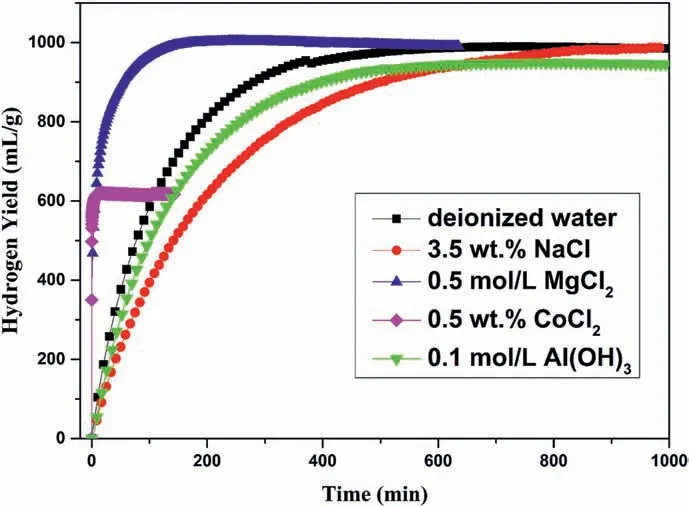
Fig.2.Hydrogen evolution curves of Zr(BH4)4·8NH3 in different hydrolysis solutions(deionized water,3.5 wt%NaCl,0.5 mol/L MgCl2,0.5 wt%CoCl2 and 0.1 mol/L Al(OH)3).
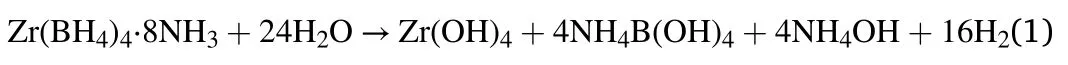
By hydrolysis reaction,Zr(BH4)4·8NH3could thoroughly suppress the release of ammonia and generate pure hydrogen at room temperature.However,the hydrolysis kinetics of Zr(BH4)4·8NH3was sluggish.To improve the hydrolysis kinetics,the effects of magnetic stirring,changing hydrolysis solution and tuning the ammonia coordination number on the hydrolysis properties of Zr(BH4)4·8NH3were studied in this work.
2.Experimental
2.1.Preparation
LiBH4(98%,Sigma-Aldrich),ZrCl4(99.5%,Alfa Aesar),NaCl (AR,Aladdin),MgCl2(99%,Alfa Aesar),CoCl2·6H2O(AR,Aladdin),Al(OH)3(AR,Tianjin Damao Chemical Reagent Factory) and NH3(99.9%,Guangdong Huate Gas Co.) were used as raw materials.
Zr(BH4)4·8NH3was prepared by a ball-milling method.Firstly,Zr(BH4)4was synthesized by ball milling LiBH4and ZrCl4using a planetary ball mill.Due to its volatility,Zr(BH4)4sublimated and deposited on the lid of the ball mill pot during ball milling process.Then,Zr(BH4)4was transported into a tube and exposed to ammonia.Finally,Zr(BH4)4·8NH3was synthesized as a white powder after ammonization for 8 h.The detailed preparation process was shown in Ref.[11].
Zr(BH4)4·xNH3(x ≤8)samples were also prepared by a similar ballmilling method,but the ammonization time was reduced to 10,60 and 180 min.The product powders with different ammonization time (10,60 and 180 min)were named as S1,S2 and S3,respectively.All handling was operated in a glove box equipped with a recirculation system,which may keep the water and oxygen concentrations below 1 and 5 ppm,respectively.
2.2.Hydrolysis test
The hydrogen generation properties of Zr(BH4)4·8NH3in different situations (with magnetic stirring,in different hydrolysis solutions and with different ammonia coordination number)were performed using in our house-made hydrolysis equipment [14].Around 0.1 g sample was loaded in a 50 mL flask,and then 10 mL hydrolysis solution was injected for hydrolysis.During the process,the generated hydrogen pushed water from Monteggia washing bottle into a beaker,and then the data of exhausting water mass against time were collected.Finally,the hydrogen evolution curves were drawn,and the hydrolysis properties could be evaluated.
