Surface study of the reconstructed anatase TiO2 (001) surface
Guanxing Li,Ke Fang,Yang Ou,Wentao Yuan ,Hangsheng Yang,Ze Zhang,Yong Wang
Center of Electron Microscopy and State Key Laboratory of Silicon Materials,School of Materials Science and Engineering,Zhejiang University,Hangzhou,310027,China
Keywords:Anatase TiO2 Surface science TiO2 (001) surface Surface reconstruction TiO2 (1×4)-(001) surface
ABSTRACT The anatase TiO2(001)surface has attracted extensive attention in surface science and for practical applications because of its excellent performance.However,the highly reactive (001) surface usually reconstructs to a (1×4)-(001) surface,which may potentially alter its properties.As a result,there are still controversial issues concerning the intrinsic performance of this surface.Recently,significant advances have been made in the surface science study of TiO2(001),which have elucidated its intrinsic physical and chemical properties.In this review,we summarize our present understanding of the structure and properties of the reconstructed TiO2(001)surface.First,we provide a brief background of the anatase TiO2 surfaces,including its low-index surface structures and the synthesis of anatase TiO2 nanocrystals with the (001) surface exposed.Next,we focus on the structure,formation mechanism,stability,and surface chemistry of the reconstructed TiO2(001)surface.Finally,we finish this review by highlighting the current challenges of this subject and providing future research perspectives.
1.Introduction
Titanium dioxide (TiO2) has been intensively studied since the first discovery that TiO2electrodes could photocatalyze water splitting [1].TiO2shows excellent performances in various applications,including thermal catalysis [2,3],photocatalysis [4],sensors [5,6],lithium ion batteries [7-9],dye-sensitized solar cells [10,11],biomedical treatments [12,13] and so on [14].Its performance is attributed to various properties,including its nontoxicity,low cost,and stability,as a wide bandgap (3.0-3.2 eV) semiconductor.In these applications,the TiO2surface plays an essential role.The critical processes (adsorption,reaction,charge separation,and charge transfer) all involve surface-molecule/atom/electron interactions.The exposure of different facets may result in dramatic discrepancies of the surface structures and performances.For instance,water molecules can dissociatively adsorb on the anatase(001)surface but molecularly adsorb on the(101)surface[15].In addition,taking a supported catalyst as an example,the interactions between the support surface and metal particles can be facet-dependent[16],resulting in different catalytic performances[17].
There are naturally four kinds of TiO2polymorphs [18]:anatase,rutile,brookite,and TiO2(B).Among them,anatase is the most active phase when applied to photocatalysis because of its slightly higher Fermi level,lower oxygen-adsorbing capacity,and higher degree of hydroxylation [19].In addition,anatase TiO2is more stable at the nanoscale because of its low surface energy [20].Compared to the thermodynamically stable(101)surface,the anatase TiO2(001)surface has a higher surface energy and is predicated to have excellent activity[15].Inspired by the fantastic properties predicted by theoretical calculations,the TiO2(001)surface has been intensively studied [21,22].
However,many experimental studies have found controversial results related to the properties of TiO2(001) [21,23-26].For instance,Pan et al.compared the low-index facets of TiO2and concluded that{001} facets had lower photocatalytic activities for OH radical generation and hydrogen evolution [26].In contrast,Wu et al.found that crystals with dominant {001} facets had better photo-oxidation activities for the degradation of methylene blue under ultraviolet(UV)light irradiation[25].Roy et al.reckoned that the synergy between the{101}and {001} facets could enhance the photocatalytic activities for the degradation of organic contaminants under UV light [23].In these studies,very littlesurface structural information was derived at the atomic level,yet the surface structure can remarkably influence the catalytic performances.The bulk-truncated anatase TiO2(001) usually reconstructs to a (1×4)-(001) structure because of its high surface energy.Whether it was reconstructed or maintained with a bulk-truncated structure in these studies was not determined.The atomic structure,formation mechanism,and corresponding properties of the reconstruction also must be clarified.In addition,the F species normally used in the synthesis can adsorb on the surface,which may also affect the surface structures and thus the performances.These uncertainties are critical reasons for the experimental discrepancies.Thus,to better understand the catalytic performance,the TiO2(001) surface and its reconstruction should be considered[27].
In the last two decades,many advances have been made in the study of TiO2(001)and reconstructed TiO2(1×4)-(001)surfaces,especially with the help of newly developed simulations andin-situcharacterization techniques,e.g.,scanning probe microscopy (SPM),density functional theory (DFT) calculations,andin-situtransmission electron microscopy (TEM).The reconstruction dynamics and mechanism of TiO2(1×4)-(001)were also unveiled[28].A comprehensive summary of this topic can help to fully understand the intrinsic properties of TiO2(1×4)-(001).Several review papers have partially mentioned the structure of the TiO2(1×4)-(001)surface.However,a comprehensive review on this topic is still lacking.
Herein,we present an overview of the anatase TiO2(1×4)-(001)surface from a surface science perspective at the atomic scale.The paper is organized as follows (Scheme 1).In Section 2,we provide a brief background of anatase TiO2,including three low-index surfaces and the synthesis of anatase TiO2nanocrystals with exposed (001) surfaces.In Section 3,we mainly focus on the structure,formation mechanism,and stability of the TiO2(1×4)-(001) surface.We review the recent advances in the surface chemistry of the TiO2(1×4)-(001) surface in Section 4.Finally,we discuss the current challenges of this topic and provide our perspective of future research.
Scheme 1.Scheme of the surface study of the reconstructed TiO2 (001) surface.
2.Background
Since the synthesis and performance of the TiO2(001)surface have been well documented in previous reviews[29,30],we only give a brief introduction of its surface structure and preparation in this section.Anatase TiO2has a tetragonal crystal structure,with theI41/amdspace group and a band gap of 3.2 eV [18].The poor absorption of visible radiation and rapid recombination of photogenerated electron/hole pairs limit its catalytic efficiency [31].Many methods have been developed to improve its catalytic properties,including doping,surface modification,controlling the size,and exposing facets.In particular,the different facets may affect the properties significantly due to the different atoms exposed or different interactions with adsorbed molecules.Thus,to better understand the underlying mechanism and to improve its performance,TiO2samples with various facets exposed have been synthesized.This includes not only low-index surfaces,such as(101),(100),and(001)[26,32,33],but also some high-index ones,such as (301),(102),and (103) [34,35].Here,we only introduce three low-index surfaces.
2.1.Low-index surfaces of anatase TiO2
The low-index facets of anatase TiO2were studied both theoretically and experimentally [26,36].According to DFT calculations of their surface energies,the stability sequence of these facets is {101} (0.44 J/m2),{100} (0.53 J/m2),and {001} (0.90 J/m2) [37],which is consistent with the density of the under coordinated Ti atoms.Compared to the (100) and (001) surfaces with all the Ti atoms being five-fold-coordinated (Ti5c),the (101) surface contains 50%six-fold-coordinated Ti (Ti6c) and 50% Ti5catoms (Fig.1).Thus,the(101) surface has the lowest surface energy and is the most stable.The atomic structures of these three surfaces (Fig.1) have been well illustrated by scanning transmission electron microscopy (STEM) experiments [34].
The activities of different surfaces have been explored.However,in the experimental results reported so far,there are still some discrepancies [25].The main difficulty in forming a consistent conclusion is that too many factors are involved in such complicated experiments.For an ideal experiment,it must be guaranteed that samples with different surfaces have the same sizes,crystallinities,defect densities,and surface cleanliness.Meanwhile,the experiments must be performed under same conditions,which is extremely difficult to achieve in practice.The first fundamental step is to synthesizehigh-qualityTiO2nanocrystals with specifically exposed facets and controllable grain sizes.
2.2.Synthesis strategies of anatase TiO2 (001)
TiO2crystals with predicted equilibrium shapes by the Wulff construction have approximately 94% exposed {101} surfaces and 6%exposed {001} surfaces [38],forming a slightly truncated bipyramid.The{001}facets usually vanish rapidly during the crystal growth due to the high surface energy.Therefore,most previous studies of TiO2(001)were focused on films that were generally grown on specific substrates,such as SiTiO3or LaAlO3[39,40].
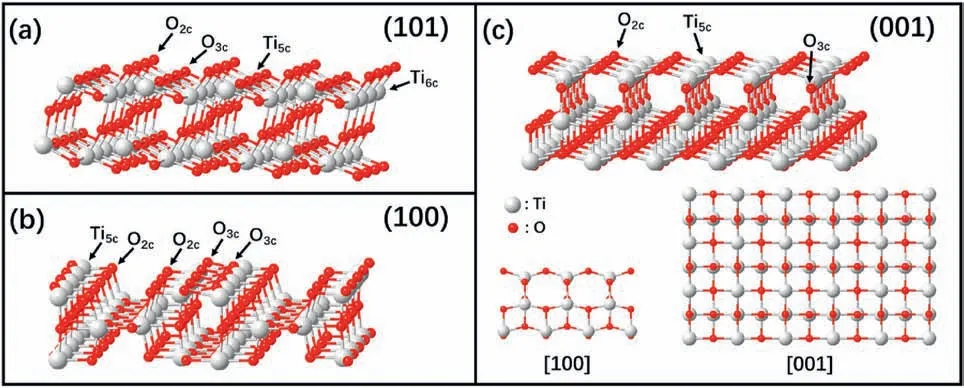
Fig.1.Ball and stick models of anatase TiO2 low-index surfaces:(a) (101) surface;(b) (100) surface,and (c) (001) surface (red:oxygen;blue:titanium).
