乳腺癌脑转移系统治疗的研究进展
张佳玲,张凤春,徐迎春
1.上海交通大学医学院附属仁济医院肿瘤科,上海200127;2.上海交通大学医学院附属苏州九龙医院肿瘤科,苏州215021
乳腺癌是女性最为常见的恶性肿瘤,占所有恶性肿瘤的30%[1]。乳腺癌易发生脑转移,仅次于肺癌,占所有脑转移的15%~20%[2-3]。近年来,乳腺癌脑转移(breast cancer brain metastases,BCBM)的发生率越来越高。发生脑转移的乳腺癌以三阴性乳腺癌(triple negative breast cancer,TNBC)和人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)阳性乳腺癌为主。BCBM发生发展的机制目前还未完全阐明,可能与多种信号分子的改变相关[4-6]。肿瘤细胞与神经元和胶质细胞的相互作用也在脑转移灶的增长中起着关键的作用[7]。
目前,局部治疗仍然是BCBM的主要治疗手段,包括手术、立体定向放射外科治疗(stereotactic radiosurgery,SRS)和 全 脑 放 射 治 疗(whole-brain radiotherapy,WBRT);治疗方式的选择主要由患者预后,是否存在神经系统症状,转移灶的数目、大小、分布情况及既往接受过何种治疗决定[8]。局部治疗对脑转移灶的针对性更强、起效更快,但不良反应也较为严重。系统治疗是指包括化学治疗、内分泌治疗、免疫治疗及靶向治疗在内的针对全身的癌症治疗方法,治疗药物可随血液循环到达身体各处从而杀伤多个部位的肿瘤细胞。BCBM的系统治疗主要取决于乳腺癌的分子亚型,即雌激素受体、孕激素受体和HER2的表达状态。尽管乳腺癌的系统治疗取得了诸多进展,BCBM的系统治疗仍然非常棘手。许多针对乳腺原发灶的药物不能通过血脑屏障(bloodbrain barrier,BBB),某些亲脂性药物易被BBB的外排泵排出,均给BCBM的治疗带来了挑战。血肿瘤屏障(blood-tumor barrier,BTB)的渗透性较BBB更高,但药物仍然不能达到有效治疗浓度。此外,BTB异质性较大,导致肿瘤各个部位的药物浓度不同[9]。如何克服BBB、BTB以及外排泵是目前BCBM治疗中亟待解决的问题。本文主要总结了一些传统和新型系统治疗药物在HER2阳性、激素受体(hormonal receptor,HR)阳性、TNBC 3种BCBM亚型中应用的研究进展,并简述了脑转移的局部治疗以及预后,以期为BCBM的治疗提供参考。
1 HER2 阳性BCBM的系统治疗
HER2上调可增强细胞外环境中生长信号的作用,通过各种下游效应促进细胞存活和增殖。靶向HER2的药物可以阻断这种下游效应,改善HER2阳性乳腺癌患者的预后,但同时中枢神经系统(central nervous system,CNS)转移也日益增多。目前主要的HER2靶向药物包括单克隆抗体(单抗)、抗体药物偶联物(antibody-drug conjugate,ADC)和酪氨酸激酶抑制剂(tyrosine kinase inhibitor,TKI)。大分子单抗可与HER2分子胞外段结合,而小分子TKI可阻断HER2分子胞内段的信号转导发挥抗肿瘤作用;ADC在靶向HER2单抗的基础上再偶联细胞毒药物,从而进一步杀伤肿瘤细胞。有多项临床试验探索了靶向HER2分子的单抗、ADC及TKI药物在HER2阳性BCBM中的疗效。
1.1 单抗
1.1.1曲妥珠单抗研究[10]显示曲妥珠单抗在未接受过放射治疗的BCBM患者的脑脊液与血浆中的浓度比为1∶420,放射治疗或手术可使其比例增加,但效果依然有限。在存在软脑膜转移的情况下,可通过鞘内注射曲妥珠单抗使药物绕过BBB在脑脊液中达到治疗浓度,从而延长患者的生存期[11]。Park等[12]的研究发现曲妥珠单抗显著改善BCBM结局,主要与其能够长期有效地控制颅外病变有关。此外,Kodack等[13]的临床前研究发现,曲妥珠单抗和小分子TKI类药物拉帕替尼联合抗血管药物可以使颅内病灶出现明显坏死,并显著延缓颅内病灶的进展。Ⅰ期NCT00543504试验[14]进一步证实了该疗法的有效性,发现在10例BCBM患者中,6例无进展生存期(progression-free survival,PFS)超过6个月,其中1例PFS超过12个月,且未发生脑转移相关的不良反应。