2.3.Characterization
The phases of hydrolysis byproducts of Zr(BH4)4·8NH3in different situations were indexed by X-ray diffraction (XRD,PANalytical) with Cu-Kα radiation.Prior to XRD test,the hydrolysis byproducts were dried by a freeze-drying machine (ALPHA 1-2,GHRIST).All the samples for measurement were loaded on our home-made XRD protective device to protect them from exposure to air.
The SEM pictures of Zr(BH4)4·xNH3(sample S1,S2 and S3) were studied by field emission scanning electron microscopy(FE-SEM,Zeiss-Supra 40).The chemical bonds of Zr(BH4)4·xNH3were determined by a Fourier transform infrared spectrometer (FT-IR,NICOLET) using KBr pellets,in the range of 500-4000 cm-1with 32 scans.
3.Results and discussion
3.1.Hydrogen properties of Zr(BH4)4·8NH3 with magnetic stirring
Magnetic stirring is one of the most common methods to improve the hydrolysis kinetics.Thus,the effect of magnetic stirring (Model CJJ 78-1,JiTe Experimental Instrument) on the hydrogen properties of Zr(BH4)4·8NH3was studied.Fig.1 presents the hydrogen evolution curves of Zr(BH4)4·8NH3with or without magnetic stirring.Results show that magnetic stirring had little improvement to enhance the hydrolysis kinetics.Hence,it can be assumed that the decomposition of Zr(BH4)4·8NH3is the rate-limiting step in the hydrolysis process of Zr(BH4)4·8NH3.In other word,the decomposition rate of Zr(BH4)4·8NH3is a key factor affecting the hydrolysis performance.Changing the hydrolysis solution or tuning the ammonia coordination number of Zr(BH4)4·8NH3may enhance the decomposition rate of Zr(BH4)4·8NH3,and then improve its hydrolysis kinetics.
3.2.Hydrolysis properties of Zr(BH4)4·8NH3 in different solutions
To verify the effects of changing the hydrolysis solution,thehydrolysis properties of Zr(BH4)4·8NH3in different hydrolysis solutions(deionized water,NaCl,MgCl2,CoCl2and Al(OH)3) were studied.Among these solutions,NaCl,MgCl2and CoCl2are common additives to improve the hydrolysis properties of MgH2and NaBH4,and Al(OH)3is an amphoteric hydroxide that may affect the pH in the hydrolysis solution.
Fig.2 exhibits the hydrogen evolution curves of Zr(BH4)4·8NH3in different hydrolysis solutions(deionized water,3.5 wt%NaCl,0.5 mol/L MgCl2,0.5 wt% CoCl2and 0.1 mol/L Al(OH)3).As shown in Fig.2,it took 600,120 and 800 min for Zr(BH4)4·8NH3to generate 1000 mL/g H2in deionized water,MgCl2and NaCl solutions,respectively.Compared to that in deionized water,the hydrolysis kinetics was significantly improved in MgCl2solution but became worse in NaCl solution.It took less than 2 min to generate 600 mL/g H2in CoCl2solution.Thus,the hydrolysis rate in CoCl2solution was quite fast but the hydrogen yield was significantly reduced.Finally,it took 600 min to generate 980 mL/g H2in the Al(OH)3solution,implying both the hydrolysis kinetics and yield got worse.In summary,the hydrolysis kinetic properties of Zr(BH4)4·8NH3were improved in the MgCl2and CoCl2solutions,however the hydrogen yield was reduced in the CoCl2solution.The hydrolysis performance data of Zr(BH4)4·8NH3in different hydrolysis solutions are summarized in Table 1.