In 2008,Yang et al.[41] made a breakthrough by synthesizing micrometer-sized TiO2single crystals with {001} facets dominant,adopting F as the capping agent.They explored the effect of a range of non-metallic adsorbate atoms on reducing the surface energy of TiO2{001} facets (Fig.2a) and the total surface area of the crystal (Fig.2b)using theoretical calculations.They found that F atoms could reduce the energy of the (001) surface dramatically and even make it more stable than the (101) surface.Accordingly,they used a titanium tetrafluoride(TiF4) aqueous solution and hydrofluoric acid (HF) to generate truncated anatase bipyramids,and they successfully grew high-quality single crystals with 47% exposed {001} facets.After this,F-containing compounds were widely used as the crystallographic controlling agents for synthesizing TiO2(001) with different sizes and percentages of the exposed {001} facets.Fig.2c indicates the morphology changes of the TiO2crystals as the percentage of the{001}facets increased,and Fig.2d shows the continuous cases with different crystal sizes and percentages of the (001)surface [41-49].
Han et al.synthesized sheet-like anatase TiO2via a simple hydrothermal route,obtaining TiO2nanosheets with up to 89%exposed{001}facets[47],with a side length of~40 nm and a thickness of~6 nm.Lv and co-workers used a similar method to grow TiO2(001) with 88%exposed {001} facets,and the as-prepared nanosheets showed high thermal stabilities (up to 1100°C) [46].Wen et al.developed a new synthesis strategy using TiF4and 1-butanol with HF to synthesize paper-like anatase TiO2nanosheets,and the percentage of exposed{001} facets could reach 98.7%[49].
As HF solutions are extremely corrosive,and F-containing compounds may induce severe environmental problems,significant efforts have been devoted to developing eco-friendly methods for synthesizing TiO2(001),which are summarized in Table 1[50-58].For instance,by usingtitanium isopropoxide (TTIP) as the TiO2precursor and acid-delaminated vermiculite (DVMT) and tetramethylammonium hydroxide (Me4NOH) as synergistic morphology-controlling reagents,Wang et al.proposed a green synthesis method for shape-defined anatase TiO2nanocrystals with fully exposed {001} and {100} facets[55],and the percentage of {001} facets could reach 40%.Using K-titanate nanowires as precursors and urea as a surface regulator,TiO2nanoparticles with 60% exposed {001} facets were obtained.The authors ascribed this fluorine-free hydrothermal route to the key role of the carbonate ions[52].A green synthesis route using a microwave-assisted method was also reported,where titanium tetrafluoride and a tetrafluoroborate-based ionic liquid were used to obtain micro-sheet anatase TiO2with 80% exposed {001} facets [50].In addition,some other unique hierarchical structures with a high percentage of {001}facets were made,with nearly 100%exposed{001} facets [59].
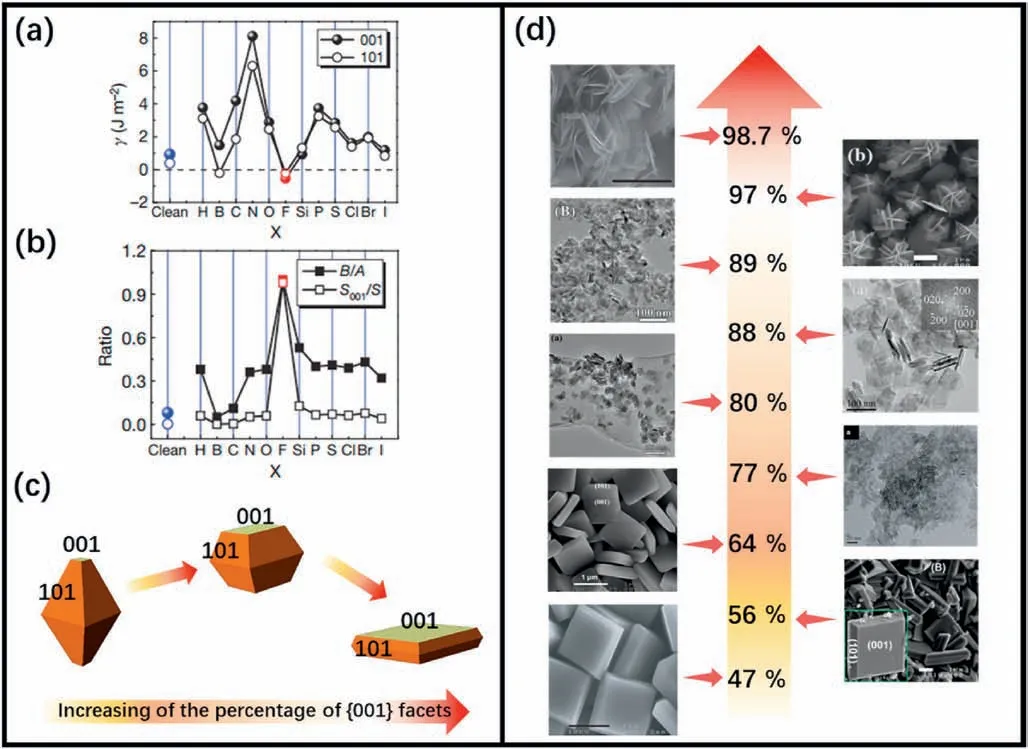
Fig.2.SynthesizedTiO2 crystals with the(001) surface.(a) Calculated energies of the(001) and (101) surfaces adsorbed by X atoms.(b)Plots of the optimized value of B/A and the percentage of {001} facets for anatase single crystals with various adsorbate atoms X (A:side lengths of the bipyramid;B:side lengths of the square {001}‘truncation’ facets).(a,b) Reproduced with permission from Ref.41,copyright:2008,Nature Publishing Group.(c) Models of crystal shape of anatase TiO2 with different percentages of {001} facets exposed.(d)Images of TiO2 samples with different percentages of {001} facets exposed.Reproduced with permission from Refs.41-49,copyright:2008,Nature Publishing Group;2009,American Chemical Society;2012,American Chemical Society;2011,Elsevier;2011,Wiley-VCH Verlag GmbH&Co;2011,Royal Society of Chemistry.
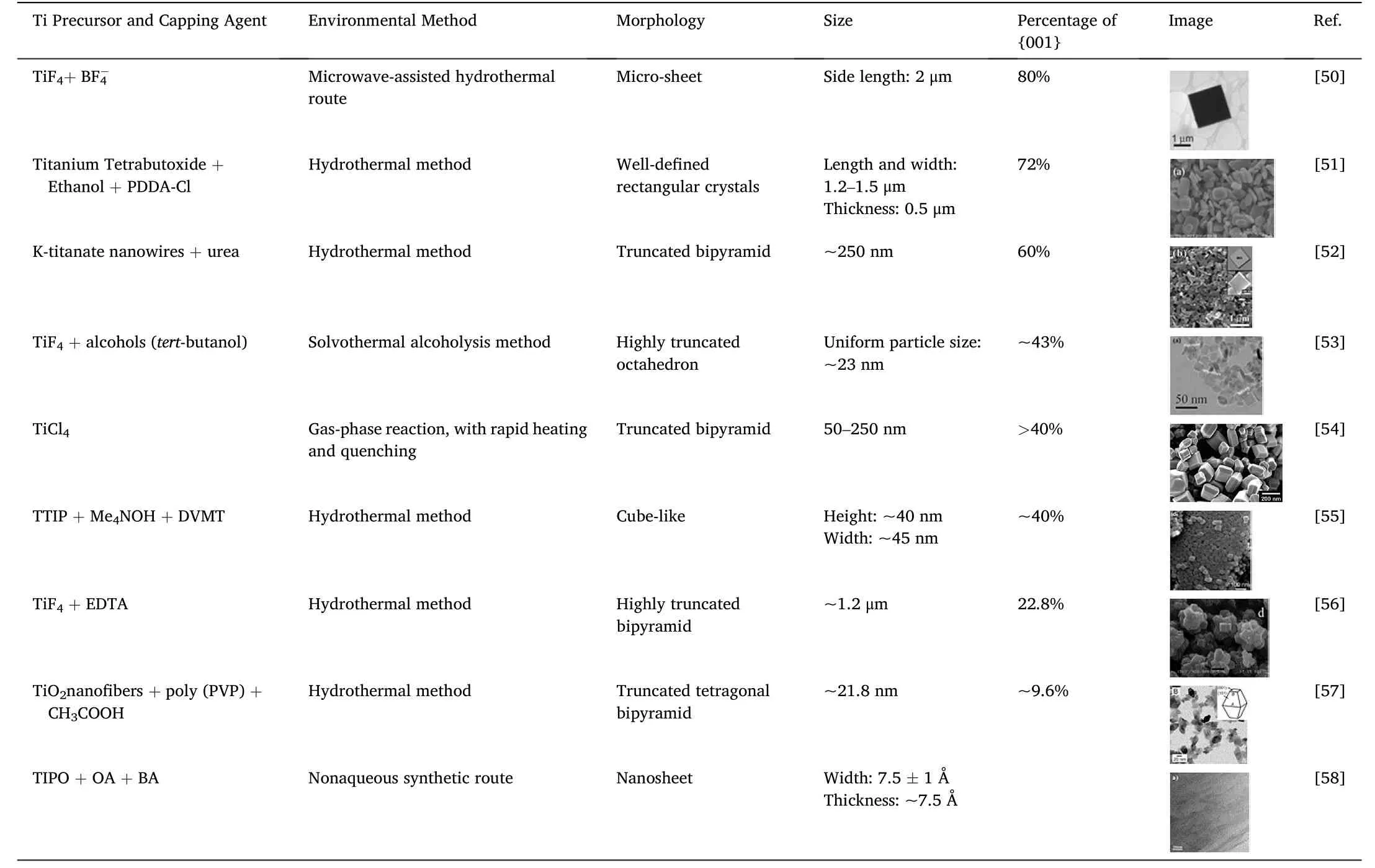
Table 1 TiO2 crystals with(001)surface synthesized by green methods.
