Brazing diamond grits onto AA7075 aluminium alloy substrate with Ag-Cu-Ti filler alloy by laser heating
Guoqin HUANG,Yinga WANG,Miqin ZHANG,Changai CUIa,b,,Zhn TONG
a Institute of Manufacturing Engineering,Huaqiao University,Xiamen 361021,China
b Fujian Engineering Research Center of Intelligent Manufacturing for Brittle Materials,Xiamen 361021,China
c National & Local Joint Engineering Research Centre for Intelligent Manufacturing Technology of Brittle Material Products,Huaqiao University,Xiamen 361021,China
d School of Mechanical and Automotive Engineering,Xiamen University of Technology,Xiamen 361011,China
e EPSRC Future Metrology Hub,Centre for Precision Technologies,University of Huddersfield,Huddersfield HD1 3DH,UK
KEYWORDS
Abstract The brazing of diamond is a promising way to fabricate grinding wheels for efficient machining and precision grinding.This work investigated the feasibility of bonding diamond grits onto Aluminium Alloy 7075(AA7075)substrate with a Ag-Cu-Ti filler alloy via laser fusion brazing.The interfacial microstructures and the strength of the brazed diamond joints were studied.The cross-section of the brazed diamond joint consists of a molten filler alloy layer,a molten pool,a heat effect zone,a columnar crystal zone and an equiaxed crystal zone.Within the interface of the filler alloy/substrate metal,microstructures observed possibly were Ag(s.s),Al(s.s),TixAl,Al2Cu and Mg intermetallic compounds.A layer of TiC with a thickness of about 30-50 nm was found at the bonding interface of the diamond/filler alloy.The averaged peak shear force of the brazed joints was found to be approximately 39.8 N.The abrasion grinding test indicated that the diamond/AA7075 brazed joint was adequate for grinding.However,the pulled-off of grit was found to be the primary failure of this type of brazed joint.This work broadened the brazing diamond technique and the range of applications of brazed diamond wheels for efficient grinding.©2020 Chinese Society of Aeronautics and Astronautics.Production and hosting by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Recently,the brazing superabrasive(diamond and cubic boron nitride)technique has been considered as one of the most promising approaches to fabricate grinding wheels.1,2Compared with the conventional counterparts bonding of abrasive particles onto substrates by electroplating or sintering,the brazed tool has shown high cutting performance,e.g.especially the lower force and the lower temperature when grinding difficult-to-cut materials such as carbides,3optical glass,4ceramics,5aluminium alloys6and stone.7These favourable cutting performance were ascribed to the metallurgical bonding between the binding agent and the diamond grits,which provides brazed diamond tools with many advantages such as high-bonding strength and large chip-storage space.8Meanwhile,due to its controllable of grain distribution,this brazing technique is also ideal for fabricating grain textured grinding wheel.9-11
Literatures review indicate that most of the brazed wheels reported so far are made by brazing diamond onto a tool’s body made of 1045 steel.12-14Seldom studies have attempt brazing diamond onto aluminium alloy.In fact,the aluminium alloy is a good choice for the construction material of tool’s body,especially for high-speed grinding wheels,owning to its low density.15Generally,the large mass of a tool’s body not only increases the difficulty of installing and disassembling procedures but also dramatically increases the difficulty of tool balancing and the load on the main shaft.16
Among aluminium alloys,AA7075 is a heat-treatable alloy based on the Al-Mg-Zn system and known to have the highest strength-to-weight ratio compared to other aluminium alloys.Thus,it can potentially serve as a body material for the brazed diamond wheel.However,to bond diamond onto such a metal,one should consider the following two factors.The first one is the filler alloy.Generally,diamond is difficult to be wetted,unless with the help of active carbide formation elements,such as Ti and Cr.17The common filler alloys for brazing diamond are mainly Ni-Cr-B-Si,18-20Ni-Cr-P,14Ag-Cu-Ti21and Cu-Sn-Ti.22,23By combining the factor above with the consideration of bonding AA7075,24the Ag-Cu-Ti alloy was chosen in this work.The other consideration is the heating method.Due to the high thermal expansion coefficient of aluminium alloys,using a furnace and induction might cause considerable inner stress on the tool’s basal body,which is harmful to the tool’s accuracy and safety.Hence,the laser heating method was chosen as the heating spot issmalland thus minimises heating effects onthe tool body asprevious studies also have shown that diamonds could be brazed onto a steel substrate by laser heating.25-28
Therefore,in the present paper,a series of demonstration works was carried out to investigate whether diamonds could be brazed onto the aluminium alloy AA7075 with the Ag-Cu-Ti filler alloy and the feasibility of using this bonding technology to develop brazed diamond grinding tools.Laser heating method was employed as the heating source to conduct the brazing process.To be exact,this is a laser fusion brazing,but hereinafter referred to as brazing.Scanning electron microscope(SEM),energy dispersive spectrometer(EDS)and transmission electron microscope(TEM)analyses were conduct to analyse the bonding status of brazed diamond,based on which the interfacial formation mechanism was discussed.A grinding test was also conducted to check the cutting performance of brazed diamond joints.It is expected that this work would contribute to the broadening of the brazing diamond techniques and application ranges of brazed diamond wheels.
2.Experiments
2.1.Laser heating setup
A laser heating device(YLR-500-MM-WC,Shenzhen Unite Winners Laser System Co.,Ltd,China)was employed and is shown in Fig.1(a).It was integrated with a 500 W fibre laser generator(IPG-YLR-500,IPG Photonics Corporation,USA)with a wavelength of 1070 nm.A special brazing chamber integrated with an argon gas protection and a pre-heater module was developed.The whole laser heating brazing system is schematically shown in Fig.1(b).
2.2.Materials and brazing process
Monocrystalline diamond grits(Element Six.Co,USA)were used with the size of 30/35 mesh(420-500μm)and the grade of sawing.A commercial Ag-based alloy(68.8Ag-26.7Cu-4.5 Ti,wt%)with a particle size of 300 US-meshed was used as the filler alloy,whose melting temperature ranged from 830 to 850℃.An AA7075 aluminium alloy(Al-5.79Zn-2.50 Mg-1.58Cu,wt%)in T6 was chosen as the substrate metal,whose melting temperature ranged from 475 to 635℃.
For interfacial microstructure observations,the substrates used were prepared in a dimension of 14 mm×14 mm×5 mm.All substrates were serially cleaned using a 15%NaOH diluted solution for 10 min followed by a HNO3diluted solution for 10 min to remove the oxide films on their surfaces.The filler alloy mixture,which was a blend of the filler alloy and organic glue with a weight ratio of 7:1,was evenly coated on the substrate with a thickness of 250μm.Then,diamonds were planted on the top of the coat in a designed order.Finally,the specimen was placed onto the pre-heater,and the laser brazing process was carried out with gas protection.The pre-heating temperature was set at 350°C during the whole brazing process.The diameter of the laser heating spot was 1.5 mm,and the defocus was set at-12 mm.The laser beam with a heating power of 140 W was CNC-driven with a scanning speed of 20 mm/min to complete the brazing process.
2.3.Characterization of the interface microstructure
A Hirox KH8700 3D digital microscope(Hiroshi,Japan)was employed to observe the monograph of brazed diamond joints.For interfacial observations,a cross-section of the brazed diamond joint was cut from the block specimen by WEDM and polished on a Struers Tegramin-25 grinder.A PhenomTMProX SEM,a Hitachi S4800 field-emission scanning electron microscope,and an FEI Tecnai G2 F20 S-TWIN field-emission TEM with an energy dispersive spectrometer(EDS)were used for microstructure observation and phase analysis.TEM sitespecific specimen in laminate was prepared using an FEI Helios Nanolab-600i dual-beam focused ion beam(FIB)milling machine.Before FIB cutting,a protective coat was applied on the diamond by a Gantan 682 coating machine.