1.1.2帕妥珠单抗帕妥珠单抗联合曲妥珠单抗和紫杉类药物是目前HER2阳性晚期乳腺癌的一线标准治疗方案。Ⅲ期CLEOPATRA试验[15]显示,与仅使用曲妥珠单抗联合多西他赛相比,曲妥珠单抗和帕妥珠单抗联合多西他赛使得转移性乳腺癌患者的中位PFS和总生存期(overall survival,OS)均显著延长。Swain等[16]发现,相比于仅使用曲妥珠单抗,曲妥珠单抗联合帕妥珠单抗组发生脑转移的时间显著延长(15.0个月vs 11.9个月,P=0.005),且发生脑转移患者的OS也有延长趋势(34.4个月vs 26.3个月,P=0.114)。鉴于曲妥珠单抗和帕妥珠单抗均能为BCBM患者带来生存获益,有必要进一步研究大分子单抗对BCBM的作用。
1.2 ADC
1.2.1 T-DM1 T-DM1由曲妥珠单抗和微管抑制剂DM1组成,是目前HER2阳性晚期乳腺癌的二线标准治疗方案。Ⅲ期EMILIA试验[17]纳入了95例接受过治疗、无症状的BCBM患者,其中45例接受T-DM1治疗;回顾性分析发现与拉帕替尼联合卡培他滨相比,T-DM1显著延长了BCBM患者的OS(26.8个月vs 12.9个月,P=0.008)。Ⅲb期KAMILLA试验[18]的亚组分析显示,在基线脑转移灶稳定的126例患者中,84例在T-DM1治疗期间脑转移灶缩小,表明T-DM1治疗BCBM有效。KATE2试验[19]显示,T-DM1联合阿特珠单抗为程序性死亡配体1(programmed death-ligand 1,PD-L1)阳性的转移性乳腺癌患者带来生存获益,但该研究没有纳入BCBM患者,因此该疗法在BCBM中的作用还需进一步研究。此外,T-DM1联合放射治疗、高能聚焦超声治疗、马西替坦和图卡替尼等都在BCBM中取得了较好的效果[20]。然而,Stumpf等[21]发现T-DM1联合SRS可能会增加放射性坏死的发生率,但鉴于该研究患者例数较少,需要更大规模的前瞻性研究评估T-DM1联合SRS的安全性以及最大耐受剂量和给药时间。
1.2.2 DS-8201 DS-8201由曲妥珠单抗和拓扑异构酶Ⅰ抑制剂deruxtecan组成。Ⅱ期DESTINY-Breast01试验[22]探索了其作为后线治疗的有效性。对既往接受过T-DM1的184例转移性乳腺癌患者使用DS-8201治疗,中位PFS达16.4个月,独立评审委员会评估的客观应答率(objective response rate,ORR)为60.9%;其中24例既往接受过治疗、无脑转移相关症状的BCBM患者,中位PFS达18.1个月。值得注意的是,DS-8201治疗后间质性肺病的发生率较高,在治疗过程中需注意监测肺部症状。
1.2.3 SYD985 SYD985由曲妥珠单抗和烷化剂duocarmycin组成,已获得美国食品药品监督管理局(FDA)快速通道认定。Ⅰ期NCT02277717试验[23]剂量扩展队列共纳入146例转移性乳腺癌患者,其中8例为BCBM,结果显示SYD985安全有效。然而目前还没有SYD985治疗脑转移的相关数据发表,因此SYD985在BCBM中的作用还需进一步研究。
1.3 小分子TKI类药物
1.3.1拉帕替尼拉帕替尼是表皮生长因子受体(epidermal growth factor receptor,EGFR)和HER2双靶点的小分子TKI。Ⅱ期LANDSCAPE试验[24]显示拉帕替尼联合卡培他滨对既往未接受过放射治疗的BCBM有效,研究主要终点CNS的ORR为65.9%;对低转移负荷、无症状或无生命危险的HER2阳性BCBM患者使用拉帕替尼联合卡培他滨治疗,可以获得8.3个月的中位PFS,后续再进行放射治疗,可获得长达17个月的OS。2018年美国临床肿瘤学会年会(American Society of Clinical Oncology,ASCO)上发布HER2阳性BCBM治疗指南指出,对于未接受过放射治疗的无临床症状、负荷小的脑转移瘤,尽管放射治疗仍是标准选择,在放射治疗前进行拉帕替尼和卡培他滨治疗也是可选方案[8]。