These F-containing and F-free synthesis strategies helped to obtain different sizes and morphologies of TiO2(001),which greatly contributed to the further investigation of the structures and corresponding properties of TiO2(001).However,with the present F-free synthesis processes,either the percentage of the(001)surface is relatively small or the TiO2crystals are too large.To obtain TiO2(001)samples with high quality,F-containing agents are still used widely.The environmentally benign F-free methods for preparing TiO2(001) must be further explored in the future.
2.3.Anatase TiO2 (001) post-treatment
As mentioned above,in most cases,the synthesis of anatase TiO2{001} surfaces with high quality inevitably involves F-containing solvents,which can closely adsorb on the surfaces.Many studies have suggested that F species on the (001) surface largely reduce the performance [5,60,61].Thus,removing the surface F impurities has become a common pretreatment procedure before property studies and is crucial for studying the intrinsic activities of TiO2(001) surfaces.Generally,there are two methods that are mostly used to remove surface F,i.e.,annealing at high temperatures and washing with an NaOH solution.Previous studies confirmed the feasibility and effectiveness of these two methods[28,41,61].However,the heating process may result in some significant structural changes of the TiO2nanocrystals,such as sintering or surface reconstruction [61,62].The details of the surface reconstruction will be discussed in the next section.
We should note that whether the adsorbed F on the {001} surface weakens the properties is still under debate.For example,Peng et al.found that surface fluorine could improve the photocatalytic H2evolution activity of TiO2(001) because of the local electronic modification imposed by the surface F[63].An increase in the photocatalytic activity of TiO2(001) for degrading gas-phase acetaldehyde and liquid-phase phenol was also found,which was ascribed to the enhanced adsorbed oxygen due to the residual HF [64].
3.(1×4) reconstruction of anatase TiO2(001)
Because of their high surface energies mentioned above,TiO2(001)surfaces usually restructure to a (1×4)-(001) surface.In particular,after removing surface contamination,reconstruction can be an important pathway for surface stabilization.In addition,it may also be a reason for the performance discrepancies.In recent decades,the atomic structure,formation mechanism,and stability of the anatase TiO2(1×4)-(001)surface have been intensively explored.
3.1.Determination of (1× 4) reconstruction
In 2000,Herman et al.[65] first observed a two-domain (1×4)reconstruction on the TiO2(001)surface after sputtering and annealing the (1×1) surface through low-energy electron diffraction (LEED)(Fig.3a).They proposed three possible structures,i.e.,the missing-row model (MRM),the added-row model (ARM),and the microfacet model(MFM).In the MRM,every fourth row of oxygen ions is removed,exposing the underlying Ti ions.The ARM is formed by adding a row with a periodicity four times the lattice constant.The MFM exposes both the (103) and (-103) planes,and the oxygen is two-fold coordinated,whereas the titanium is 4-or 5-fold coordinated.However,the comparison of the experimental and calculated angle-resolved mass spectroscopy data ruled out the former two models,and the MFM gave the best agreement.Their X-ray photoelectron spectroscopy (XPS) results also supported the auto-compensated MFM.
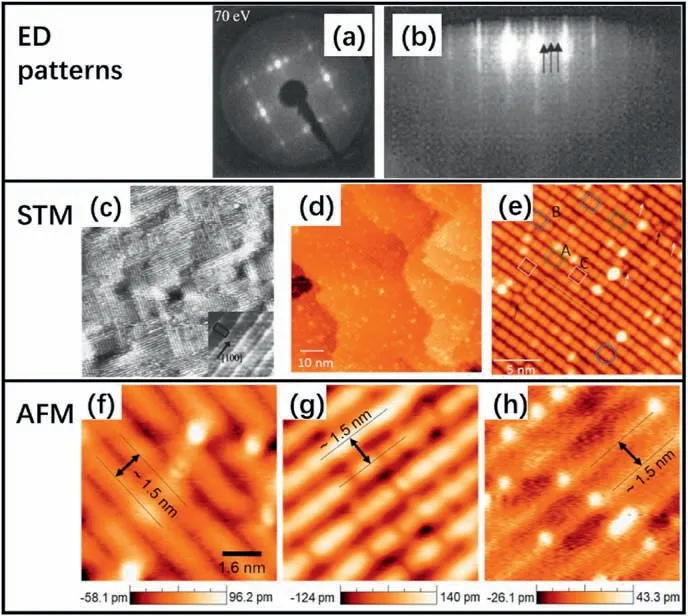
Fig.3.(a)Two-domain(1×4)low-energy electron diffraction(LEED)pattern for the TiO2(001)surface.Reproduced with permission from Ref.65,copyright:2000,American Physical Society.(b) A reflection high-energy electron diffraction(RHEED)pattern obtained during the anatase film growth[66].(c)A large-scale scanning tunneling microscopy (STM) image showing (1×4)/(4×1) domains on anatase TiO2(001),1200×1200 Å2.(b,c) Reproduced with permission from Ref.66,copyright:2001,American Physical Society.STM images of (001) thin film surface with (d) an 80 nm×80 nm image and (e) a high-resolution image (20 nm×20 nm) showing three types (A,B,and C) of features.(d,e) Reproduced with permission from Ref.67,copyright:2013,American Chemical Society.(f-h) Three different types of topographic images measured by non-contact atomic force microscopy (AFM) on TiO2 (001) surface:(f) protrusion mode,(g) hole mode,and (h) neutral mode.(f-h) Reproduced with permission from Ref.[68],copyright:2019,IOP Publishing Ltd.
Later,a missing oxygen-row model was proposed but not verified[69] based on the result that the reconstructed (1×4) surface would recover to(1×1)when it was exposed to oxygen or air.However,Liang and co-workers[66]found that the reconstruction was stable not only in an ultrahigh vacuum but also in oxygen-rich environments and even during the growth of the TiO2film based on scanning tunneling microscopy (STM),reflection high-energy electron diffraction (RHEED),XPS,and LEED results (Fig.3b and c).They reckoned that the (1×4)reconstruction was fully oxidized,which ruled out the missing-oxygen rows model.In addition,they found that the atomic rows in the high-resolution STM images were not consistent with the microfacet{103} model.Based on this information,they proposed an“added-and-missing-row”model(AMR),which was also called the microfacet{014}model.In the AMR,each surface unit cell has one added Ti-O row,two added oxygen rows,and one missing oxygen row.All atomic positions were derived from the bulk structure,and the surface was stoichiometric and auto-compensated in charge,which was consistent with the XPS results.The AMR is easily adapted to different periodicities,such as(1×3)and (1×6)observed in the STM images.
Although the MFM and AMR are consistent with the experimental results,they fail to explain the energy.Both the MFM and AMR introduce four-fold coordinated Ti (Ti4c),while the Ti atoms on the original(1×1)surface are all Ti5c.This means the occurrence of reconstruction is not energetically favored.Based on DFT calculations,Lazzeri et al.[70]proposed an energetically favorable model,i.e.,the“ad-molecule”(ADM).In this model,surface bridging oxygen of the(1×1)surface are replaced by TiO3rows periodically,which can be easily adapted to(1×n) periodicities.They also calculated the surface energies of the MFM,AMR,and ADM and found that the ADM was the only one that had a lower energy than that of the unreconstructed surface.In addition,they confirmed that the(1×4)periodicity was the most stable one compared to other (1×n) periodicities.The calculated STM images of the ADM were highly consistent with the experimental images[66],and the(1×4) reconstruction could effectively relieve the surface tensile stress existing on the(1×1)surface.