Fig.1 Setup of laser brazing system.
2.4.Grinding performance tests
The bonding strength of the brazed diamond was evaluated by a single-grit shearing test,which is illustrated in Fig.2.The shear-force(Fs)was measured by a piezoelectric dynamometer(Kistler 9265B,Switzerland).The shearing height(SH)was set to 150μm,and the shear speed(Sv)was set to 0.5 mm/s.Twenty brazed diamonds were tested to gain the averaged peak shear force Fs-av.
For grinding performance testing,end grinding wheels were fabricated,and they are shown in Fig.3(a).The grinding test was carried out on a DJ033 vertical machining centre,and its setup is shown in Fig.3(b).A medium hard granite(100 mm×20 mm×500 mm)was used as workpiece.As shown in Fig.3(b),the grinding parameters of the rotation speed(ns)were 2500 r/min;the feed speed(vf)was 600 mm/min;and the pass depth(gd)was 40μm.The coolant used was water.The worn states of all brazed diamonds on the wheel were inspected by the Hirox KH-8700 microscope mentioned above.The inspection intervals were set with series of the accumulative workpiece Material Removal Volume(MRV)reached 0,4,18,58 and 106 cm3.
3.Results and discussion
3.1.Monograph and cross-section of the brazed diamond joint
Fig.4 is the image of a laser-brazed specimen after cleaning of the un-melted filler alloy.Along the line-scanning route of the laser heating spot,the filler alloy was heated and cladded onto the substrate in line.Meanwhile,diamond grits that were preplanted on the laser scanning route were brazed onto the substrate.An optical morphology micrograph of one of the brazed diamond joints is shown in Fig.4.Brazed diamond joint gained looks well bonded by the filler alloy in a hill shape and had a high protrusion,this is quite similarly to the diamonds brazed on the steel substrate by laser heating.27

