Differential degeneration of rod/cone bipolar cells during retinal degeneration in Royal College of Surgeons rats
Yan-Ming Huang, Mei Yang, Rong-Di Yuan
Department of Ophthalmology, Xinqiao Hospital of Army Medical University, Chongqing 400038, China
Dear Editor,
Royal college of surgeons (RCS) rat is an inherited retinal degeneration rat caused by mutation of Mertk in the retinal pigment epithelial (RPE) cells[1]. In this dystrophic rat, the dysfunction of RPEs leads to the progressive death of photoreceptors, with rods initially affected. Many studies have attempt to transplant various stem cells, photoreceptors and retinal explants to reconstruct the structure and function of the dystrophic retina[2]. However, the ability of deafferented bipolar cells to establish functional synapses with photoreceptors is poorly understood. In the present study, we investigated the degeneration of rod bipolar cells (RBs) and type II, type VIII cone bipolar cells (CBs) during retinal degeneration in RCS rats. Beneath the morphologic changes, we find RBs and CBs exhibit markedly different responses to deafferentation. These implications might provide some clues and directions for the development of new therapies.
RBs Withdraw Normal Dendrites Early and Sprout New Dendrites LaterRBs were identified using antibodies against Protein kinase C alpha (PKCα). In P30 control rats, PKCα immunoreactive RBs had fine dendritic terminals (Figure 1A, arrowhead). In RCS rats, with the degeneration of rods,RBs start to withdraw their fine dendrite processes around P30 (Figure 1B); all the normal dendrites disappeared until P45 (Figure 1C). By P60, RBs sprouted abnormal dendritic processes into the subretinal space fulfilled with debris of photoreceptors (Figure 1D). The amount of abnormal dendritic processes increased at P90 (Figure 1E).Previous studies revealed that in RCS rats, rod degenerate shortly after eye open and almost all the rods have disappeared by P90. As the output signal from rods diminishing, normal dendrites of RBs were gradually lost. At late stage of retinal degeneration, RBs sprouted new dendrites to seek new input signals to maintain their survival. The phenomenon of dendritic sprouting of RBs has been seen in other genetically degenerated rats[3]. In a focal photoreceptor lesion model, the deafferented RBs of rabbits reconstructed thickened processes directed toward the healthy photoreceptors at the lesion edge[4].
Phenomenon of Dendrite Sprouting in CBs was not Obvious Compared to RBsRecoverin immunoreactivity demonstrates type II and type VIII CBs as well as all the photoreceptors. The axons of type II CBs made a dense continuous plexus in sublamina-a of the inner plexiform layer(IPL; Figure 2A, arrowheads), which is considered to be the OFF-CB layer. Type VIII CBs were ON-CBs with a diffuse plexus of axons terminating in sublamina-b of the IPL. As recoverin is positive not only for these two types of CBs, but also all the photoreceptors. It might not be able to identify the dendrites of CBs clearly. Thus, we can’t observe early changes of dendrites of CBs by recoverin immunoreactivity. However,at late stage of degeneration, with rare photoreceptors left, the recoverin positive CBs still do not undergo such changes as we observe in RBs. At P90, no obvious dendritic sprouting was found in these two types of CBs (Figure 2E). In rabbits with focal photoreceptor lesion, the deafferented RBs project their newly dendrites toward the lesion edge, but deafferented secretagogin-positive CBs do not direct their dendritic fields toward remaining photoreceptors[4]. In another genetically dystrophic rat retina, mixed-input (type III and IV) OFF CBs sprout to ectopic sites while true cone-selective type I and II OFF CBs did not show sprouting[5]. This is consistent with what we found in the present study. Type II OFF CBs did not sprout new dendrites in response to photoreceptor degeneration. In the above study, they assume that true coneselective bipolar cells never sprout while bipolar cells with mixed rod/cone input have the ability to sprout. Therefore, not all the CBs in dystrophic rat retina lack the ability of dendritic sprouting. Further investigations concerning the dendritic sprouting ability of subtypes of CBs are need.
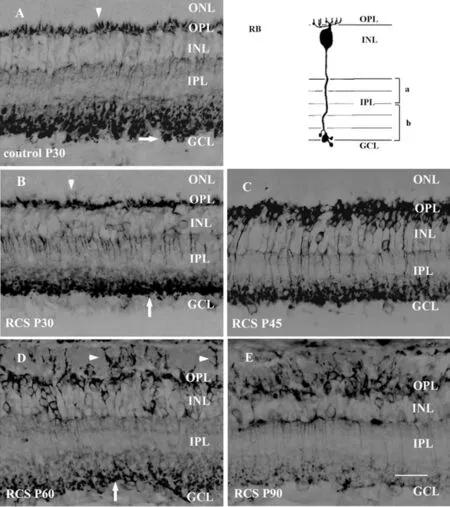
Figure 1 PKCα positive RBs in control (A) and RCS rats during retinal degeneration (B-E, scale bar=20 μm) Compared with control rats (A), the dendritic terminals of RBs were less profuse in P30 RCS rats (B) and almost all the dendrites were lost by P45 (C). In P60 RCS rats, RBs sprouted thick processes into the subretinal space (D),and more dendritic processes were seen by P90 (E). In control rats, the axon terminals of RBs had characteristic varicosities (A). The amount and size of the terminal varicosities in P30 RCS rats decreased (B)and continue to decline (C&D) until no varicosities were seen by P90 (E).