1.3.2来那替尼来那替尼能够不可逆地抑制HER1、HER2和HER4的活性。NEfERT-T试验[25]比较了来那替尼联合紫杉醇与曲妥珠单抗联合紫杉醇作为转移性乳腺癌一线治疗方案的效果,结果发现两者疗效相近,但来那替尼联合紫杉醇能延缓和减少脑转移的进展。Ⅱ期TBCRC022试验[26]进一步证明了来那替尼在HER2阳性BCBM中的疗效:入组时,超过90%的患者有CNS进展,在未接受过和接受过拉帕替尼治疗的患者中,CNS的ORR分别为49%和33%,中位PFS分别为5.5个月和3.1个月。Ⅲ期NALA研究[27]发现,相较于拉帕替尼,来那替尼联合卡培他滨显著延长了转移性乳腺癌患者的PFS,且需要干预脑转移症状的患者减少。
1.3.3图卡替尼图卡替尼是一种对HER2具有高度选择性的可逆TKI,在临床前和临床研究中均显示出了很好的颅内活性。Ⅱ期HER2CLIMB试验[28]显示与安慰剂联合曲妥珠单抗和卡培他滨相比,图卡替尼联合曲妥珠单抗和卡培他滨可显著降低疾病进展和死亡风险。在291例BCBM患者中,图卡替尼组与安慰剂组1年PFS分别为24.9%和0,中位PFS分别为7.6个月和5.4个月。基于HER2CLIMB试验的结果,图卡替尼联合曲妥珠单抗和卡培他滨获得了FDA优先审批,用于治疗既往接受过3种及以上HER2靶向治疗后进展的局部晚期不可切除或转移性HER2阳性乳腺癌患者,包括BCBM。此外,一项Ⅰb期试验[29]发现图卡替尼联合T-DM1同样对BCBM有效,且不良反应较轻。
1.3.4吡咯替尼吡咯替尼是我国自主研发的广谱抗HER1、HER2和HER4胞内段小分子TKI。吡咯替尼联合卡培他滨对比安慰剂联合卡培他滨治疗HER2阳性转移性乳腺癌的Ⅲ期临床试验[30]数据显示,吡咯替尼组较安慰剂组PFS显著延长(11.1个月vs 4.1个月);对于基线无脑转移患者,吡咯替尼组较安慰剂组出现新发脑转移的比例更低(1.2% vs 3.6%),且至新发脑转移出现的时间更长(397.5 d vs 132.0 d);对于基线存在未治疗的脑转移患者,吡咯替尼组脑转移进展的患者比例更低(73.3% vs 87.5%),至脑转移进展的时间更长(168.0 d vs 127.0 d)。表1列出了本文涉及的小分子靶向药物及相关的临床研究。
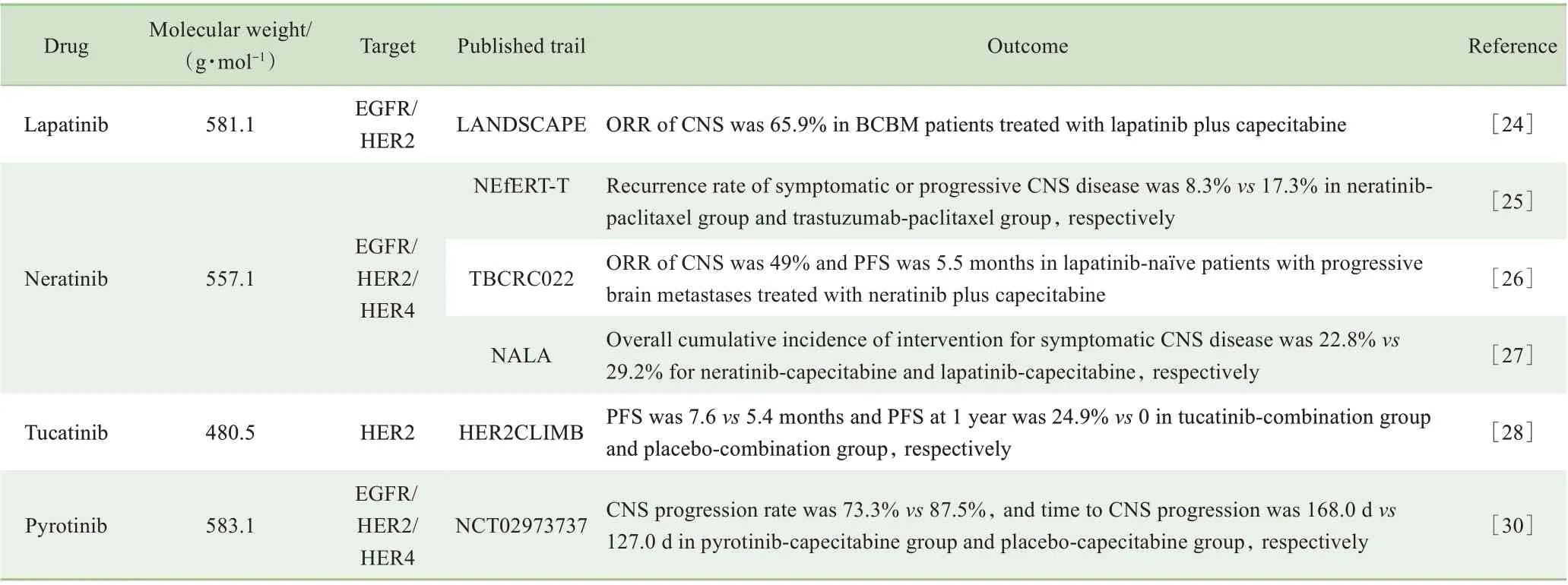
表1 BCBM小分子靶向药物及相关临床研究Tab 1 Small-molecule targeted drugs used in BCBM and relevant clinical trails

Continued Tab
2 HR阳性BCBM的系统治疗
内分泌治疗是HR阳性乳腺癌的重要治疗手段,但其在脑转移中的作用尚不清楚。近年来,细胞周期蛋白依赖性激酶4/6(cyclin-dependent kinase 4/6,CDK4/6)抑制剂的应用使HR阳性乳腺癌的治疗发生了巨大的转变。磷脂酰肌醇3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白(phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin,PI3K/Akt/mTOR)通路抑制剂也给BCBM患者带来了更多的选择。
2.1 内分泌治疗
内分泌治疗在BCBM中的作用尚不明确。尽管他莫昔芬亲脂性较强,易通过BBB,但脑转移发生时肿瘤可能已经对内分泌治疗抵抗,而且约40%的脑转移灶中HR的表达状态与原发灶不一致,因此内分泌治疗对BCBM是否有效还需进一步研究[33]。
2.2 CDK4/6抑制剂
约50%的HR阳性乳腺癌患者在内分泌治疗过程中会出现耐药,通过抑制CDK4/6阻断下游信号通路是一种应对耐药的治疗策略。在现有的CDK4/6抑制剂中,玻玛西林对BBB的渗透能力较强[2]。Ⅱ期NCT02308020试验[31]纳入既往接受过多线治疗的BCBM患者,结果显示经玻玛西林单药治疗后CNS的ORR为6%,临床获益率为25%,中位PFS为4.4个月。研究[34]表明,使用CDK4/6抑制剂治疗后,视网膜母细胞瘤基因1失去功能的突变显著增多,PI3K/Akt、细胞周期和Hippo信号通路的改变也较治疗前增多,这些基因组的改变可能与CDK4/6抑制剂耐药相关。了解CDK4/6抑制剂治疗耐药的机制有助于更有针对性地研发和使用药物,应对CDK4/6抑制剂耐药。
2.3 PI3K/Akt/mTOR抑制剂
HR阳性乳腺癌的复发常常与PI3K/Akt/mTOR通路的激活相关。PI3K/Akt/mTOR抑制剂在转移性乳腺癌中的疗效可观,但其治疗BCBM的临床数据较少。PI3K/mTOR抑制剂GDC-0084、Akt抑制剂GDC-0068都在乳腺癌细胞株和BCBM小鼠模型展示出了较好的抗肿瘤活性,其能够通过BBB,降低肿瘤细胞的存活率,诱导凋亡并抑制小鼠BCBM的生长[35-36]。