Fig.2 Illustration of single-grit shearing test.
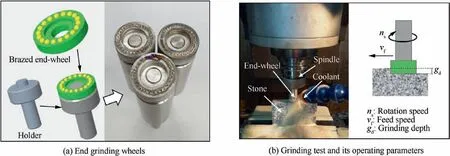
Fig.3 Setup of grinding test.

Fig.4 Optical morphology of brazed diamond joints.
To understand the bonding conditions at the interfaces of diamond,the filler alloy and the substrate metal,a crosssection of the brazed diamond joint was prepared,and its SEM micrograph is provided in Fig.5.It was quite similar to the cross-section of the coat/substrate by laser cladding.29At the diamond/filler alloy interface,it is clear that the diamond was bonded onto the melted filler alloy layer(MFAL).Part of substrate metal melted and was mixed with the molten filler alloy,producing a molten pool(MP)that located under the diamond.Four different microstructures were also identified,such as the substrate metal,the heat-affected zone(HAZ),the narrow columnar crystal zone(CCZ),and the small equiaxed crystal zone(ECZ).These four features are quite similar to the cross sections observed in laser cladding and laser welding.Thus,the whole cross-section of the brazed joint consisted of seven typical features,namely diamond,MFAL,MP,ECZ,CZZ,HAZ and the substrate metal.It was also found that the top surface of the substrate sunk as indicated by the dashed curve in Fig.5.This might be due to the fusion of the substrate metal within the MP.

Fig.5 SEM of the cross-section of a brazed diamond joint(merged by two SEM images).
3.2.Microstructures of the filler alloy/substrate