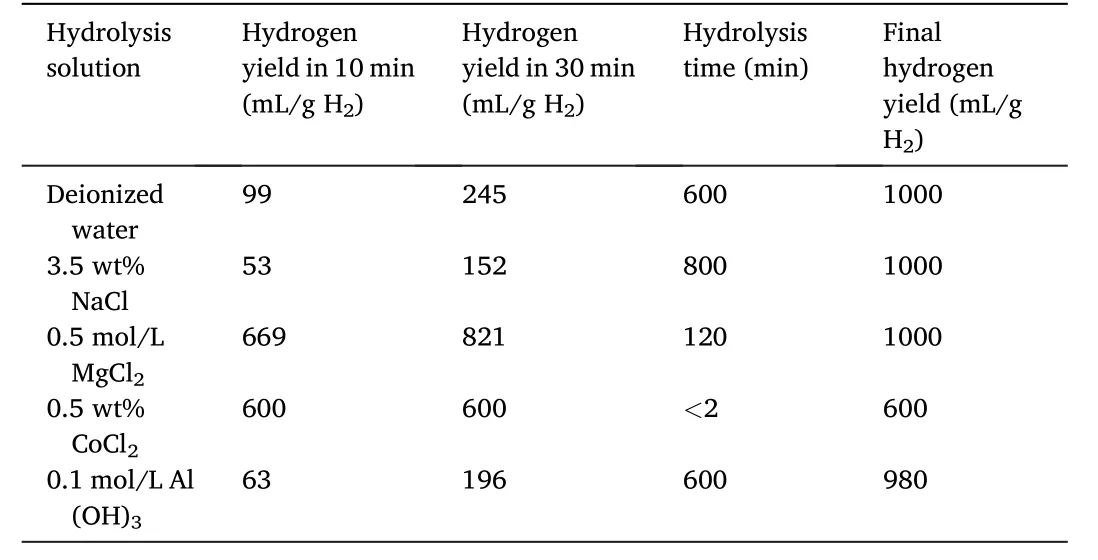
Table 1 Hydrolysis performance data of Zr(BH4)4·8NH3 in different hydrolysis solutions(deionized water,3.5 wt%NaCl,0.5 mol/L MgCl2,0.5 wt%CoCl2 and 0.1 mol/L Al(OH)3).

Table 2 Theoretical hydrogen yields of Zr(BH4)4·xNH3 (x ≤8).
To elucidate the hydrolysis mechanisms of Zr(BH4)4·8NH3in different hydrolysis solutions,the hydrolysis byproducts were collected and analyzed.Fig.3 shows the XRD patterns of the hydrolysis byproducts of Zr(BH4)4·8NH3in(a)3.5 wt%NaCl,(b)0.5 mol/L MgCl2,(c)0.5 wt%CoCl2(inset:picture of the hydrolysis byproduct solution)and(d)0.1 mol/L Al(OH)3solutions.
As shown in Fig.3(a) and (b),the hydrolysis byproduct in 3.5 wt%NaCl solution was mainly unreacted NaCl,while the byproduct in 0.5 mol/L MgCl2solution was composed of MgCl2(H2O)6and NH4(Mg(H2O)6)Cl3.The poor hydrolysis kinetics in NaCl solution may be ascribed to the salt-effect of NaCl.Due to the decomposition of NaCl,the average ionization degree of NH4OH (intermediate product in the first step of hydrolysis mechanism of Zr(BH4)4·8NH3)was raised,resulting in higher pH in the solution and worse hydrolysis kinetics.By contrast,MgCl2reacted with NH4OH and formed Mg(OH)2sediment and NH4Cl in MgCl2solution.Since the formation of Mg(OH)2sediment,the pH in the MgCl2solution was reduced and the hydrolysis rate of Zr(BH4)4·8NH3was improved.