3 三阴性BCBM的系统治疗
TNBC侵袭性高,多见于年轻女性。由于缺乏可作用的靶点,TNBC治疗手段有限,预后较差。尽管化学治疗仍然是治疗TNBC的主要方法,抗血管生成药物、新型制剂和免疫检查点抑制剂已经显示出了广阔的发展前景。
3.1 细胞毒药物
以顺铂为基础的多种化学治疗方案已经在临床试验中得到了研究。其中,顺铂联合依托泊苷、环磷酰胺、长春瑞滨和吉西他滨对BCBM有效[37]。此外,一项Ⅱ期临床试验[38]表明,伊立替康联合替莫唑胺治疗脑转移或软脑膜转移进展的患者有效。一些病例报告[39-44]报道了艾日布林单药或联合WBRT对BCBM治疗效果显著,但尚需大样本临床试验证实。
3.2 抗血管生成药物
阿帕替尼和贝伐单抗等抗血管生成药物在多种亚型BCBM中都展示出了很好的疗效。Hu等[45]报道了1例既往接受过五线治疗的TNBC患者,在接受阿帕替尼联合伊立替康和S-1的六线治疗后,颅内病灶部分缓解。贝伐单抗联合卡铂以及贝伐单抗联合依托泊苷和顺铂也都在Ⅱ期临床试验[46-47]中表现出了较强的抗肿瘤活性,CNS的ORR分别为63%和77%。
3.3 新型制剂
3.3.1 PARP抑制剂聚腺苷二磷酸核糖聚合酶(poly-ADP-ribose polymerase,PARP)抑制剂能阻碍DNA单链损伤的修复,在乳腺癌易感基因1/2(breast cancer susceptibility gene 1/2,BRCA1/2)突变的肿瘤中疗效显著。PARP抑制剂奥拉帕利的临床研究OlympiAD未入组脑转移的患者。而EMBRACA研究[32]仅包含63例BCBM患者,结果显示与医师选择的治疗(卡培他滨、艾日布林、吉西他滨或长春瑞滨单药治疗)相比,PARP抑制剂他拉唑帕尼组中位PFS显著延长(8.6个月vs 5.6个月,HR=0.54),ORR提高(62.6% vs 27.2%)。此外,他拉唑帕尼治疗所获得的PFS受益在预先指定的各个亚组中均表现一致,包括有脑转移病史的患者。
3.3.2 ANG1005 ANG1005由3个紫杉醇分子和脑靶向肽angiopep-2连接而成,通过识别低密度脂蛋白受体相关蛋白1诱导转胞吞作用穿过BBB。一项Ⅱ期研究[48]纳入72例复发性BCBM患者,结果表明ANG1005能够有效地控制颅内外病灶,改善脑转移症状,延长生存期,尤其对软脑膜转移的患者疗效显著。
3.3.3 Etirinotecan pegol Etirinotecan pegol(EP)为聚乙二醇修饰的伊立替康剂型,是一种长效拓扑异构酶Ⅰ抑制剂。Ⅲ期BEACON试验[49]评估了EP治疗转移性乳腺癌的效果,发现EP组中位OS较医师选择的治疗(艾日布林、伊沙匹隆、吉西他滨、长春瑞滨或紫杉醇)延长,差异无统计学意义(12.4个月vs 10.3个月,P=0.084),但在脑转移亚组中,EP组中位OS显著延长(10.0个月vs 4.8个月,P<0.01)[50]。
3.4 免疫检查点抑制剂
免疫检查点抑制剂在非小细胞肺癌和黑色素瘤脑转移中都取得了较好的疗效[51-52]。在使用PD-L1/程序性死亡受体1(programmed death 1,PD-1)抗体治疗转移性TNBC的IMpassion130Ⅲ期 临 床 试 验[53]中,共 入 组902名患者,其中61例有脑转移,随机给予阿特珠单抗/安慰剂联合白蛋白结合紫杉醇;结果显示与安慰剂组相比,阿特珠单抗组PD-L1阳性患者的OS延长了7个月(25.0个月vs 18.0个月),但PD-L1阴性的患者几乎没有生存获益(19.7个月vs 19.6个月)。阿特珠单抗没有让BCBM患者获益,尚须前瞻性大样本研究证实。此外,一项Ⅱ期试验[54]发现卡瑞丽珠单抗联合阿帕替尼也在晚期TNBC中取得了较好的疗效。
4 BCBM的局部治疗
