A TEM study on the microstructure of spark plasma sintered ZrB2-based composite with nano-sized SiC dopant
Mehi Shhei Asl ,Abbs Sbhi Nmini ,Seye Ali Delbri ,Quyet Vn Le ,Mohmmrez Shokouhimehr,*** ,Mohsen Mohmmi
a Marine Additive Manufacturing Centre of Excellence (MAMCE),University of New Brunswick,Fredericton,NB,E3B 5A1,Canada
b Department of Engineering Sciences,Faculty of Advanced Technologies,University of Mohaghegh Ardabili,Namin,Iran
c Department of Engineering Sciences,Faculty of Advanced Technologies,Sabalan University of Advanced Technologies (SUAT),Namin,Iran
d Department of Materials Science and Engineering,Research Institute of Advanced Materials,Seoul National University,Seoul,08826,Republic of Korea
e Institute of Research and Development,Duy Tan University,Da Nang,550000,Vietnam
Keywords:Spark plasma sintering ZrB2 Nano-sized SiC Glassy phases
ABSTRACT Sintering behavior of ZrB2 ceramic with nano-sized SiC dopant was studied.ZrB2-25 vol% nano-sized SiC was selected as the starting mixture to fabricate the composite.The manufacturing process was accomplished at 1800°C for 5 min under 25 MPa via spark plasma sintering(SPS).The as-sintered sample reached a relative density of 99%.Besides the initial phases,namely ZrB2 and SiC,the high-resolution X-ray diffraction (HRXRD)was used to study the formation of an in-situ ZrC phase.The possible chemical interactions during the ZrC phase formation were scrutinized.The microstructure of the composite was studied by the field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM).Elemental analysis through FESEM evaluations revealed the formation of amorphous phases,rich in Zr,C,Si,B,and O elements,which was in harmony with the thermodynamical assessments.TEM studies endorsed the formation of such phases,containing a glassy bed of Si-B-O with ZrC and C islands dispersed therein.
1.Introduction
Zirconium diboride (ZrB2) belongs to a group of substances named ultra-high-temperature ceramics(UHTCs),which offers elevated melting temperatures(higher than 3000°C)[1-3].These unique materials have captivated quite a number of researchers owing to their combination of properties,including high electrical conductivity,good thermal conductivity,superior thermal shock resistance,excellent hardness,chemical stability,and high elastic modulus [4-6].With having such characteristics,ZrB2is considered as a vital substance for the wing leading edges and thermal protection systems of hypersonic flight vehicles,molten metal containers,and high-temperature electrodes [7-9].However,ZrB2shows low oxidation resistance and poor fracture toughness,which are considered as main drawbacks of this group of UHTCs.Besides,the sintering of ZrB2in monolithic form is challenging due to several factors,such as the presence of oxide layers in its powder particles,the strong covalent bonding among its constituent atoms,and poor diffusion rates in grain boundaries and lattices[10].
Introducing secondary phases,and/or developing/adjusting the sintering processes are among the main strategies that scientists implemented to address the limitations of ZrB2.The impacts of lots of metallic additives(i.e.,La,V,Ni Zr,Ti,and Al),and ceramic reinforcements(i.e.,SiC,ZrC,ZrO2,ZrSi2,Si3N4,C3N4,B4C,TiC,TaC,HfB2,VB2,AlN,SiAlON,carbon fiber,carbon black,graphite,diamond,graphene,and CNT)have been studied on the sintering behavior,mechanical properties,and microstructural characteristics of ZrB2[11-16].In addition,the implementation of high-technology sintering processes,including spark plasma sintering (SPS) and reactive hot pressing (RHP),can be cost-effective,and at the same time,result in a remarkable enhancement in as-produced composite samples[17-21].
Silicon carbide(SiC)is the most frequently studied reinforcement by various researchers.Akin et al.[22] fabricated ZrB2-based composites through introducing different weight percentages of SiC as an additive.Relative densities higher than 99% were secured when 20-60 wt% SiC was added to the ZrB2matrix.These samples were sintered at 2000 and 2100°C for 3 min through the SPS method.It was also reported that,when the sintering temperature was lower than 2120°C,the microstructures of composites comprised of equiaxed ZrB2and α-SiC grains.Nevertheless,rising sintering temperatures higher than those mentioned above led to a change in SiC grains morphology from equiaxed to elongated forms.A comparison was made between SiC whisker-doped ZrB2materials fabricated using two different processes,namely SPS and HP[23].For this objective,20 vol%SiCwwas added to ZrB2,and the HPed sample was sintered at 1800°C for 1 h under an external pressure of 30 MPa,whereas the other specimen was SPSed at 1600°C for 5 min under 30 MPa.The composite fabricated through the HP technique presented a better relative density (98%) than the SPSed one (95%).In addition,X-Ray diffraction technique (XRD) and microstructural evaluations revealed the formation of in-situ ZrC in both ceramics.