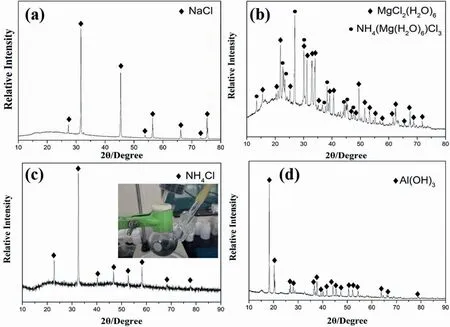
Fig.3.XRD patterns of the hydrolysis byproducts of Zr(BH4)4·8NH3 in (a) 3.5 wt% NaCl,(b) 0.5 mol/L MgCl2,(c) 0.5 wt% CoCl2 (inset:picture of the hydrolysis byproduct solution) and (d) 0.1 mol/L Al(OH)3 solutions.
Fig.4.Preparation of Zr(BH4)4·xNH3 (x ≤8) by a ball-milling method with different ammonization time (10,60 and 180 min).
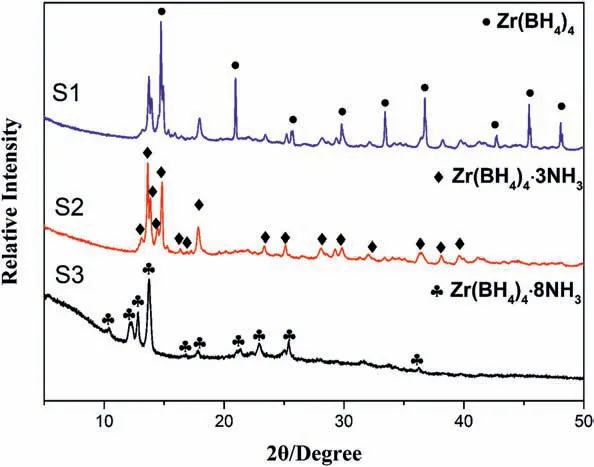
Fig.5.XRD patterns of S1,S2 and S3.
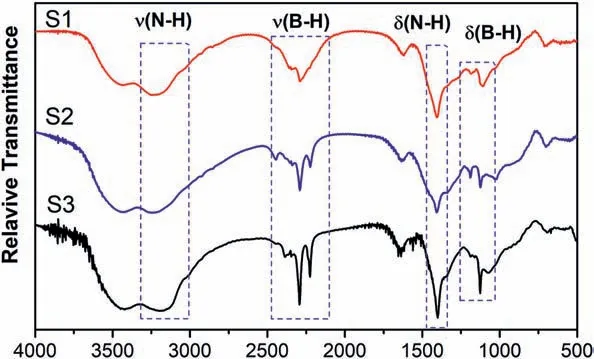
Fig.6.FT-IR curves of S1,S2 and S3.
The hydrolysis byproduct in 0.5 wt% CoCl2solution could be indexed as NH4Cl,as shown in Fig.3(c).Additionally,its hydrolysis byproduct solution showed a black color(inset of Fig.3(c)),which was different from others commonly in white[14].Zr(BH4)4·8NH3may have a different hydrolysis mechanism in CoCl2solution.Because the standard electrode potential of H-(Eo=-0.8277)is lower than Co2-(Eo=-0.73) in alkaline solution,the H-in Zr(BH4)4was oxidized by Co2+instead of H+,to form H2.Thus,the hydrolysis reaction of Zr(BH4)4·8NH3in CoCl2solution turns to be:
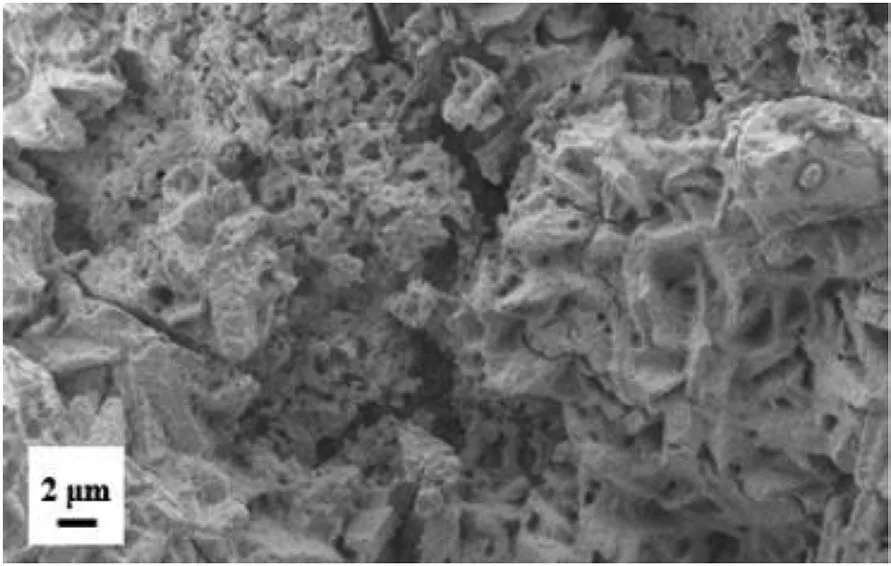
Fig.7.SEM picture of Zr(BH4)4·8NH3.