Later,Tanner et al.[71] proposed a {101}-microfacet model that satisfied the following criteria obtained in their experiments:it was stoichiometric,Ti atoms were centered at the crests of the rows,and the surface had more preferable Ti5c.However,their model had difficulty matching all the characterization results of previous experiments.For example,the corrugation in the{101}-microfacet model is significantly greater than that observed in STM images,and it is not clear why molecules do not adsorb in the troughs.This model can also be ruled out by the dramatic difference in the electronic structures between the (101)and (001) surfaces obtained by Thomas et al.[72] via resonant photoemission investigations.
High-resolution STM images acquired by Xia et al.[67]showed that the atomic corrugation of the bright rows on the TiO2(1×4)-(001)surfaces were not uniform (Fig.3d and e),which was quite different from all the previous models mentioned above.They proposed a modified added molecule model (M-ADM) for this reconstruction,which consisted of two types of atomic building blocks:the AMR and the ADM.
In 2013,Wang et al.[73] studied the reconstructed TiO2(001) surface through the STM and spectroscopic measurements.Oxidized(as-grown film on SrTiO3(001)substrates) and reduced (Ar+sputtered followed by annealing at 873 K) TiO2(1×4)-(001) surfaces were characterized.Their XPS,ultraviolet photoelectron spectroscopy(UPS),and STM results indicated only the defect Ti3+sites were active and the oxidized(1×4)surface was inert.Based on these results,they proposed an“ad-oxygen atoms”model (AOM),in which the terminal Ti at the ridges was six-fold coordinated instead of four-fold coordinated in the ADM.It could explain the inactive performance of the(1×4)surface in the experiments,which were quite different from the predicted results[70].The defect sites and corresponding properties of their model were studied by DFT calculations [74].Though the AOM could explain the experimental results well,the surface energy was relatively large compared to that of ADM [28,75].
Xu et al.[75]used theoretical methods to study the ADM and AOM,and their simulated STM images coincided with previous experimental observations.The ADM was stable in most of the oxygen potential range,and the AOM more easily formed in oxygen-rich conditions.From the MD results,they found that the ADM surface was more active than the AOM surface in terms of photoreactivity,which was related to the surface atomic coordination and the electronic structure.Chen et al.[76]also found that the surface structures(ADM or AOM)could be different,depending on the temperature and oxygen pressure.
Among these proposed models (Fig.4),the ARM and MRM models are not consistent with the XPS and UPS results [65].The MFM cannot adequately explain the(1×n)periodicities(n=3,4,5,6…)observed in experiments [66].The M-ADM contains approximately 2.5% more oxygen atoms than the stoichiometric surface[67],and it was proven to be less stable by theoretical work[77].The surface energy of the AOM is relatively high in most cases,which means it is not stable and thus very hard to form[28,75],and the ADM is the most widely accepted model.However,based only on the top-view experimental results via the SPM(Fig.3c-h),it is difficult to distinguish these models.The differences in the structural features between these models is small,only involving the accuracy of the positions of Ti and O rows parallel to the surface.Thus,further structural information from the side view should be provided to determine this structure.
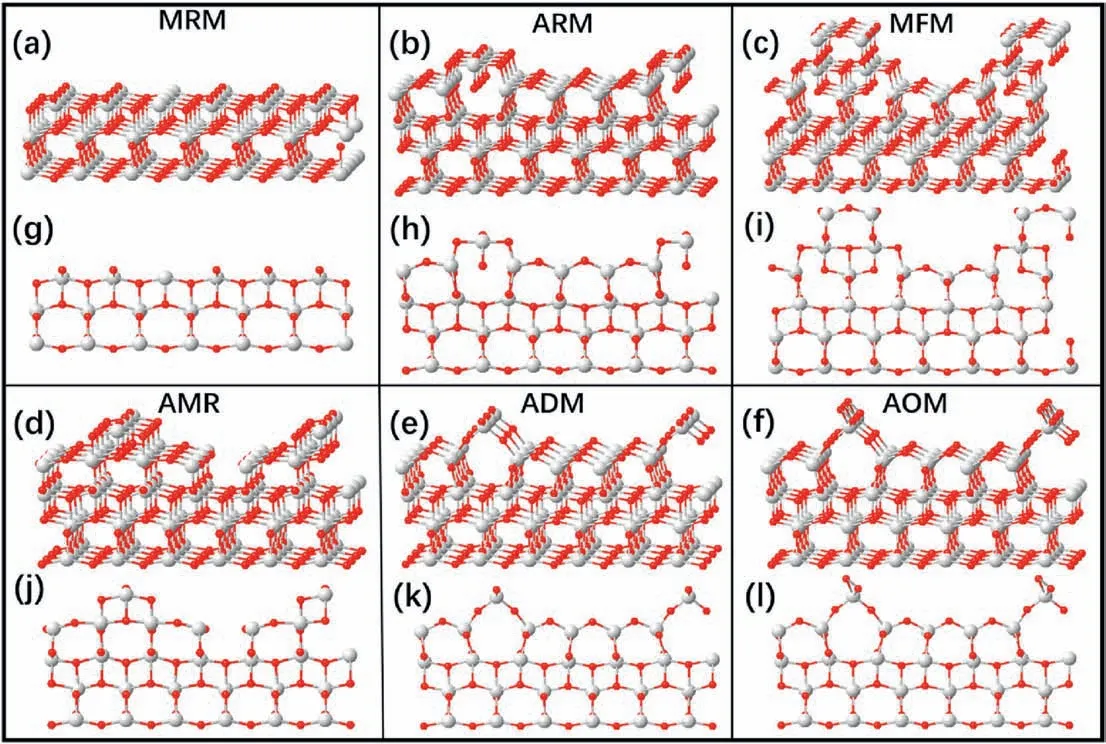
Fig.4.Proposed models for TiO2 (1×4) reconstructed (001) surface:(a-f) side views of the missing-row model (MRM) [65],added-row model (ARM) [65],microfacet model (MFM) [65],added-and-missing row model (AMR) [66],ad-molecule model (ADM) [70],and ad-oxygen model (AOM) [73],respectively.(g-l)Views along the [100] direction of the MRM,ARM,MFM,AMR,ADM,and AOM,respectively.
Based on this,Yuan et al.performed anin-situatomic-scale observations of the reconstructed TiO2(001)surface from a side view using an aberration-corrected STEM and a heating holder system [78].The sample used in the experiment was a TiO2nanorod whose tips exposed a small proportion of(001)surfaces.At room temperature,the high-angle annular dark-field(HAADF)-and bright field(BF)-STEM images showed that the (001) surface was the bulk-truncated structure.Afterin-situheating of the sample to~750°C in a vacuum (10-5Pa),the reconstructed (001) surfaces appeared,and the side-view STEM images showed accurate positions of the surface Ti-O and O columns,as shown in Fig.5a and b.Most of the previous surface structural characterizations by conventional surface analytical methods were from the top view.This side-view STEM observation at the atomic level provided the most convincing evidence for the ADM.These results were supported by the simulated HAADF and BF images(Fig.5c and d).Different periodicities of the(1×n)structures were also observed in the atomic-scale images.Thus,the ADM is the first model supported by both top-view and side-view experimental evidence,which is also energetically favorable.
Another TEM study was performed by Ek et al.[79].First,the(1×4)reconstruction was imaged by STM under ultra-high-vacuum (UHV)conditions,which proved that oxygen was not the necessary condition for reconstruction.Next,in anin-situTEM study,they used an exit wave(EW) reconstruction to improve the quality of their TEM images.With this method,they obtained extremely clear TEM images,which showed that there were no additional asymmetrical O or Ti atoms on the top of the row or in the void below the ADM(Fig.5e and f).Thus,the AOM and M-ADM were ruled out,and ADM was confirmed,which agreed with Yuan’s STEM images [78].
3.2.Formation and stability of reconstruction structure
Understanding the reconstruction mechanism is an important prerequisite to tune the surface atomic structure and even the performances of functional nanocrystals.As the nanocrystal will be inevitably exposed to different environments,the stability of its surface structure is also crucial in terms of potential applications.Studying the mechanism and stability of the reconstructions can help to select the optimized application conditions.

Fig.5.(a,b) Atomic resolution high-angle annular dark-field-scanning transmission electron spectroscopy (HAADF-STEM) and the corresponding bright-field (BF)-STEM images of the (1×4) reconstructed (001)surface,viewed along the [010] axis.(c,d)Simulated HAADF-and BF-STEM images of the (1×4) reconstructed (001) surface.(a-d) Reproduced with permission from Ref.78,copyright:2017,American Chemical Society.(e) Exit wave (EW) phase image of the reconstructed (001) surface after annealing at 870 K.(f) STM image of the (1×4) reconstructed terrace on the (001)surface.(e,f) Reproduced with permission from Ref.79,copyright:2018,Royal Society of Chemistry.