Ikegami et al.[24] studied the effects of size and amount of SiC reinforcements on the microstructure and consolidation behavior of ZrB2specimens.The study exposed the role of SiC additive in enhancing the sinterability of ZrB2,in a way that relative densities higher than 98%were attained for the SiC-doped composites SPSed at 1900°C under 30 MPa for 2 min.On the other hand,an increment in SiC amount resulted in a decrease in the grain size of as-produced ceramics.The impacts ofsintering temperature and time on the relative density and open porosity percentage of SPSed ZrB2-nano-sized SiC were evaluated by Eatemadi et al.[19].It was observed that increasing the sintering temperature from 1600°C to 1700°C had no noticeable effect on the reduction of remained porosity percentage.Nonetheless,rising the sintering temperature to levels higher than 1800°C had a significant impact on eliminating the open porosities.The authors stated that particle reorientation and pore filling owing to grain rotation and boundary sliding were responsible for such a phenomenon.Finally,Jaberi et al.[25]investigated the effects of SiC particle size and hot pressing variations on the consolidation behavior of ZrB2-based composites doped with 25 vol% SiC.The examinations revealed that the applied pressure was the most determinant factor on the relative densities of samples.They studied several variables for various factors,including sintering temperatures (1700,1775,and 1850°C),applied pressure (8,12,and 16 MPa),dwelling time (30,60,and 90 min),SiC particle size (20,200,and 5000 nm),and the optimal hot pressing circumstances were ascertained 1850°C,90 min,16 MPa,and 200 nm for temperature,dwelling time,applied pressure,and dopant particle size,respectively.
2.Experimental procedure
In this study,commercially available powders of ZrB2and nano-sized SiC were used as starting materials to fabricate a ZrB2-25% vol% SiC composite sample.The relevant suppliers’data are presented in Table 1.The morphology and relevant XRD results of these powders are presented in Fig.1.Although only peaks related to hexagonal ZrB2were detected for the corresponding powder,two various species of SiC(hexagonal and cubic) and also two species of SiO2(hexagonal and tetragonal) were identified for SiC particles.To avoid agglomeration,nano-sized SiC powder was first dispersed in ethanol environment by an ultrasonic device for 60 min.Then,25 vol% of this dispersed powder was added to ZrB2,and subsequently,the prepared mixture was ball-milled by cup and balls out of polyethylene and WC,respectively,in an ethanol medium at 90 rpm for 60 min.Next,the resulting slurry was dried using a rotating drier,and ground by an agate mortar.The resulting mixture(Fig.2)was charged into a graphite mold.To avoid any possible chemical reaction between the powder mixture and mold,the internal surface of graphite die was lined by thin foils out of graphite.All graphite surfaces were also covered using a lubricant (boron nitride) to facilitate the exit of the sample after the sintering process.The SPS procedure was accomplished by a sintering furnace (Nanozint 10i,Khala Poushan Felez Co.,Iran)under a vacuum of 5×10-2Pa at a temperature of 1800°C for 5 min underwent an external pressure of 25 MPa.After cooling down in the furnace,the SPSed pellet was removed.The samples with dimensions of 20 mm in diameter and 5 mm in thickness were fabricated for this study.
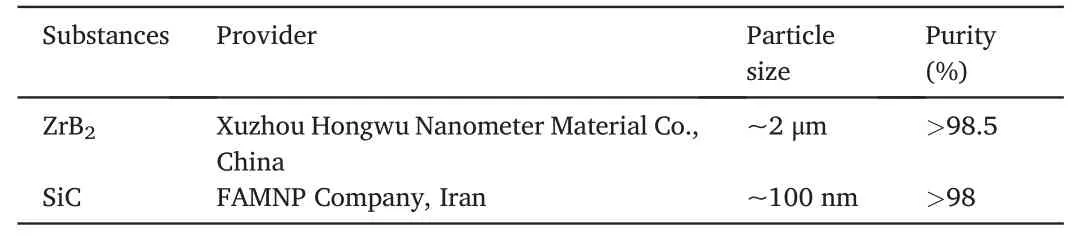
Table 1 Relevant data of starting powders.