目前局部治疗仍然是BCBM治疗的基石。如何将局部治疗与系统治疗进行有机整合仍是一个亟待解决的问题。局部治疗可以破坏BBB,从而增加药物在脑组织中的浓度,更好地控制颅内病变。Niwińska等[55-56]的研究表明,在局部治疗后应用系统治疗可显著延长BCBM生存期。2018年ASCO发布的HER2阳性BCBM治疗指南也建议,对于局部治疗后进展的BCBM,可选择易于透过BBB的药物进行系统治疗[8]。此外,同时应用HER2靶向治疗和SRS也能够延缓局部复发,但应关注可能出现的严重不良反应[57]。对于颅外病变快速进展的患者,应首先进行系统治疗,待颅外病灶稳定后再考虑颅内病灶的局部治疗;而对于脑转移症状严重的患者,可先行局部治疗降低颅内压,缓解症状后再用系统治疗维持。对于治疗过程中颅外病变稳定、颅内病变进展的患者,可考虑保留系统性治疗方案不变,加强局部治疗干预。总之,BCBM治疗方法的选择和治疗顺序的确定应个体化,将脑转移灶及颅外病灶的特征、患者身体和经济状况以及既往接受过何种治疗等因素纳入考量。
5 BCBM的预后
BCBM患者的预后与多种因素有关。准确的预后评估能够指导局部治疗和系统治疗的布局,并可作为前瞻性临床试验筛选入组患者的工具。Breast-GPA(breastgraded prognostic assessment)是目前应用较广泛的BCBM预后评分系统,包含卡诺夫斯凯计分(Kanofsky performance score,KPS)、分子分型和年龄3个因素[58];评分越低,预后越差。Subbiah等[59]对breast-GPA进行了改良,纳入了脑转移灶数目这一影响因素,使预后评估更加准确。Griguolo等[60]纳入668例患者的回顾性分析也证明了脑转移灶数目对预后的预测价值。总体而言,体力状况评分和分子分型是影响治疗选择和预后的重要因素。在多学科合作确定合理个体化治疗的框架下,应对不同分子分型BCBM开展前瞻性临床研究,以便为脑转移患者确立合理的治疗方案,延长生存期。
6 小结
随着转移性乳腺癌诊断水平的不断提升,BCBM的发生越来越常见。在转移性乳腺癌系统治疗不断改善的同时,脑转移灶的治疗也需要不断跟进。应根据脑转移灶的解剖学特征、分子分型及预后等因素,为BCBM患者制定合适的局部治疗和系统治疗方案。化学治疗、内分泌治疗等传统治疗对BCBM具有一定的效果,一些新型药物例如ADC、抗HER2小分子TKI、CDK4/6抑制剂、PARP抑制剂和免疫检查点抑制剂等也会为BCBM患者带来更大的生存获益。目前,关于BCBM的多个临床试验正在进行中,将不断为BCBM患者带来新的希望。
参·考·文·献
[1]Siegel RL,Miller KD,Jemal A.Cancer statistics,2020[J].CA Cancer J Clin,2020,70(1):7-30.
[2]Fecci PE,Champion CD,Hoj J,et al.The evolving modern management of brain metastasis[J].Clin Cancer Res,2019,25(22):6570-6580.
[3]Waks AG,Winer EP.Breast cancer treatment:a review[J].JAMA,2019,321(3):288-300.
[4]Xing F,Liu Y,Sharma S,et al.Activation of the c-met pathway mobilizes an inflammatory network in the brain microenvironment to promote brain metastasis of breast cancer[J].Cancer Res,2016,76(17):4970-4980.
[5]Sirkisoon SR,Carpenter RL,Rimkus T,et al.TGLI1 transcription factor mediates breast cancer brain metastasis via activating metastasis-initiating cancer stem cells and astrocytes in the tumor microenvironment[J].Oncogene,2020,39(1):64-78.
[6]Choy C,Ansari KI,Neman J,et al.Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases[J].Breast Cancer Res,2017,19(1):51.