To explore the reconstruction mechanism,Yuan et al.used environmental TEM (ETEM) to observe the reconstruction process [28].In their experiments,TiO2nanosheets dominated by the(001)surface were used.To remove surface adsorbed F,the samples were washed with an NaOH solution and deionized water.The (1×4) reconstruction appeared on the surfaces afterin-situheating to 500°C in an oxygen environment (0.05 Pa).During the observation,the processes through which the(1×4)reconstruction formed and the metastable(1×3)and(1×5) patterns transformed into the (1×4) stable structure were unraveled.Previous investigations mainly focused on micrometer-sized surfaces under UHV conditions.This work confirmed that this reconstruction was also stable on nanosized crystals in an oxygen environment.
The reconstruction process in an oxygen environment is briefly summarized(Fig.6).After the removal of the amorphous capping layer on the surface,the randomly adsorbed TiOxspecies formed small rows/islands and then formed regular (1×n) patterns.This step occurred quickly because it was driven by the tendency to reduce the number of dangling bonds(DBs)of lowly coordinated atoms on the surface,which could significantly reduce the total energy.The(1×n)patterns became a partially transformed state (PTS) and finally transformed to (1×4)patterns completely.This step was stress driven and occurred much slower [28].In addition,during the reconstruction process under a vacuum (not an oxygen environment),a metastable surface called the“(001)-HT”structure was involved in the pathway for the (1×4)reconstruction.Notably,the (1×4) reconstruction could be formed in both oxygen and vacuum environments,which also indicated that oxygen was not a necessary condition for the reconstruction.The oxygen in the ETEM experiments served as a protective gas,compensating for the oxygen loss in TiO2induced by electron beam irradiation.
By systematically studying the different factors that may influence the reconstruction[27],it was found that a clean surface and sufficient energy (provided by elevated temperature in experiments) were necessary for the reconstruction.The (1×4) surface could form from a vacuum up to an oxygen pressure of 10-2Pa at a temperature window of 300-800°C.In addition,with the same oxygen pressure and electron-beam dose,the higher the temperature was,the more easily the reconstruction was.The reconstruction conditions in the literature are summarized in Table 2[27,28,65-67,69,71-73,78-81].
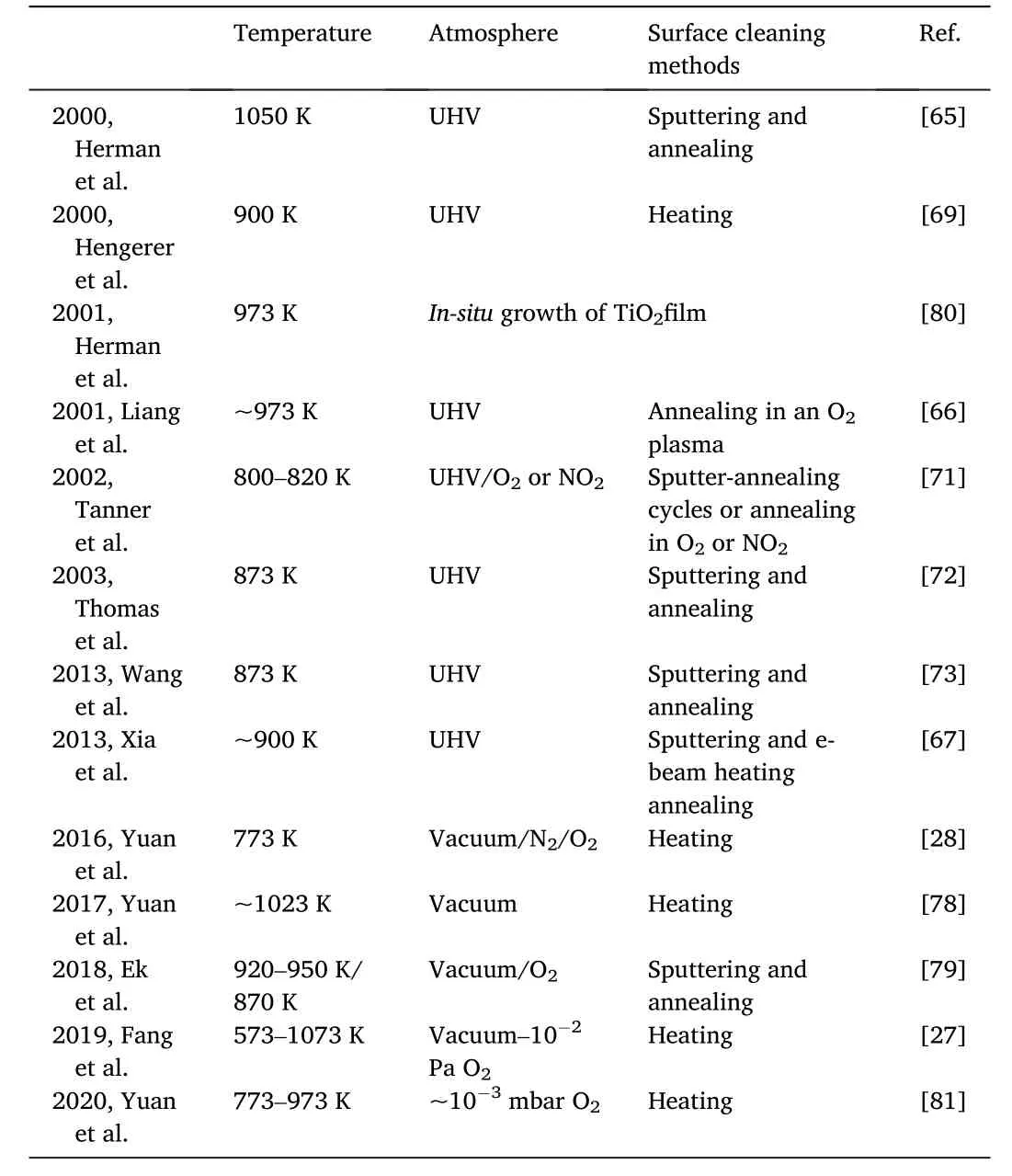
Table 2 Reconstruction conditions reported in the literature.
As shown in Fig.7,the surface energies of the different models were calculated by DFT,which showed that the ADM was the most stable one under environment with oxygen chemical potential less than-0.34 eV.Experiments showed that the reconstruction could survive in vacuum(5×10-5Pa) and oxygen (pressures:0.005-0.05 Pa) at differenttemperatures (300-800°C) [27].It could even survive under atmospheric pressure conditions[27].Herman et al.[80]also confirmed that when the reconstructed(001)surface was cooled to room temperature,the structure could still exist stably,which meant the reconstructed surface was indeed a stable structure because of its low surface energy(0.51 J/m2).
During TEM observations,electron-beam irradiation is often involved,which may damage the surface structures of TiO2[82].Thus,the damage process of the reconstruction was investigated using a high electron-beam dose (140 A/cm2and higher),and a layer-by-layer decomposing mechanism was found [27].The series ofin-situimages showed a decomposition process with a preferred orientation.To qualitatively estimate the damage degree,the electron-beam induced structural damage was divided into three degrees,i.e.,intrinsic surface(without notable surface damage),surface damaged(only surface layer damage),and bulk-structure damaged (even the bulk structure was damaged).The structural evolution diagrams were obtained as a function of electron current density and temperature.The above experiments were within the limited conditions of a small pressure/temperature range,and further studies should be explored under other conditions.
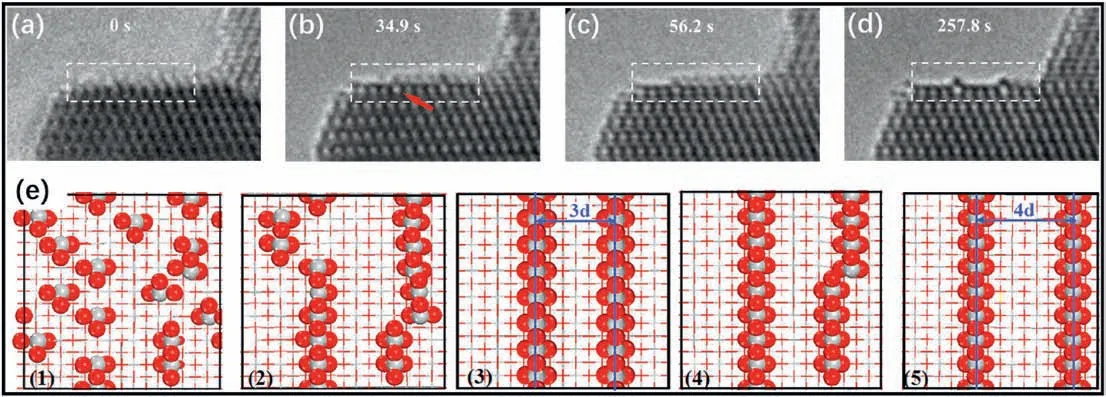
Fig.6.Atomic evolution of the(1×n)reconstructions:(a-d)Sequential HRTEM images of the dynamic structural evolution,viewed from[010]direction,with the red arrows indicating the unstable states.(e) Proposed reconstruction mechanism.Adsorbed TiO2 molecules and some of the surface oxygen are shown as balls(O,red;Ti,gray),and the major TiO2 substrate is shown as lines.Reproduced with permission from Ref.28,copyright:2016,American Chemical Society.