The available graphite layers on the specimens were eradicated through grinding,and polished up to 1 μm finish using diamond slurries.The bulk and theoretical density values of specimens were calculated using the Archimedes principle and the rule of mixtures,respectively.The real densities of initial phases were considered as 3.2 and 6.1 g/cm3for SiC and ZrB2,respectively.The relative density of the final produced composite and the ratio of bulk and theoretical densities were estimated for the measurement.A DXP-X10P Digital X-ray Processor was utilized to evaluate the chemical compositions of the starting materials and the SPSed composites.Finally,the microstructure of the as-produced samples was investigated by a Tescan Mira3 FESEM (Czech Republic) and a Philips Tecnai F20 S-TWIN TEM (Netherlands).The specimen preparation for TEM analysis was carried out using a Gatan 601 focused ion beam(FIB)instrument.
3.Results and discussion
The XRD pattern associated with the SPSed sample(Fig.3)shows that the sintering process led to a phase transformation from hexagonal to cubic in the SiC phase.According to Fig.1,the initial SiC powder consisted of two species of SiC(hexagonal and cubic),and sintering process led to turning all SiC content into the cubic form.On the other hand,the SiO2peaks disappeared after the sintering process,which could be a sign of full consumption of such a phase over the SPS process.The high amount of available SiO2in the initial powder was owing to the tiny size of SiO2particles with oxidized surfaces.
Measuring the theoretical density and bulk density of the SPSed composite specimen manifested that the sample reached a relative density of 99%.It was reported that,the rearrangement and fragmentation of particles were responsible as predominant densification mechanisms over the low sintering temperatures,while grain boundary diffusion and plastic deformation fortify the consolidation stage at higher temperatures.This relative density value obtained for the sample is in agreement with other figures recorded for ZrB2-based composites reinforced by 20-30 vol%SiC by other scientists[26,27].
Although no oxide phases,such as ZrO2and B2O3,were detected in the XRD analysis of initial ZrB2powder,it is well-known that ZrB2particles are covered by oxide layers [28].XRD was not able to identify these phases owing to their low contents.Oxide contaminations not only discourage reaching a fully dense material but inspire grain growth.On the other side,the SiC particles can also act as a sintering aid,which can enhance the sintering behavior of ZrB2through producing an intergranular liquid phase at the grain boundaries.Accordingly,the characteristics (viscosity,wettability,distribution,content,composition,etc.) of this liquid phase determine its contribution to the consolidation stage[24].
Over the SPS procedure,the chemical reaction presented in Eq.(1)may be thermodynamically favorable,leading to carbon formation.The gaseous phase(SiO)can leave the SPS system due to the applied vacuum in the furnace,and the produced carbon can reduce both ZrO2/B2O3oxide layers at temperatures close to 1500-1670°C in the standard condition[29].
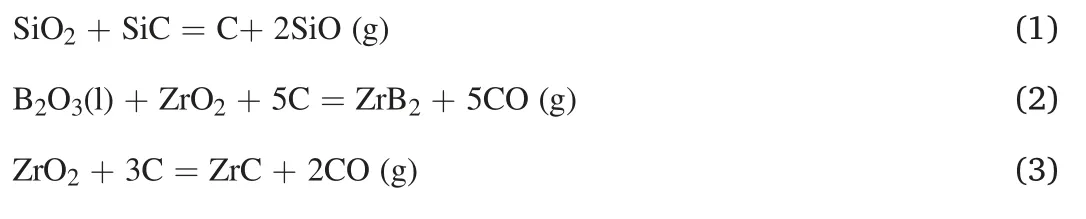
According to Eq.(2),the chemical interaction between available oxide layers on ZrB2grains and carbon resulted in the formation of in-situ ultra-fine ZrB2grains along with CO gas.This phenomenon can boost both densification and sinterability at the same time.Moreover,the progression of Eq.(3)can lead to the formation of in-situ ZrC phase.B2O3may also vaporize under mild vacuum circumstance at around 1500°C based on Eq.(4).