Fig.6 SEM and EDS observations of brazed joint.
The interfacial microstructures of the filler alloy/substrate resembled those in the laser cladding filler alloy/substrate because the melting temperature of the filler alloy is higher than that of the substrate metal and both of them were fused during heating.A further SEM examination on the filler alloy/substrate interface is shown in Fig.6.Three square zones marked in Fig.6(a)were magnified and shown in Fig.6(b)-(d)respectively.Z1 is located at the top of the MP and is close to diamond.Z2 is located in the middle of the MP.Z3 is located at the bottom of the MP and is close to the HAZ.In addition,the SEM of the substrate metal far from the HAZ is also shown in Fig.6(e).FESEM-EDS mapping results of the top MP are also presented in Fig.7.Twelve selected spots(S1-S12)in the cross-sectional zones shown in Fig.6(b)-(e)and their corresponding EDS spot analysis results are listed in Table 1.The EDS-line measurements of L1,L2 and L3 indicated in Fig.6(a)are presented in Fig.8.
From Table 1,it was found that S1 refers to the diamond phase,whereas S12 is the substrate metal phase.Due to the excellent light transmittance and thermal conductivity of diamond,the effect of diamond on the filler alloy/substrate side can be omitted.27When the laser heated the filler alloy,the filler alloy melted and formed a molten filler alloy layer(MFAL).The MFAL continued to absorb the laser heat energy and transfer it to the substrate metal,increasing the temperature of the adjunct part of the substrate metal.At the centre of the laser heating spot,the temperature was high enough to melt the filler alloy and the substrate metal,producing a melt metal pool(MP).Due to the intense heat flux induced by the laser heating,the alloys in the MP were greatly mixed,causing the molten filler alloy and the molten substrate metal to blend and diffuse into each other.Thus,phases of S2(Ag(s.s)from the filler alloy)and S2(Al(s.s)from the substrate metal)were distributed primarily in the entire MP.However,due to the scanning movement of laser heating,these blending and diffusion processes were still not enough,leaving the compositions from the filler alloy distributed mainly to the top of the MP,whereas those from the substrate metal were distributed predominantly to the lower half of the MP.This result was confirmed by the EDS-line measurements of Ag and Al elements shown in Fig.8.Meanwhile,because of the lower melt point of the substrate material compared to that of the filler alloy,the substrate material was molten more fully and as a result the metal grain size became larger during solidification.In addition,the columnar grains were found at the bottom of the MP,and some equiaxed grains were found in the middle of the lower half of the MP.Generally,the wetting of the solid parent metal by the liquid presents a favourable situation for initiation of columnar growth without having to overcome a significant nucleation energy barrier.Very low levels of undercooling will promptly result in columnar growth,which locates along the bottom boundary of the MP.The direction of the maximum temperature gradient is perpendicular to the molten pool boundary,so the columnar crystals tend to grow perpendicular to the molten pool boundary and toward the inside of the MP.Close to the middle of the MP,the cooling rate and temperature gradient are very small,and the nucleation conditions are almost the same,which results in the equiaxed crystals.30In SEM images of the Z1 and Z2 zones,a dark grey phase(S5)was also distributed within the whole pool.Since the atomic ratio of Al to Cu was found to be 2:1,S5 could be inferred as the eutectic phase of Al2Cu.31,32Based on Fig.7(e)and(f),it could be inferred that Al2Cu was largely distributed in the MP.It is worth mentioning that a needleshaped phase(S4)was found near the top of the MP,shown in the Z1 zone.According to Ref.[33],this phase might be the intermetallic compound TixAl that was formed due to the higher temperature on the top part of molten alloys.The HAZ was easily distinguished from its coarse appearance near the MP.The microstructure outside the HAZ(Fig.6(e)),however,appeared to be a white phase(S10)and distributed in a discontinuous network structure(Fig.6(d)).According to Ref.[34],this grey phase was the segregation of Mg intermetallic compounds(Mg IMCs,possibly Al-Cu-Mg and Zn-Mg)from the substrate metal.In addition,this phase was also found to be a precipitated phase in the MP(S6)in Fig.6.

Fig.7 FESEM-EDS elemental maps distributed on top of MP.

Table 1 FESEM-EDS spot analysis of the 12 spots shown in Fig.6(b)-(e)(at%).

Fig.8 FESEM-EDS-line measurements of L1,L2 and L3.
Based on the above results,the formation of the filler alloy/substrate metal under laser scanning heating can be summarized and illustrated in Fig.9,whereby the microstructural phases with the filler alloy/substrate metal were found to be mainly Ag(s.s),Al(s.s),TixAl,Al2Cu and Mg IMCs.
3.3.Microstructures at the diamond/filler alloy interface
The EDS elemental profile of Ti along line L1 shown in Fig.8 and FESEM-EDS elemental maps Fig.7(c)evidently indicates an enrichment of Ti at the diamond/filler alloy interface.This enrichment reveals that the active Ti in the Ag-Cu-Ti filler alloy was segregated and immigrated to the diamond grit region.According to the reaction Ti+C→TiC,ΔG0(kJ/mol)=-183.1+0.01T,35the Gibbs free energy of forming TiC is approximately-171.3 kJ/mol at 900℃.This negative value suggests that the formation of TiC on the diamond surface was thermodynamically possible.Therefore,the enrichment of Ti might be attributed to the formation of TiC.
To confirm the phase formation at the interface,a TEM lamella including the cross-section of the diamond/filler alloy interface was prepared with a thickness of approximately 37 nm by the FIB technique as shown in Fig.10(a).Meanwhile,Fig.10(b)shows the STEM observation of the microstructure of the interfacial formation region.The STEM-EDX measurements of the four spots indicated in Fig.10(b)are listed in Table 2.By analysing the elemental composition,it can be concluded that the possible phases are as following:P1 is evidently diamond;P2 is dominated by the element of Ag and thus could be filler alloy;P3 is Al2Cu(according to the atom ratio of Al to Cu);and P4 is dominated by the element of Al and could thus be inferred to be the substrate metal.