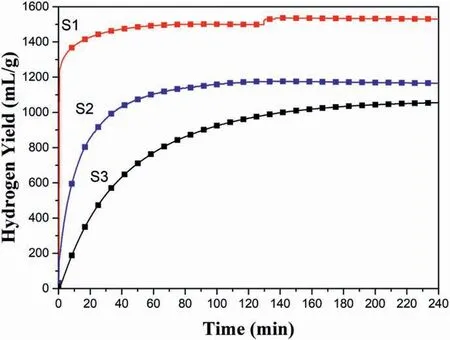
Fig.8.Hydrogen evolution curves of the hydrolysis of S1,S2 and S3.
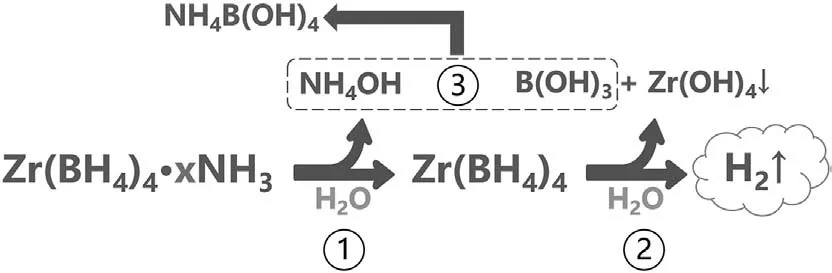
Fig.9.Hydrolysis mechanism of Zr(BH4)4·xNH3 (x ≤ 8).Similar to Zr(BH4)4·8NH3,the hydrolysis equation of Zr(BH4)4·xNH3 (x ≤8) can be summarized as follows.
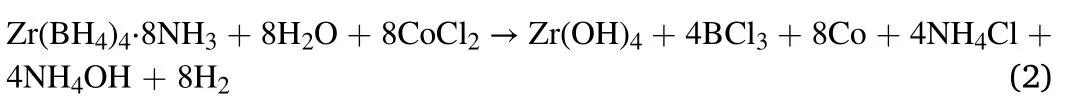
And then,Co partly reacted with BCl3to form CoCl2and B.

In summary,the hydrolysis equation of Zr(BH4)4·8NH3in CoCl2solution should be:
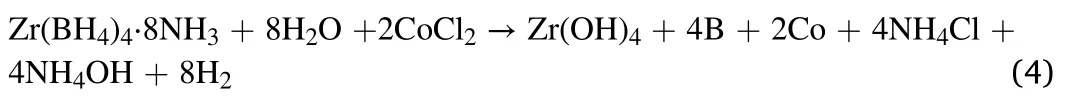
Since NH4OH was pumped out by freeze-drying,and Zr(OH)4,B and Co were amorphous or very fine nanocrystalline,only NH4Cl could be indexed in XRD pattern of hydrolysis byproduct.Both Co and B are blackparticles,consequently the hydrolysis byproduct solution showed a black color.According to its hydrolysis mechanism,the theoretical hydrogen yield of Zr(BH4)4·8NH3in CoCl2solution should be 626 mL/g H2,which is closed to the observed hydrogen yield,as Fig.2 shows.