He gave me a sick look. I ve only been in town two days. We were going to meet and then drive down South where I ve got a job. She hasn t any address for me. He touched the telegram.


Fig.2.SEM micrograph of the prepared powder mixture.
Fig.3.XRD result of the as-fabricated ZrB2-SiC composite specimen.
Fig.4.Low-and high-magnification SEM micrographs of(a,b)the polished surface,and(c,d)the fracture surface of as-fabricated ZrB2-SiC composite,as well as(e)the relevant EDS analysis of the glassy phase.
The progress of the abovementioned reaction(Eq.(4))can hinder Eq.(1),leading to remaining some ZrO2phases in the structure.Consequently,this residual zirconium dioxide can participate in a reaction with carbon from Eq.(2),resulting in forming an in-situ ZrC and a gaseous phase (CO).It is reported that,this reaction is thermodynamically favorable above 1675°C [30].Although the formation of the in-situ phases,such as ZrC and new ZrB2,can improve the sintering behavior of ZrB2-based substances,the gaseous by-products may have a negative impact on the relative density if they are not able to escape from the material.It is worthy to note that,some other reactions are suggested by Hu et al.[31],which can occur in the ZrB2-SiC system,are presented in Eqs.(5)-(7)as follows:
Based on calculations implemented by Hu et al.[31],these reactions may be thermodynamically favorable at temperatures~1530-1760°C.Since no evidence was found for the formation of the B4C phase,Eqs.(5)and(7) are possibly prohibited by other reactions.
However,the main reason for improving the sinterability of ZrB2-based ceramic with adding SiC additive,as mentioned before,is due to the formation of a liquid phase.According to the SiO2-B2O3phase diagram [32],the viscosity of the liquid phase of Si-B-O enhances with more SiO2content.Ikegami et al.[24]studied the impact of SiC particle size on the properties of this phase.An increment in content of SiO2was reported by using finer SiC particles.Thus,smaller SiC particle size provided higher viscosity for the resultant intergranular liquid phase.Subsequently,the availability of a liquid phase with a higher viscosity led to a lower shrinkage rate over the sintering process.The use of finer SiC powder was desirable for both enhancement of the packing density and the rearrangement of particles.Moreover,tiny SiC particles can occupy the existent voids between multiple ZrB2grains,promoting the consolidation behavior[24].
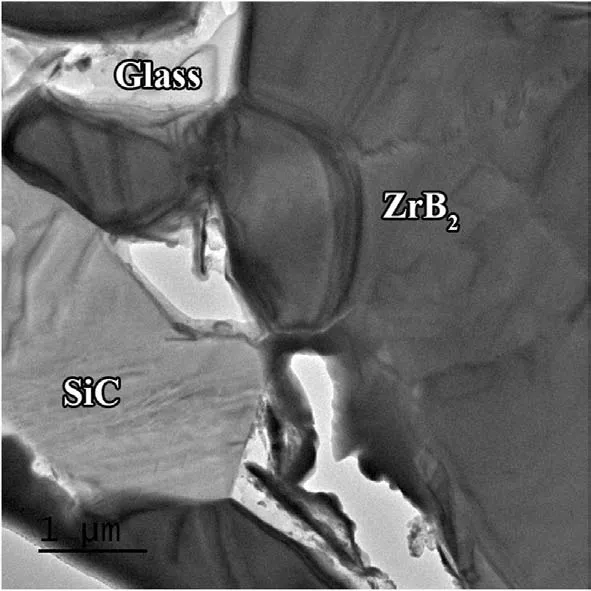
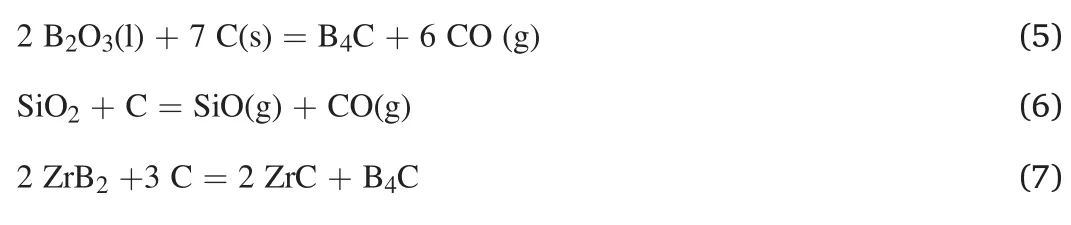
Fig.5.Low-magnification TEM image of ZrB2,SiC,and glassy phases.