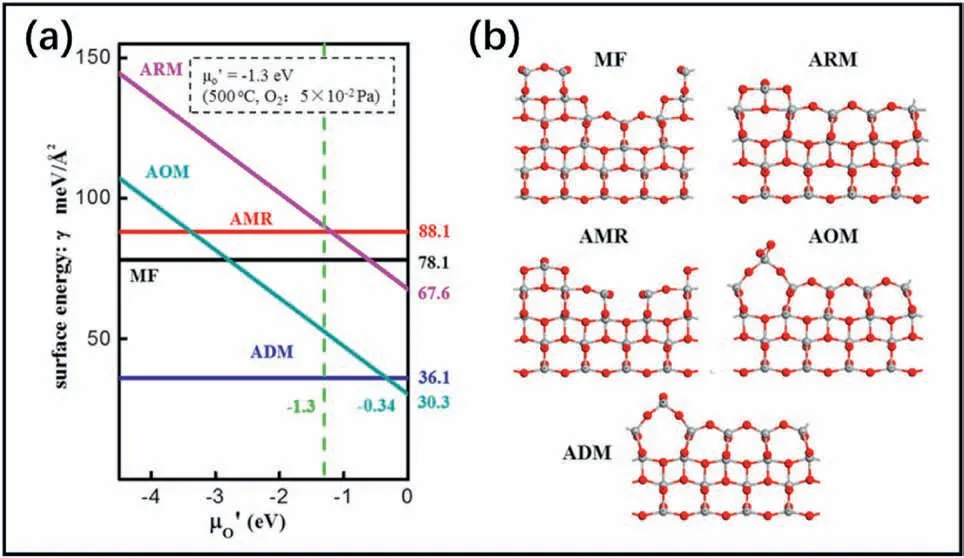
Fig.7.(a) Density functional theory (DFT) calculated surface energies of the models proposed previously vs.oxygen chemical potential (μO').(b) Relaxed slab models of the TiO2(1×4)-(001) surface.The vertical dashed green line at μO'=-1.30 eV corresponds to the typical experimental conditions (oxygen pressure of 0.05 Pa and temperature of 500 °C).Reproduced with permission from Ref.28,copyright:2016,American Chemical Society.
4.Surface chemistry of TiO2 (1×4)-(001)
Since unique dissociative adsorption of water molecules on the anatase TiO2(001)surface was suggested via DFT calculations[15],this surface has attracted tremendous interest,especially the interaction of a TiO2surface and small molecules such as H2O,HF,CH3OH,and HCHO.The surface adsorption,impurities,and reactions were extensively investigated.There is presently no consensus regarding the properties of the(1×1)-(001)and(1×4)-(001)surfaces.According to the ADM,the(1×4)-(001)surface would be more active due to the four-coordinated Ti atoms exposed [70].In addition,the enhanced dye-sensitized solar cell property of the TiO2nanosheet was also attributed to this reconstruction [83].However,through ab initio calculations,Giorgi et al.[84]found that the anatase TiO2with unreconstructed(1×1)surfaces exposed was more active during photocatalysis,because the probability that electrons/holes were available at surface sites on the(1×4)surface was significantly smaller compared to that on the (1×1) surface.Recently,as summarized below,extensive investigations of the intrinsic properties of the unreconstructed (1×1) and reconstructed (1×4)-(001)surfaces have been made.
4.1.Adsorption of H2O and HF
The issue of greatest concern is how H2O molecules adsorb on the(1×4)-(001)surface.Blomquist et al.first experimentally investigated the H2O adsorption on the TiO2(001)surface[85].The O1s spectra(Fig.8a)revealed the temperature-and coverage-dependent adsorption of water on the(1×4)reconstructed surface.At higher temperatures(above 230 K),dissociative adsorption of water was detected,which was believed to be adsorbed on the highly reactive ridges of the ADM reconstruction(Fig.8b).At lower temperatures (below 230 K),both dissociated and molecular water was found when the coverage was high.However,at low coverage,only dissociated water was detected.The conclusion that water dissociated only on the ridges but not terraces of the ADM reconstruction was consistent with previous calculations[86].
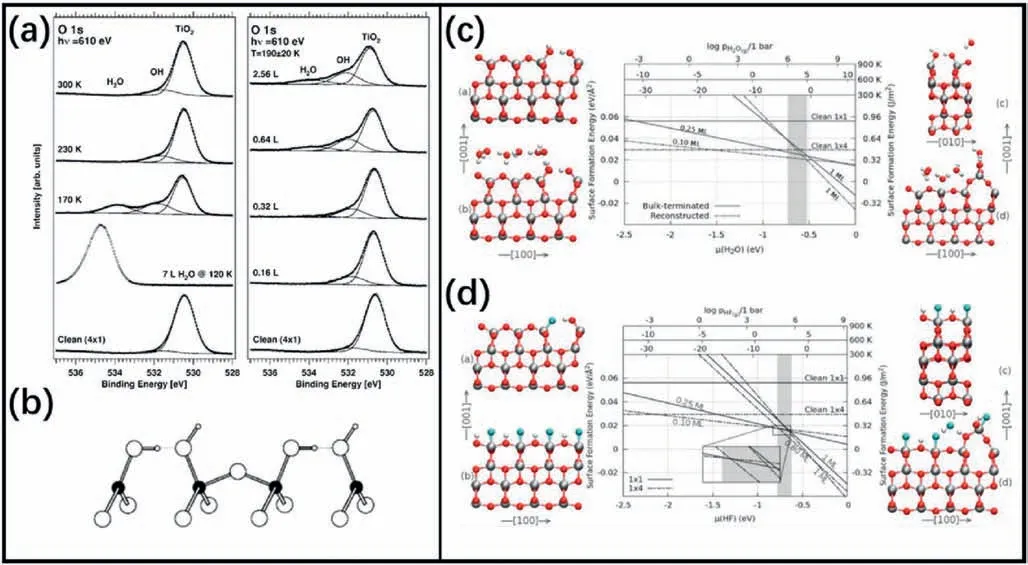
Fig.8.(a)O 1s spectra for water adsorption on TiO2 (1×4)-(001) recorded in grazing emission.The individual components associated with TiO2 and adsorbed OH and H2O obtained by curve fitting are included.Left:heating series.Right:growth series at a sample temperature of about 190 K.(b)Hydroxylation of the ridge Ti sites.(a,b)Reproduced with permission from Ref.85,copyright:2008,American Chemical Society.(c)Free energy diagram of(001)surface in the presence of water vapor (center) and selected water adsorption structures e.(d)Free energy diagram of (001) surface interacting with gaseous HF(center)and selected HF adsorption structures.(c,d) Reproduced with permission from Ref.62,copyright:2013,American Chemical Society.
Selcuk and Selloni[62]studied the adsorption of H2O on the TiO2(1×1) and (1×4) reconstructed (ADM structure) surfaces using DFT calculations and first principles molecular dynamics (FPMD) simulations.Different coverage of water and HF adsorbed on these two surfaces were considered using an extended surface model(Fig.8c and d).Under low coverage,the water adsorbed on (1×1) surface was completely dissociated.When the coverage was higher (1 ML),mixed dissociated-molecular adsorption was found,which differed from previous studies based on smaller unit cells.For the(1×4)surface,water preferred to dissociatively adsorb on the ridges of the ADM,which was consistent with previous studies [85,86].However,it showed significantly less reactivity than that on the (1×1) surface,which was also different from the previous results based on smaller unit cells [87].
In the STM and XPS results of later work [88],water could barely adsorb on the (1×4) surface at room temperature (RT).At 120 K,a mixture of molecular water and hydroxyl (OH) groups generated from water dissociation was found.The authors ascribed the reactivity of the(1×4)surface toward water to the Ti4catoms on the ridges acting as the dissociation sites.After annealing at 230 K,a(3×4)periodic structure was observed,which resulted from the ordered pairs of terminal OH groups along the ridges after water dissociation.The DFT calculations revealed the underlying dissociation mechanism and the dependence on the water coverage(Fig.9).
The adsorption of HF on(001)surface was also studied[79],which showed HF dissociatively adsorbed at both low and high coverages on the (1×1)-(001) surface.On the (1×4) surface,HF adsorbed dissociatively on the ridges,and terrace sites were occupied by a mixture of dissociative and molecular HF.When HF-water mixtures adsorbed on the surfaces,HF was always dissociative and water formed H-bonds with the surface.Further FPMD simulations indicated that the ridges of the(1×4) surface showed structural fluctuations in an HF solution,which suggested that the reconstructed surface was unstable in this solution.According to their calculations,when the samples were heated at 400-600°C,the stable (1×1) surface would transform to the (1×4)reconstruction because of the removal of the adsorbed fluorine.This supported the notion that a common heating process would induce the reconstruction,and it also explained why TiO2with dominant {001}facets sometimes did not exhibit enhanced photocatalytic activity.