Finally,the hydrolysis byproduct in 0.1 mol/L Al(OH)3solution was unreacted Al(OH)3,as shown in Fig.3(d).Al(OH)3could reduce the pH only in strong alkaline solution (pH>12),but the pH in hydrolysis solution of Zr(BH4)4·8NH3in deionized water was only 10.5[14].Thus,Al(OH)3did not reduce the pH but suppressed the hydrolysis reaction of Zr(BH4)4·8NH3.
3.3.Hydrolysis properties of Zr(BH4)4·xNH3 (x ≤8)
To test the effect of tuning the ammonia coordination number on the hydrolysis performance,Zr(BH4)4·xNH3(x ≤8)were prepared by a ballmilling method with different ammonization time(10,60 and 180 min),as shown in Fig.4.The product powders S1,S2 and S3 were determined by XRD (Fig.5).Results show that S1 was composed of mixture of Zr(BH4)4and Zr(BH4)4·3NH3,while S2 and S3 were Zr(BH4)4·3NH3and Zr(BH4)4·8NH3,respectively.Thus,the ammonia coordinate number(x)of S1,S2 and S3 were x<3,x=3 and x=8,respectively.
Fig.6 presents the FT-IR results of S1,S2 and S3.As shown in Fig.6,S1,S2 and S3 had the same infrared absorption peak positions corresponding to the B-H bonds (stretching:2180-2470 cm-1;bending:1080 cm-1) and N-H bonds (stretching:2950-3330 cm-1;bending:1405 cm-1),indicating that they all had B-H and N-H bonds.However,as the ammonia coordinate number(x)decreased,the shape of infrared absorption peaks became broader.
SEM pictures of Zr(BH4)4·xNH3(sample S1,S2 and S3) were tested to measure their particle sizes.As Fig.7 shown,the particle size of Zr(BH4)4·8NH3(S3)is about 2-4μm.The particle sizes of S1 and S2 may be less than S3.However,due to S1 and S2 were very volatility in air,we failed to measure their particle sizes.When S1 and S2 were exposed to the air,they reacted with moisture and produced white smoke immediately.
To evaluate the hydrolysis properties of Zr(BH4)4·xNH3(x ≤8),S1,S2 and S3 were hydrolyzed in deionized water at room temperature.Fig.8 presents the hydrogen evolution curves of S1,S2 and S3.As seen in Fig.8,the hydrolysis kinetics was significantly improved as the ammonia coordinate number(x)decreased.The hydrogen yields of S1,S2 and S3 in 10 min were 1380,644,and 222 mL/g H2,respectively.Interestingly,the final hydrogen yields were also boosted as the ammonia coordinate number(x)decreased.The final hydrogen yields of S1,S2 and S3 were 1495,1174 and 1054 mL/g H2,respectively.Thus,tuning the ammonia coordinate number (x) can not only regulate the hydrolysis kinetics but also tailor the hydrogen yield.
The improvements of hydrolysis properties are clarified by the hydrolysis mechanism of Zr(BH4)4·xNH3(x ≤8),as shown in Fig.9.Zr(BH4)4and NH3in Zr(BH4)4·xNH3(x ≤8)have different functions in the hydrolysis process:the former provides H-to form H2;while the latter combines with water and forms NH4OH,which can regulate the hydrolysis rate.Consequently,reducing the ammonia coordinate number(x)has two effects:on one side,raising the percentage of Zr(BH4)4in Zr(BH4)4·xNH3(x ≤8),which leads to higher hydrogen yield;on the other side,producing less NH4OH,which results in more H+in solution and faster hydrolysis kinetics.Thus,as the ammonia coordinate number(x)decreased,both the hydrogen yield and the hydrolysis kinetics were improved in the Zr(BH4)4·xNH3(x ≤8) samples.