Fig.9 Illustration of laser heating zone.

Fig.10 TEM observations of cross-section of diamond/filler alloy interface.
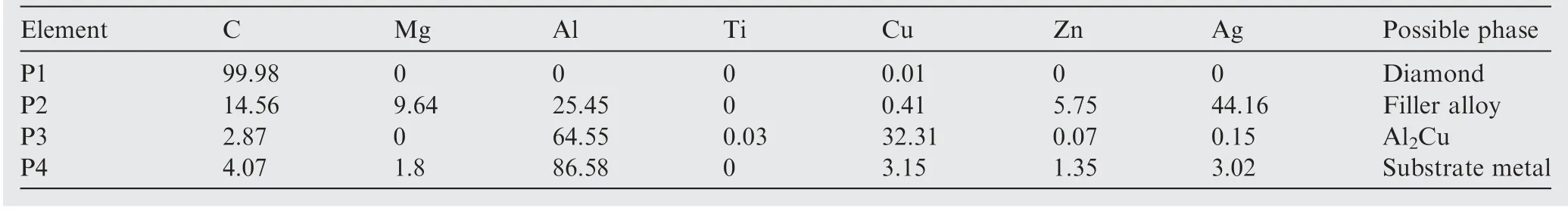
Table 2 STEM-EDX elemental measurements of five spots shown in Fig.10(at%).

Fig.11 TEM micrographs of diamond/filler alloy interfacial region.
A bright-field TEM micrograph of the interfacial region of the diamond/filler alloy is presented in Fig.11(a),while Fig.11(b)and(c)provide the TEM-HAADF(High-angle annular dark-field imaging)micrographs of both two diamond/filler alloy interfaces marked as T1 and T2 in Fig.11(a).From Fig.11(a),it is clear that the light grey layer corresponds to the diamond,whereas the darker grey layer refers to the filler alloy;this result was similar to the TEM observations reported by Refs.[21],[36]and[37].The EDX elemental distribution maps of these two zones are also presented in Figs.12 and 13.Obviously,Ti-rich belts adjacent to the surface of diamond in Figs.12(c)and 13(c)reveal that the interface consisted of Tirich formation layer,as also greatly supported by Ti enrichment exhibited in Figs.7(c)and 8.
Further TEM analyses on T1 zone are presented in Figs.14 and 15.From Fig.14,it can be found that a dark interfacial layer comprising of black grains were located on the bonding surface of the diamond,which is similar to the result reported in Ref.[21].For STEM-EDX elemental measurements,five spots were selected within the black grains.The atomic ratio of Ti to C was close to 1:1,indicating the formation in black grain is TiC particle,whose crystal structure is cubic system.The high-magnification TEM images the diamond grain(HR1 area boxed in Fig.14(b))and the black grain(HR2 area boxed in Fig.14(b))are shown in Fig.15.High resolution TEM image along the zone axis of[1 1 0]acquired from the diamond grit close to the interface is shown in Fig.15(a)and(b)and its corresponding Fast Fourier Transform(FFT)result is shown in Fig.15(c).The groups of lattice planes are denoted as(0 0 2)diamondand(-1 1 1)diamond,which have the interplanar spacing of 0.174 nm and 0.207 nm,respectively.While the high resolution TEM image obtained inside the black grain is shown in Fig.16(a)and(b),and its FFT result is shown Fig.16(c).The interplanar spacings are measured as 0.212 nm and 0.250 nm,thus the lattice planes can be verified as(0 0 2)TiCand(-1 1 1)TiC,respectively.38These results confirm the TiC formation generated at the diamond/filler alloy interface.
It is of great interest to notice that the lattice planes of TiC grain have similar orientations with that of diamond.The dashed line in Fig.15(b)has the same tilting angle with that of the dashed line in Fig.16(b)representing the orientation of the(0 0 2)TiClattice planes.The angle between the dashed line and the solid line in Fig.15(b)is measured as 2°indicating that the misorientation of(0 0 2)TiCand(0 0 2)diamondlattice planes is very small.This are further confirmed by the FFT results as shown in Figs.15(c)and 16(c),the patterns have similar orientation.Considering that the formed TiC and diamond both have the fcc structure,as evidenced by the high resolution TEM images and FFT results in Figs.15 and 16,only the interplanar spacings are different,it is thus rational to conclude that the TiC has a preferable growth orientation on the diamond surface,having its(0 0 2)TiClattice plane nearly parallel to the(0 0 2)diamondlattice plane which is the lattice plane of the diamond grit surface in this case.The epitaxial growth of the TiC grains must be more preferable from the view point of formation energy and this reaction layer may lead to a strong bonding.