Fig.4a-b exhibits FESEM images captured from polished surface of ZrB2-25 vol% SiC sample SPSed at 1800°C.As is apparent,the SiC particles are dispersed in the ZrB2matrix owing to the EDS analysis(not shown here).The bright color phases are associated with the matrix,and the dark color ones are related to the SiC secondary phases.Moreover,some areas can be observed in the similar color of SiC,which seem to be amorphous.These areas may deal with the glassy phase explained before,resulted in an interaction between SiO2and B2O3phases[33,34].On the other hand,although a relative density of 99% was calculated for the composite specimen,no porosity can be detected in these micrographs.Moreover,some of the remained pores that could be related to the tiny gaseous phases were entrapped into the grains or at the grain boundaries.Such pores are relatively difficult to detect by low magnification SEM images.This hypothesis is also considered in the investigation in the TEM analysis.As shown in the low and high magnification fractographs of the SPSed sample (Fig.4c-d),necks formed among the particles,a sign of diffusion-controlled sintering.However,the presence of some deformed grains with smooth polygon faces implies the role of plastic deformation in the densification of the sample owing to the application of an external pressure at high temperatures.The remained porosity is utterly obvious in these micrographs.Reaching a fully dense ZrB2-based material is known to be very difficult without using a suitable sintering aid.It was reported that,sintering temperatures higher than 2100°C were required to obtain a single-phase ZrB2material with a high-density value [24].Although SiC is a reinforcement,it can also act as a sintering aid.As noted above,the presence of SiO2oxide layers inhibits the evaporation of B2O3phase and forms a stable liquid phase.In other words,wherever SiO2and B2O3coexist,the mentioned liquid phase(Si-B-O)can be produced.The inability of this intergranular phase to fill all the remained pores could be due to the available SiO2and B2O3as the initial ingredients.According to the size of initial SiO2and the relevant XRD pattern (Fig.1),possibly a relatively high amount of this oxide phase was present.By contrast,it seems that the content of B2O3phase was not as much as needed for perfect sintering.The absence of such a peak in the XRD analysis of initial ZrB2powder (shown in Fig.1) fortifies this assumption.As can be observed in Fig.4c-d,the amorphous glassy phase covered some zones,but its amount was not sufficient to fill the available remaining