4.2.Adsorption of CH3OH,HCHO,and HCOOH
Because TiO2has important applications for degrading organic pollutants,the adsorption of organic molecules such as formate,formic acid(HCOOH),and methanol (CH3OH) on TiO2(1×4)-(001) has been studied extensively.Tanner et al.[89] investigated the absorption of HCOOH on TiO2(1×4)-(001) thin films.As shown in Fig.10a and b,formate adsorbed at under-coordinated Ti sites in the highest points of the ridges instead of the trenches of the(1×4)structure.Their results were consistent with the ADM,in which formate adsorbed at the Ti4cpositions.
Gong et al.[86]studied the adsorption of HCOOH on the TiO2(001)(1×1) and (1×4) surfaces.By systematically studying the different adsorption configurations (Fig.10c),they found that HCOOH could dissociate spontaneously on both bulk-truncated and (1×4) reconstructed surfaces with various configurations.Among them,the bidentate adsorption configurations,in which the formate moiety bound to the surface through two Ti-O bonds,were more preferable.
Wang et al.[73]investigated the structure and activity of the TiO2(1×4)-(001) surface.They first investigated the chemical activity using CH3OH as a probe molecule [90].They found that the perfect (1×4)lattice sites were quite inert for the reaction of methanol,which was consistent with their AOM.Two types of defect sites (Fig.11a and b),reduced Ti pairs (dR-Ti) and partially oxidized Ti pairs (O-bridged Ti pairs(dO-Ti)),were active in this thermally driven reaction(Fig.11c),as proven by the STM and temperature programmed desorption (TPD)results.
The same group used another probing molecule,formaldehyde(HCHO),to detect the activity and photoactivity of this reconstructed structure[91].The thermally driven reaction could occur at the reduced defect sites (Ti4c),producing C2H4,but the perfect lattice sites were thermally inactive.The photocatalytic decomposition of HCHO was detected after UV irradiation,forming Hadand HCOO-species.When the temperature was elevated,the HCOO-species could be thermally dissociated to CO,and the Hadcould pick up the O atoms from the surface to form H2O.The loss of O atoms transformed AOM sites to ADM sites temporarily,which could act as the reactive sites for the dissociative adsorption of HCHO and the production of C2H4.They claimed that the AOM could explain the discrepancy of the thermally driven and photocatalytic reactions on the perfect (1×4) surface(Fig.11d).
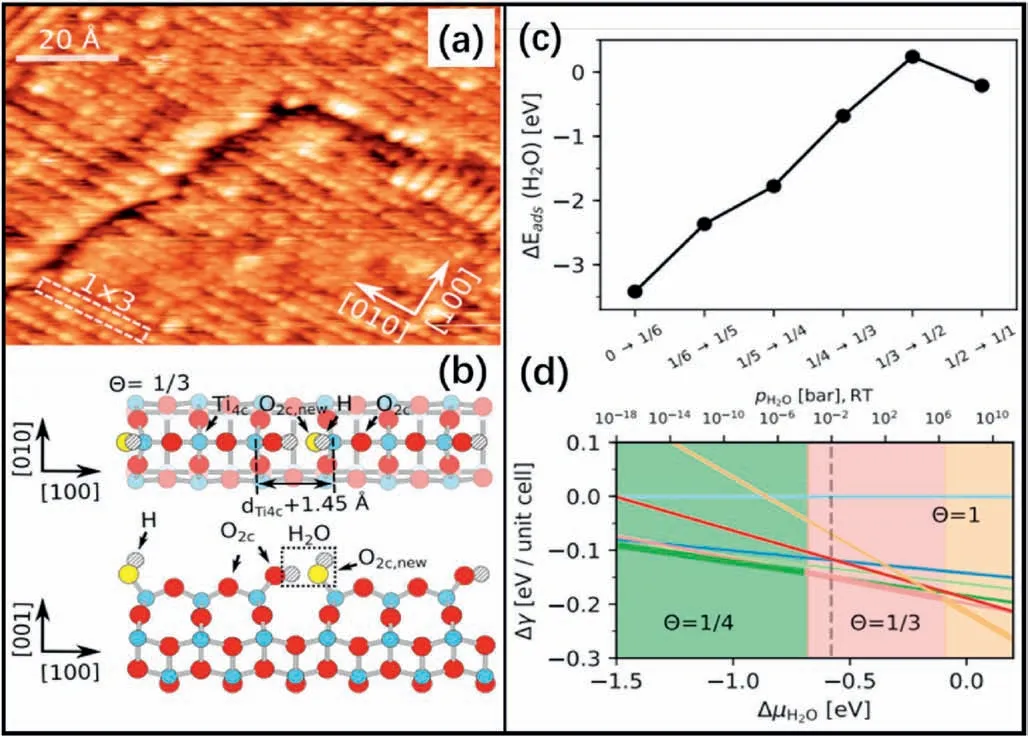
Fig.9.(a) STM image of a surface that was exposed to~1 L of H2O at 120 K,followed by annealing at 230 K for 5 min.(b)Top and side view on the optimized structure of dissociatively adsorbed water on TiO2(1×4)-(001) with a water coverage of Θ=1/3 along the ridge.(c) Differential adsorption energy (ΔEads) for subsequent increases in coverage.(d) Variation in surface free energy with respect to bare TiO2(1×4)-(001)evaluated as a function of the chemical potential of H2O.Reproduced with permission from Ref.88,copyright:2018,American Physical Society.
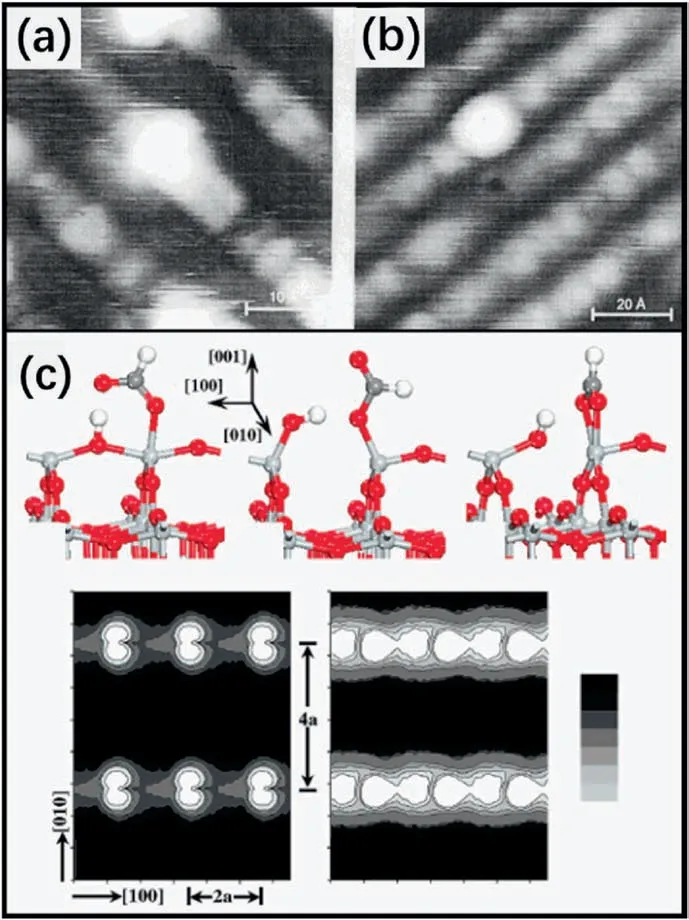
Fig.10.(a)STM and(b)AFM images of submonolayer coverage of HCOOH on TiO2(1×4)-(001) surface.Reproduced with permission from Ref.89,copyright:2002,American Chemical Society.(c) Top:structures of dissociated HCOOH on the ridge of the TiO2(1×4)-(001) surface in different configurations.Bottom:Simulated STM images of two configuration species.Reproduced with permission from Ref.86,copyright:2006,American Chemical Society.
Later,this group further investigated the site-dependent photocatalytic behaviors of CH3OH on the (1×4)-(001) surface under UV irradiation [92].According to their results,the perfect (1×4) lattice sites were active for the photocatalytic reaction of CH3OH.On the reduced surface,the dR-Tisites were photoinactive,while the dO-Tisites showed active properties for photocatalytic reaction of methoxy groups(dissociative adsorbed CH3OH),producing HCHO,which was slightly different from that in the thermally driven reactions (Fig.11e).However,these results disagreed with the conclusions of other work [93],which indicated that the Ti4cbased on the ADM was the photocatalytically active site for CH3OHdissociation.
4.3.Adsorption and incorporation of nonmetal impurities
Based on DFT calculations,Lee et al.[94]investigated the adsorption and incorporation of nitrogen(N)and carbon(C)on the TiO2(001)(1×4) surface based on ADM (Fig.12a and b).The central roles of the surface structure and the local stress in determining the preferential adsorption and incorporation sites of N and C were discussed,as were the favorable incorporation pathways and intermediates.They found that the adsorbed species could migrate to the subsurface oxygen vacancies,forming substitutional impurities,and the nonexposed O site on the surfaces played a crucial role in the incorporation of the N and C impurities.These findings could help to design anion doped TiO2(001)catalysts.