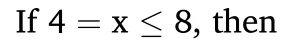
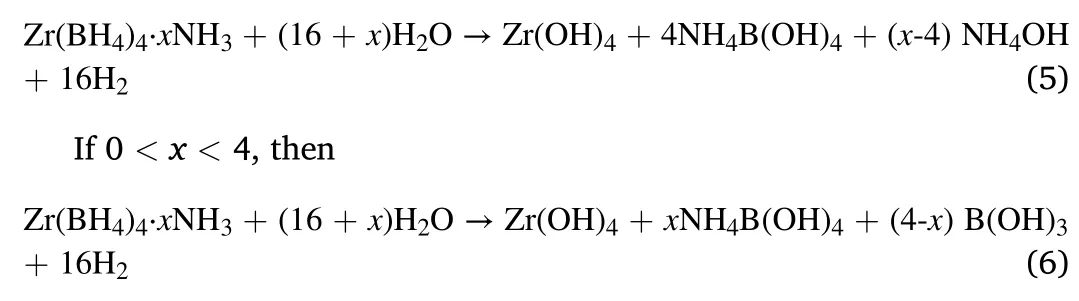
Then the theoretical hydrogen yields of Zr(BH4)4·xNH3(x ≤8)can be calculated,as shown in Table 2.As the ammonia coordinate number(x)decreased from 8 to 1,the theoretical hydrogen yields remarkably raised from 1252 to 2144 mL/g H2,which was basically in agreement with the experimental results.In conclusion,tuning the ammonia coordinate number (x) is a useful way to control the hydrolysis properties of Zr(BH4)4·xNH3(x ≤8).
4.Conclusions
The effects of magnetic stirring,changing hydrolysis solution and tuning the ammonia coordination number on the hydrolysis properties of Zr(BH4)4·8NH3were investigated.Results show that magnetic stirring had little effect on enhancing the hydrolysis kinetics of Zr(BH4)4·8NH3.The hydrolysis kinetic properties of Zr(BH4)4·8NH3were improved in MgCl2and CoCl2solutions,but the hydrogen yield was reduced in the CoCl2solution.Zr(BH4)4·xNH3(x ≤8)samples were prepared by a ballmilling method with different ammonization time(10,60 and 180 min).Both the hydrolysis kinetics and hydrogen yield of Zr(BH4)4·xNH3(x ≤8) were significantly improved as the ammonia coordinate number (x)decreased.Thus,tuning ammonia coordination number (x) is an effective way to control the hydrolysis properties of Zr(BH4)4·xNH3(x ≤8).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Key R&D Program of China (No.2018YFB1502101),the Foundation for Innovative Research Groups of the National Natural Science Foundation of China(No.NSFC51621001),National Natural Science Foundation of China Projects(Nos.51771075)and by the Project Supported by Natural Science Foundation of Guangdong Province of China (2016A030312011).It was also supported by MOST Scientific Project (No.2018YFE0100700),Guangdong Scientific Project (No.2017B030314081) and the GDAS Project of Science and Technology Development (No.2017GDASCX-0506,2019GDASYL-0503005).H.Shao acknowledges the Science and Technology Development Fund,Macau SAR(FDCT 0062/2018/A2)and MYRG2019-00055-IAPME from University of Macau.
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Surface study of the reconstructed anatase TiO2 (001) surface
- Effect of Na+ in situ doping on LiFePO4/C cathode material for lithium-ion batteries
- Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors
- Electric transmission behavior of self-assembled Cu-W nano multilayers
- A hybrid hydrogel/textile composite as flame-resistant dress
- A TEM study on the microstructure of spark plasma sintered ZrB2-based composite with nano-sized SiC dopant