pores.The elemental analysis of the point indicated by a white arrow in Fig.4d is presented in Fig.4e.According to the chemical analysis,this glassy phase,which covered some of ZrB2and SiC grains,was rich in Zr,C,B,Si,and O elements.If it is assumed that the bed of this amorphous phase would be Si-B-O,consequently,some other in-situ formed phases must be present as well.The reliability of this theory can be checked through TEM evaluations.In terms of fracture mode,it seems that the intergranular fracture was the predominant mode in this composite.In other words,the presence of intergranular liquid phase promoted the intergranular fracture mode due to not providing a strong bond with other compounds.
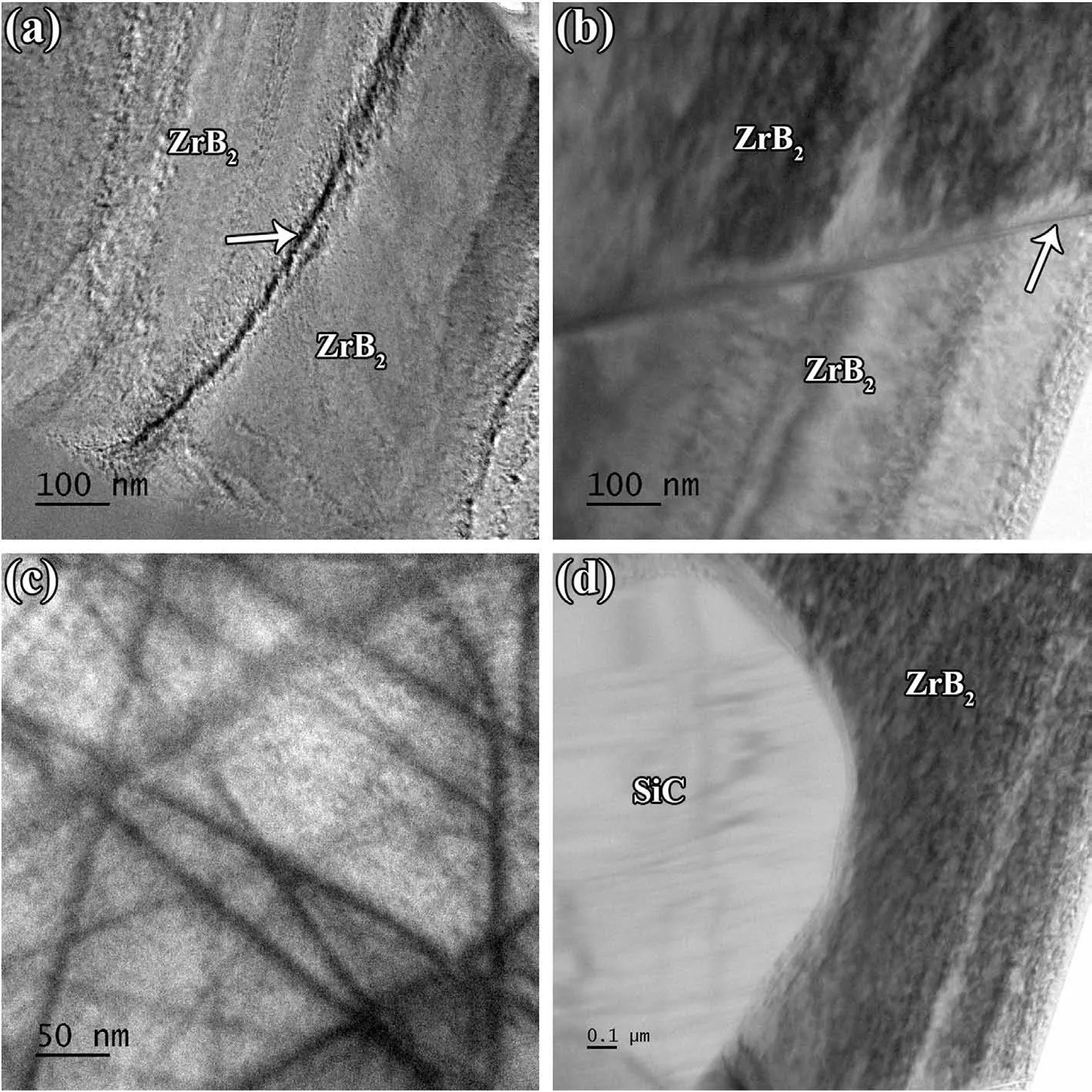
Fig.6.TEM images indicating (a,b) high-resolution views of different interfaces between ZrB2 grains,(c) dislocations in a ZrB2 grain,and (d) a clean interface between ZrB2 and SiC phases.
To validate several hypotheses assumed over the discussion,the microstructure of the composite sample was studied by TEM,especially at the interfaces.To have a clear perspective,a low magnification TEM image of the prepared FIB sample is indicated in Fig.5,where various phases of ZrB2,SiC,and glass can be seen clearly (based on EDS map analysis,which is not shown here).It is also obvious that the glassy phase formed,wherever a SiC particle was available.Two different ZrB2/ZrB2interfaces were observed,as shown in Fig.6a-b.A clean interface is shown in Fig.6b,while that presented in Fig.6a does not seem to be a clean one.The difference between these interfaces could derive from the accessibility of the formed glassy phase among the particles.In brief,the formation of such a phase has a significant role in activating various mass transfer mechanisms.More oxide layers reduce,and subsequently,more liquid phase is produced through this phenomenon,namely facilitated mater transfer.The products of chemical interaction can easily leave the interfaces,letting them form strong bonds.Moreover,the composition of such a phase varies in a different zone,which can be beneficial in driving it out of the grain boundaries owing to the gradient created in surface tension[35].Obviously,triple junctions are the most favorable places for this liquid phase to accumulate.Based on the discussion above,it can be concluded that a clean interface was formed wherever the liquid phase had the chance to form and leave the interface;conversely,the absence of such a phase led to the remaining oxide impurities,and accordingly,creating an unclean interface.