[7]Witzel I,Oliveira-Ferrer L,Pantel K,et al.Breast cancer brain metastases:biology and new clinical perspectives[J].Breast Cancer Res,2016,18(1):8.
[8]Ramakrishna N,Temin S,Chandarlapaty S,et al.Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases:ASCO clinical practice guideline update[J].J Clin Oncol,2018,36(27):2804-2807.
[9]Osswald M,Blaes J,Liao YX,et al.Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases[J].Clin Cancer Res,2016,22(24):6078-6087.
[10]Stemmler HJ,Schmitt M,Willems A,et al.Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier[J].Anticancer Drugs,2007,18(1):23-28.
[11]Bonneau C,Paintaud G,Trédan O,et al.PhaseⅠfeasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis[J].Eur J Cancer,2018,95:75-84.
[12]Park YH,Park MJ,Ji SH,et al.Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients[J].Br J Cancer,2009,100(6):894-900.
[13]Kodack DP,Chung E,Yamashita H,et al.Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases[J].Proc Natl Acad Sci USA,2012,109(45):E3119-E3127.
[14]Falchook GS,Moulder SL,Wheler JJ,et al.Dual HER2 inhibition in combination with anti-VEGF treatment is active in heavily pretreated HER2-positive breast cancer[J].Ann Oncol,2013,24(12):3004-3011.
[15]Swain SM,Miles D,Kim SB,et al.Pertuzumab,trastuzumab,and docetaxel for HER2-positive metastatic breast cancer(CLEOPATRA):end-of-study results from a double-blind,randomised,placebo-controlled,phase 3 study[J].Lancet Oncol,2020,21(4):519-530.
[16]Swain SM,Baselga J,Miles D,et al.Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab,trastuzumab,and docetaxel:results from the randomized phaseⅢstudy CLEOPATRA[J].Ann Oncol,2014,25(6):1116-1121.
[17]Krop IE,Lin NU,Blackwell K,et al.Trastuzumab emtansine(T-DM1)versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases:a retrospective,exploratory analysis in EMILIA[J].Ann Oncol,2015,26(1):113-119.