Fig.12 STEM-EDX elemental maps distributed on the T1 zone.

Fig.13 STEM-EDX elemental maps distributed on T2 zone.

Fig.14 TEM micrograph of interfacial layer of T1 zone.

Fig.16 High resolution TEM images of HR2 boxed in Fig.14(b).
From above discussion,it can be concluded that C atoms from the diamond and Ti atoms from the filler alloy reacted to form a layer of TiC at the diamond/filler alloy interface during laser heating,as illustrated in Fig.9(c).However,it should be pointed out that the reaction layer in T1 was located between diamond and Ag(s.s),whereas that in T2 was within the diamond and the Al2Cu phase.Based on the width of Ti elemental distribution,the thickness of the reaction layer in zone T1 was approximately 50 nm,whereas that in zone T2 was approximately 30 nm.This difference reveals that the interfacial layer of TiC was first formed at the interface.The Al2Cu phase might be in-situ separated out or moved from another position through the convection of the MP.The existence of Al2Cu adjacent to the diamond not only occupied the space but also blocked the supply of the Ti element for the interfacial TiC formation,resulting in the restricted TiC formation layer.It is also noticed that the interfacial layer’s thickness was only about one-fourth of that obtained by Ref.[36],in which a TiC layer with a thickness of 200 nm was obtained by furnace heating.This difference might cause by the short heating time when scanning with the laser beam.
3.4.Bonding strength of brazed diamonds
Typically,the bonding strength of a brazed diamond is usually determined by the peak shear-force(Fs)obtained in its singlegrain shearing test as shown in Fig.2.It was found through shear test that the averaged Fsof brazed diamonds was approximately 39.8 N(Fig.17(a)).To check whether this bonding strength was sufficient for grinding process,the diamond particles were laser-brazed in an end grinding wheel as shown in Fig.3,and then the wheel was tested in abrasion grinding process.Basing on the classification reported in Ref.[39],during the grinding test the grain wear types were identified as strong(including intact and micro-cracked grains),macro-cracked and abnormal wears(including fractured and pulled-off,which are respectively shown in Fig.18).The corresponding percentage of changes versus the accumulative material removal volume are shown in Fig.17(b).With the increase of MRV,diamond grains gradually got worn and leading to the reduction of the percentage of strong grain bonding on the wheel.From the tendency of the strong grain percentage shown in Fig.17(b),it can be found that the brazed diamond-AA7075 joint sufficiently fulfilled the wear requirement for the grinding wheel to have a high percentage of strong grains(69.6%when MRV reached 200 cm3).In other words,the brazed diamond layer could be maintained at a high percentage level during abrasion grinding process.It well known that the qualified abrasive tools should gradually get worn in application,which means the tools can maintain a high rate of strong grains for long time in working.40Thus it can be inferred that this type of brazed diamond joints can be used for grinding.