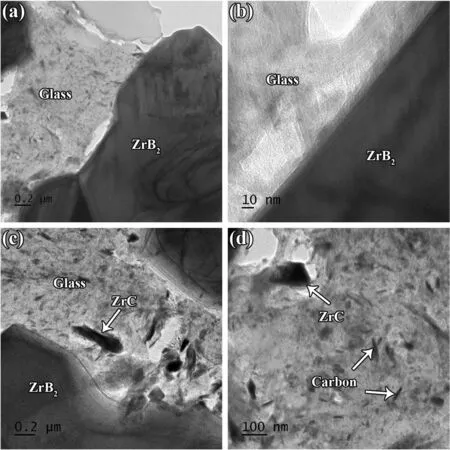
Fig.7.Low-and high-magnification TEM images of(a,b)an interface between ZrB2 matrix and a glassy phase,and(b,c)the in-situ formed glassy phase with ZrC and C islands therein.
Fig.6c shows a high magnification TEM image from inside a ZrB2grain.A compact network of dislocations can be seen in this image,which confirms the role of plastic deformation of ZrB2particles as one of the main densification mechanisms at elevated temperatures.It implies the role of high pressure in compacting the particles over the densification stage[36-39].An interface between ZrB2and SiC grains is presented in Fig.6d.The lack of glassy phase at this grain boundary proves the impact of convection in transferring the phase to other areas,namely pores,triple junctions,etc.Such phenomenon was reported by Shahedi et al.[40].However,the footprint of atomic defection is apparent at both sides of the shown grain boundary.In short,atomic diffusion may lead to the vacancy orientation in these kinds of boundaries,forming a different texture at both sides of the interface.As shown in Fig.3,XRD evaluation revealed the formation of a cubic phase as the final SiC form in the as-sintered sample.The non-formation of a completely clean interface by ZrB2and SiC could be attributed to the difference between their crystalline structures (cubic for SiC and hexagonal for ZrB2).Low and high magnification TEM micrographs captured from ZrB2and glassy phase interfaces are depicted in Fig.7a-b.Although the diffusion bond can be seen at the ZrB2side(Fig.7b),the interfacial area is relatively clean.The presence of a liquid phase was apparently successful in eradicating the oxide layers in this area,and removed the reaction products from the interfacial zone.However,the glassy phase seems to have some other phases,and their closer views are exhibited in Fig.7c-d.It was noted in the discussion that ZrC,C,and gaseous phases of CO and SiO are the main by-products of chemical reactions between the oxide impurities along with the glassy phase of Si-B-O.The EDS analysis (not shown here)confirmed that these nano-sized particles dispersed into the glassy phase are associated with in-situ formed ZrC and carbon.It is worthy to note that,the lack of carbon peaks in the XRD result(Fig.3) could be due to their low content.
4.Conclusions
The SPS process has been accomplished on the ZrB2-25 vol% nanosized SiC composite sample at 1800°C for 5 min under 25 MPa external pressure.The evaluations of sinterability and microstructure of this ZrB2-based composite result in the following outcomes:
-The as-fabricated material reaches a relative density of 99%,indicating the positive influence of nano-sized SiC on the sintering behavior of ZrB2.
-XRD evaluations unveil the formation of the in-situ ZrC phase along with the initial phases,namely ZrB2and SiC.
-The FESEM and thermodynamical investigations reveal the formation of a liquid phase containing Si,B,O,Zr,and C elements over the sintering process.
-Fractographs indicate that the amount of the formed liquid phase is not sufficient to eliminate all remained porosity.
-The TEM assessment approves the formation of such a liquid phase during the SPS process,which contains a Si-B-O glassy matrix with dispersed islands of ZrC and carbon.
-Ultimately,the TEM images endorse the role of the liquid phase on facilitating matter transfer,which lead to the formation of relatively clean interfaces wherever it is available.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Mohsen Mohammadi acknowledges the funding received from Natural Sciences and Engineering Research Council of Canada (NSERC)Grant No.RGPIN-2016-04221.Mehdi Shahedi Asl thanks the McCain Foundation for providing enough funding through the McCain Foundation Postdoctoral Fellowship in Innovation program to conduct this work.Quyet Van Le appreciates the fund of the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.05-2020.15.
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Surface study of the reconstructed anatase TiO2 (001) surface
- Effect of Na+ in situ doping on LiFePO4/C cathode material for lithium-ion batteries
- Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors
- Electric transmission behavior of self-assembled Cu-W nano multilayers
- A hybrid hydrogel/textile composite as flame-resistant dress
- Improvement on hydrogen generation properties of Zr(BH4)4·8NH3