[18]Montemurro F,Ellis P,Delaloge S,et al.Safety and efficacy of trastuzumab emtansine(T-DM1)in 399 patients with central nervous system metastases:Exploratory subgroup analysis from the KAMILLA study[C]//Proceedings of the 2016 San Antonio Breast Cancer Symposium,December 6-10,2016.San Antonio,TX.Philadelphia:AACR.Cancer Res,2017,77(4 Suppl):P1-12-10.
[19]Emens LA,Esteva FJ,Beresford M,et al.Overall survival(OS)in KATE2,a phaseⅡstudy of programmed death ligand 1(PD-L1)inhibitor atezolizumab(atezo)+trastuzumab emtansine(T-DM1)vs placebo(pbo)+T-DM1 in previously treated HER2+advanced breast cancer(BC)[J].Ann Oncol,2019,30:v104.
[20]Dong RR,Ji JL,Liu H,et al.The evolving role of trastuzumab emtansine(T-DM1)in HER2-positive breast cancer with brain metastases[J].Crit Rev Oncol Hematol,2019,143:20-26.
[21]Stumpf PK,Cittelly DM,Robin TP,et al.Combination of trastuzumab emtansine and stereotactic radiosurgery results in high rates of clinically significant radionecrosis and dysregulation of aquaporin-4[J].Clin Cancer Res,2019,25(13):3946-3953.
[22]Modi SN,Saura C,Yamashita T,et al.Trastuzumab deruxtecan in previously treated HER2-positive breast cancer[J].N Engl J Med,2020,382(7):610-621.
[23]Banerji U,van Herpen CML,Saura C,et al.Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer:a phase 1 dose-escalation and dose-expansion study[J].Lancet Oncol,2019,20(8):1124-1135.
[24]Bachelot T,Romieu G,Campone M,et al.Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer(LANDSCAPE):a single-group phase 2 study[J].Lancet Oncol,2013,14(1):64-71.
[25]Awada A,Colomer R,Inoue K,et al.Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer:the NEfERT-T randomized clinical trial[J].JAMA Oncol,2016,2(12):1557-1564.
[26]Freedman RA,Gelman RS,Anders CK,et al.TBCRC 022:a phaseⅡtrial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases[J].J Clin Oncol,2019,37(13):1081-1089.
[27]Saura C,Oliveira M,Feng YH,et al.Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with≥2 HER2-directed regimens:phaseⅢNALA trial[J].J Clin Oncol,2020,38(27):3138-3149.
[28]Murthy RK,Loi S,Okines A,et al.Tucatinib,trastuzumab,and capecitabine for HER2-positive metastatic breast cancer[J].N Engl J Med,2020,382(7):597-609.
[29]Borges VF,Ferrario C,Aucoin N,et al.Tucatinib combined with adotrastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer:a phase 1b clinical trial[J].JAMA Oncol,2018,4(9):1214-1220.
[30]Jiang ZF,Yan M,Hu XC,et al.Pyrotinib combined with capecitabine in women with HER2+metastatic breast cancer previously treated with trastuzumab and taxanes:a randomized phaseⅢstudy[J].J Clin Oncol,2019,37(15_suppl):1001.
[31]Anders CK,Le Rhun E,Bachelot TD,et al.A phaseⅡstudy of abemaciclib in patients(pts)with brain metastases(BM)secondary to HR+,HER2-metastatic breast cancer(MBC)[J].J Clin Oncol,2019,37(15_suppl):1017.
[32]Litton JK,Rugo HS,Ettl J,et al.Talazoparib in patients with advanced breast cancer and a germline BRCA mutation[J].N Engl J Med,2018,379(8):753-763.
[33]Jung J,Lee SH,Park M,et al.Discordances in ER,PR,and HER2 between primary breast cancer and brain metastasis[J].J Neurooncol,2018,137(2):295-302.
[34]Razavi P,dos Anjos CH,Brown DN,et al.Molecular profiling of ER+metastatic breast cancers to reveal association of genomic alterations with acquired resistance to CDK4/6 inhibitors[J].J Clin Oncol,2019,37(15_suppl):1009.
[35]Ippen FM,Alvarez-Breckenridge CA,Kuter BM,et al.The dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CAmutant breast cancer brain metastases[J].Clin Cancer Res,2019,25(11):3374-3383.
[36]Ippen FM,Grosch JK,Subramanian M,et al.Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases[J].Neuro Oncol,2019,21(11):1401-1411.
[37]Shah N,Mohammad AS,Saralkar P,et al.Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases[J].Pharmacol Res,2018,132:47-68.
[38]Melisko ME,Assefa M,Hwang J,et al.PhaseⅡstudy of irinotecan and temozolomide in breast cancer patients with progressing central nervous system disease[J].Breast Cancer Res Treat,2019,177(2):401-408.