4.4.Surface reaction on TiO2 (1× 4)-(001)
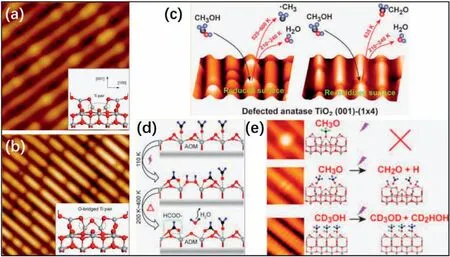
Fig.11.(a)Reduced and(b)reoxidized surfaces of TiO2(1×4)-(001).The insets show the proposed structural models of the two defect structures[90].Schematic diagrams of (c) CH3OH thermal desorption [90],(d) CH2O photodecomposition [91],and (e) CH3OH photocatalytic dissociation reactions [92].Reproduced with permission from Ref.90,91,and 92,copyright:2017,American Chemical Society.
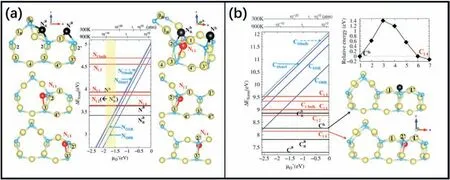
Fig.12.(a)Formation energies(eV)vs.oxygen chemical potential or O2 pressure at fixed temperatures(T=300 and 900 K,top)for N impurities on the TiO2(1×4)-(001)surface.(b)Formation energies(eV)vs.oxygen chemical potential or O2 pressure at fixed temperatures(T=300 and 900 K,top)for C impurities on the TiO2(1×4)-(001) surface.Reproduced with permission from Ref.94,copyright:2013,American Physical Society.
Recently,some exciting advances related to the surface reactions have been reported via the state-of-the-art spherical aberrationcorrected (Cs-corrected)ETEM.Yuan et al.taking the advantage of the unique structure of the TiO2(1×4)-(001) surface,realized the visualization of H2O molecules dissociating and reacting on the active sites viain-situCs-corrected ETEM [81].Imaging surface-reacting gaseous molecules with TEM is a great challenge,which is also significant for heterogeneous catalysis[95-98].During TEM observations,the contrast of the molecules adsorbed on the catalyst surface is too weak to identify.Therefore,to obtain an enhanced contrast,the reacting molecules should be arranged regularly on the catalyst surface along the viewing direction,which requires ordered active sites.Based on their previousin-situTEM studies[27,28],the TiO2(1×4)-(001)surface was obtained with protruding Ti4crows (Fig.13a),which could exactly serve as the active rows.After H2O vapor was introduced at an elevated temperature,two additional small protrusions appeared on the Ti4crows when the water pressure was 1 mbar or more,and they disappeared when the H2O vapor was evacuated.The twin protrusions were attributed to adsorbed water species,namely,hydroxyl species,which was also confirmed byin-situFourier-transform infrared spectroscopy (FTIR) results.The adsorbed model is shown in Fig.13b.
Next,the water vapor environment was converted to a mixed (CO and H2O vapor in a 1:1 ratio;pressure:5 mbar) gas atmosphere under which the water-gas shift reaction could occur.The disappearance and reappearance of the twin protrusions indicated that the hydroxyl species were reacting with CO molecules and thus confirmed that the reaction sites were Ti4csites (Fig.13c).DFT calculations illuminated that the dynamic contrast changes of the twin protrusions were caused by the hydroxyl species consumption by CO gas and supplemented by H2O vapor repeatedly.Thein-situmonitoring of water molecules dissociating and reacting during the catalytic process shows the great potential of ETEM for visualizing gas reactions occurring on the highly ordered active sites.
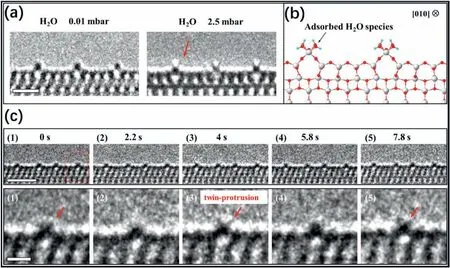
Fig.13.(a) Aberration-corrected in situ environmental TEM(ETEM) images show the same area of the TiO2 (001) surface at 700 °C under oxygen (left,0.01 mbar;right,2.5 mbar)conditions;scale bar,1 nm.(b)Atomic structure of the adsorbed H2O species on the TiO3 rows.(c)Dynamic structural evolution of the(1×4)-(001)surface in the water-gas shift reaction.Top:sequential ETEM images acquired in the mixed gas environment(1:1 ratio of CO and H2O vapor;gas pressure:5 mbar;temperature:700 °C),viewed from the[010]direction;scale bar,2 nm.Bottom:enlarged ETEM images show the dynamic structural evolution of the Ti row outlined by the dotted rectangle in the top image;scale bar,0.5 nm.Reproduced with permission from Ref.81,copyright:2020,American Association for the Advancement of Science.
5.Conclusions and future perspectives
During catalysis research,correlating the catalyst structures and their corresponding properties is a great challenge,especially for surface structures,which play a key role in the catalytic performance.In recent years,with the help of many emerging techniques in surface science,particularlyin-situSTM,in-situXPS,HRTEM,andin-situ(S)TEM,determining the intrinsic structures of the catalyst surfaces under reaction conditions becomes possible.Attributed to the successful synthesis of the high-quality TiO2(001) surface (sizes from nanometers to micrometers),the intrinsic surface structures of TiO2(001) and their surface reconstructions have been extensively studied.Currently,most of the methods for synthesizing high-quality TiO2(001)nanocrystals involve F species,which strongly attach on the surface and inevitably affect the estimates of its performance.As a result,removing surface F contamination has become a common procedure before estimating its performance,which may trigger the TiO2(1×4)-(001)reconstruction.Thus,characterization of the surface atomic structure before the property tests is important to establish its structure-property relationships.However,this is rarely done.
Many critical pieces of evidence have been obtained over nearly two decades of continuous research on the TiO2(1×4)-(001) reconstruction.Several models(e.g.,ARM,MRM,MFM ADM,and AOM)have been proposed.Currently,the most widely accepted model is the ADM suggested by DFT calculations,which is energetically favorable and consistent with the top-view results via SPM as well as recent side-view results via aberration-corrected (S)TEM.The AOM proposed based on STM experiments can also explain the inert properties of the TiO2(1×4)-(001)surface.According to DFT calculations,this structure may exist under O-rich conditions.However,new side-view evidence at the atomic level may help to better confirm this model in the future.
The formation mechanism and stability of the(1×4)surface are also well documented,which may be helpful for designing controllable surface structures.This reconstructed surface has been demonstrated to be able to survive under various conditions,which is important for its application,while further study is necessary to illustrate its stability in practical application environments (e.g.,photocatalysis,lithium ion batteries,and dye-sensitized solar cells).
The surface chemistry of the(1×4)surface has been studied,both in theory and experiment.Ti4cis suggested to be active in dissociative adsorption and degradation for various molecules.DFT calculations and MD simulations have indicated that H2O,HCOOH,and HF can dissociate spontaneously on the ridges of the (1×4) surface,depending on the coverage and temperature,which is supported by experiments.Some experiments on the thermally driven and photocatalytic reaction of methanol and formaldehyde adsorbed on (1×4) surface have been carried out systematically.Moreover,this surface can also serve as a model catalyst,providing well-aligned Ti4cactive rows,above which the dissociating and reacting molecules during the reaction can be captured by the state-of-the-art Cs-corrected ETEM.This provides a new way to monitor a reaction viain-situTEM,which encourages researchers to design and manipulate a reaction for better investigation.Moreover,insituTEM can also be helpful for the further comprehension of the catalysis mechanism,even under practical conditions.
Overall,great progress has been achieved in the development of the(1×4)reconstruction of the TiO2(001)surface,including the structure determination,formation mechanism,stability,and surface chemistry.However,the understanding of the TiO2(1×4)-(001) surface is still insufficient,and thus,further investigations are important.This review may shed light for future exploration and application of this unique structure.
Conflicts of interest
The authors declare no competing financial interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the support of the Zhejiang Provincial Natural Science Foundation of China (LD19B030001),National Natural Science Foundation of China (51801182,51971202 and 51872260)and China Postdoctoral Science Foundation(2018M642407,2019T120502) and the Fundamental Research Funds for the Zhejiang Provincial Universities (2019XZZX003-01).
 Progress in Natural Science:Materials International2021年1期
Progress in Natural Science:Materials International2021年1期
- Progress in Natural Science:Materials International的其它文章
- Effect of Na+ in situ doping on LiFePO4/C cathode material for lithium-ion batteries
- Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors
- Electric transmission behavior of self-assembled Cu-W nano multilayers
- A hybrid hydrogel/textile composite as flame-resistant dress
- Improvement on hydrogen generation properties of Zr(BH4)4·8NH3
- A TEM study on the microstructure of spark plasma sintered ZrB2-based composite with nano-sized SiC dopant