Fig.17 Bonding strength assessment of brazed joints.
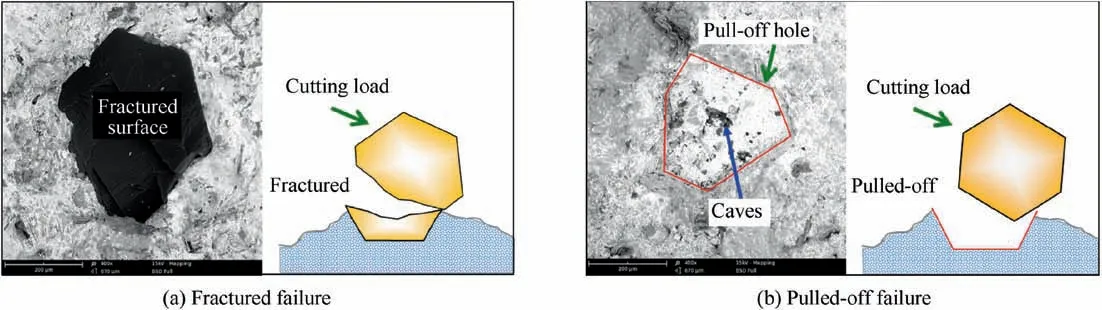
Fig.18 Abnormal wear of grits.
When it comes to the diamond wear,it should be pointed out in previous studies that the major failure in brazed diamonds was due to the fractured mode.13,41By contrast,the failure of the diamonds brazed in this work was mainly due to the pulled-off mode.As shown in Fig.17(b),when the MRV reached 200 cm3,22.3%of the brazed grains were pulled off by the cutting load in grinding,but the fractured was only 0.9%.Generally,the pulled-off brazed diamond is seldom found in the brazed diamond with a Ni-Cr base filler alloy,but it has been reported in the brazed diamond with a lowmelting-point filler alloy,such as Ag-based and Cu-based alloys.42One possible reason might be that the pulled-off failure was greatly ascribed to the low stiffness of the Ag-Cu-Ti filler alloy.43The other consideration is the bonding defects within the bonding interface.As shown in the holes in Fig.18(b),there are lots of small caves inside the pulled-off hole left on the side of filler alloy.
4.Conclusion
The objective of this work was to investigate the feasibility of using laser heating method to braze diamond grits onto the AA7075 substrate metal with the Ag-Cu-Ti filler alloy.The following conclusions can be drawn from the experimental results:
(1)Diamond grits can be brazed onto the AA7075 substrate alloy with high grain protrusion by employing laser heating with the Ag-Cu-Ti filler alloy.
(2)The cross-section of the brazed diamond joint from the diamond to the substrate metal consisted of diamond,a MFAL,a MP,a heat affect zone and a substrate metal.Within the MP and close to the heat affect zone,the columnar crystal and ECZs were found.
(3)Within the filler alloy/substrate metal,the phases of Ag(s.s),Al(s.s),TixAl,Al2Cu and Mg IMCs were found.Within the diamond-filler alloy interface,an interfacial layer of TiC with a thickness of 30-50 nm was formed which served as the reliable bonding media.
(4)The abrasion grinding test confirmed that the diamond-AA7075 brazed joint was qualified for grinding.The pulled-off of grit was found to be the primary failure of this brazed diamond joint type.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors appreciate the financial supports from National Natural Science Foundation of China(Nos.51975221,U1805251 and 51575198).
 CHINESE JOURNAL OF AERONAUTICS2021年6期
CHINESE JOURNAL OF AERONAUTICS2021年6期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Performance evaluation of creep feed grinding ofγ-TiAl intermetallics with electroplated diamond wheels
- Thermomechanical coupling effect on characteristics of oxide film during ultrasonic vibration-assisted ELID grinding ZTA ceramics
- Modeling and experiment of grinding wheel axial profiles based on gear hobs
- Electrochemical machining on blisk channels with a variable feed rate mode
- Framework and development of data-driven physics based model with application in dimensional accuracy prediction in pocket milling
- Electrode design using revolving entity extraction for high-efficiency electric discharge machining of integral shrouded blisk