[39]Jung SU,Jeon CW,Choi JH.Long-term survival with eribulin monotherapy after whole brain radiation therapy in a patient with brain metastasis from breast cancer[J].Asian J Surg,2020,43(10):1008-1009.
[40]Byun KD,Ahn SG,Baik HJ,et al.Eribulin mesylate combined with local treatment for brain metastasis from breast cancer:two case reports[J].J Breast Cancer,2016,19(2):214-217.
[41]Matsuoka H,Tsurutani J,Tanizaki J,et al.Regression of brain metastases from breast cancer with eribulin:a case report[J].BMC Res Notes,2013,6:541.
[42]Nieder C,Aandahl G,Dalhaug A.A case of brain metastases from breast cancer treated with whole-brain radiotherapy and eribulin mesylate[J].Case Rep Oncol Med,2012,2012:537183.
[43]Chang AY,Ying XX.Brain metastases from breast cancer and response to treatment with eribulin:a case series[J].Breast Cancer(Auckl),2015,9:19-24.
[44]Catania G,Malaguti P,Gasparro S,et al.Activity of eribulin mesylate in brain metastasis from breast cancer:a stone in a pond?[J].Oncology,2018,94(Suppl 1):29-33.
[45]Hu T,Liu CW,Li QH,et al.Apatinib+CPT-11+S-1 for treatment of refractory brain metastases in patient with triple-negative breast cancer:case report and literature review[J].Medicine(Baltimore),2018,97(15):e0349.
[46]Lin NU,Gelman RS,Younger WJ,et al.PhaseⅡtrial of carboplatin(C)and bevacizumab(BEV)in patients(pts)with breast cancer brain metastases(BCBM)[J].J Clin Oncol,2013,31(15_suppl):513.
[47]Lu YS,Chen TW,Lin CH,et al.Bevacizumab preconditioning followed by etoposide and cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy[J].Clin Cancer Res,2015,21(8):1851-1858.
[48]Kumthekar P,Tang SC,Brenner AJ,et al.ANG1005,a brain-penetrating peptide-drug conjugate,shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases[J].Clin Cancer Res,2020,26(12):2789-2799.
[49]Perez EA,Awada A,O'Shaughnessy J,et al.Etirinotecan pegol(NKTR-102)versus treatment of physician′s choice in women with advanced breast cancer previously treated with an anthracycline,a taxane,and capecitabine(BEACON):a randomised,open-label,multicentre,phase 3 trial[J].Lancet Oncol,2015,16(15):1556-1568.
[50]Cortés J,Rugo HS,Awada A,et al.Prolonged survival in patients with breast cancer and a history of brain metastases:results of a preplanned subgroup analysis from the randomized phaseⅢBEACON trial[J].Breast Cancer Res Treat,2017,165(2):329-341.
[51]Goldberg SB,Schalper KA,Gettinger SN,et al.Pembrolizumab for management of patients with NSCLC and brain metastases:long-term results and biomarker analysis from a non-randomised,open-label,phase 2 trial[J].Lancet Oncol,2020,21(5):655-663.
[52]Kluger HM,Chiang V,Mahajan A,et al.Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phaseⅡtrial[J].J Clin Oncol,2019,37(1):52-60.
[53]Schmid P,Rugo HS,Adams S,et al.Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable,locally advanced or metastatic triplenegative breast cancer(IMpassion130):updated efficacy results from a randomised,double-blind,placebo-controlled,phase 3 trial[J].Lancet Oncol,2020,21(1):44-59.
[54]Liu JQ,Jiang ZF,Li Q,et al.Efficacy and safety of anti-PD-1 antibody SHR-1210 combined with apatinib in patients with advanced triple-negative breast cancer[J].J Clin Oncol,2019,37(15_suppl):1066.
[55]Niwińska A,Murawska M,Pogoda K.Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases[J].Cancer,2010,116(18):4238-4247.
[56]Niwińska A.Brain metastases as site of first and isolated recurrence of breast cancer:the role of systemic therapy after local treatment[J].Clin Exp Metastasis,2016,33(7):677-685.
[57]Miller JA,Kotecha R,Ahluwalia MS,et al.Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies[J].Cancer,2017,123(12):2283-2293.
[58]Sperduto PW,Kased N,Roberge D,et al.Summary report on the graded prognostic assessment:an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases[J].J Clin Oncol,2012,30(4):419-425.
[59]Subbiah IM,Lei XD,Weinberg JS,et al.Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases[J].J Clin Oncol,2015,33(20):2239-2245.
[60]Griguolo G,Jacot W,Kantelhardt E,et al.External validation of Modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases:a multicentric European experience[J].Breast,2018,37:36-41.