RBs might be better candidates for reintroduced photoreceptorsPeanut agglutinin (PNA) preferentially binds to the cone ISs, OSs and cone pedicles in the mammalian retina. We have used PNA to investigate early morphological changes of cones in RCS rats[6]. In the present study, we found that, with the development of rod degeneration, the relationship between the abnormal dendrites of RBs and the residual cone pedicles became closer during retinal degeneration in RCS rats.At P60, abnormal processes of RBs were found to embrace the enlarged cone pedicles (Figure 2D, 2G). By P90, they overlap each other, indicating that synaptic connections might be formed between RBs and cones in dystrophic retina (Figure 2E, 2F). The newly sprouted dendritic of bipolar cells have the potential to synapse with residual or implanted photoreceptors.Thus, the ability of sprouting new dendrites could be seen as a sign of neural plasticity of bipolar cells[7]. Previous work has shown that PKCα-positive RBs sprout dendrites deep into a retinal transplant in rd mice[8]. In vitro study of RBs have also shown that they could extend their dendrites in culture. And the dendrites are often decorated with varicosities and smaller spine-like extensions, suggests that RBs have the potentiality to make synapse with transplants[9]. The ability of RBs to sprout new dendrites and make synapse with residual cones indicate that RBs might be able to synapse with reintroduced photoreceptors. Compared to type II and type VIII CBs without the ability of dendrite sprouting, RBs might be better candidates for reintroduced photoreceptors in future transplant study.
Axon Terminals of Rod Bipolar Cells Decrease While New Dendrites IncreaseIn control P30 rat, PKCα immunoreactive RBs had an axon that ran perpendicularly through the IPL ending in sublamina-b of the IPL with some terminal varicosities (Figure 1A, arrow). In RCS rats, at P30 the axonal arborizations in the IPL were simpler and the terminal varicosities were smaller than that in control rats (Figure 1B, arrow). At P45 (Figure 1C) the amount and size of the terminal varicosities further decreased. Changes in axon terminals became very evident at P60 (Figure 1D)as degeneration progressed. Axon terminal varicosities still can be found but were further reduced in size and number(Figure 1D, arrow). By P90, the characteristic axon terminal varicosities had disappeared (Figure 1E) and the complexity of the axon terminals reduced further. Axon terminal varicosities are specialized presynaptic structures that contain chemical neurotransmitter. In the retina, RBs are presynaptic to retinal ganglion cells (RGCs) and amacrines. The gradual loss of varicosities indicates that, in dystrophic rat, output signals from RBs to RGCs and amacrines decrease gradually. The decrease of axon terminal varicosities of RBs is in great contrast to the quickly increase of the new dendrites. The cause of the contrast is that these newly sprouted dendrites can’t gather enough information to maintain normal morphology and function.We suggest that cell or tissue transplant therapy should be conducted at this time window. Only that the reintroduced photoreceptors could make synapse with the newly sprouted dendrites of RBs and transmit the signal to postsynaptic neurons.
Axon terminals of OFF CBs are better preserved than ON CBsIn normal rat, the axons of type II CBs made a dense continuous plexus in sublamina-a of the IPL (Figure 3A,arrowheads), which is considered to be the OFF-CB layer.Type VIII CBs were ON-CBs with a diffuse plexus of axons terminating in sublamina-b of the IPL. In RCS P30 rats, the plexus in sublamina-a was not significantly different from that seen in the control P30 rats. The axon terminals in sublamina-b appeared more sparsely distributed. This arrangement in the two layers appeared to change very little between P30 and P45. At P60, the plexus in sublamina-a became discontinuous,with vacancies created by the loss of type II CB axon terminals(Figure 3D, arrowheads) and the density of axons and axon terminals of type VIII CBs decreased further. By P90, the decrease in axon and axon terminals of type VIII CBs became more evident. However, less obvious changes could be seen when the axon terminals of type II CBs were compared with those at P60.
The axon terminals of RBs and type II CBs both ramify in sublamina-b and thus belong to OFF bipolar cells; conversely,type VIII CBs that ramify in sublamina-a are ON bipolar cells.The change pattern of type VIII CBs is similar to the situation with RBs. They both belongs to ON bipolar cells. The above results indicated that axon terminals of OFF CBs are better preserved than that of ON CBs. The early reduction of axon terminals of RBs can be attributed to the early degeneration of rod photoreceptors in RCS rats. The differential changes of the axon terminals of type II CB and type VIII CB might be attributed to the following two reasons. First, it may be due to the differential degeneration of cone subtypes presynaptic to type II and type VIII CBs. Second, it might be due to the differential degeneration of type II and VIII CBs itself within the degenerating RCS retina. Previous studies revealed that oxygen consumption in the inner retina in both RCS and P23H rats is higher in the OFF pathway compared to the ON pathway. As photoreceptor degeneration proceeds, oxygen levels in the inner retina significantly increased[10]and this may make the OFF pathway more resistant to oxidative stress in degenerating retina.In the mammalian visual system, bipolar cells are functionally crucial neurons that comprise the middle component of the vertical transduction pathway in the retina. They form a connecting link between photoreceptors, which perceive light stimuli, and RGCs, which output visual information to higher visual centers. Retinal bipolar cells in rats can be divided into two major classes: nine types of cone bipolar cell and one type of rod bipolar cell, being postsynaptic to the cones and rods, respectively. It was generally accepted that photoreceptor degeneration is followed by significant morphological changes in the second-order retinal neurons, including bipolar cells.

Figure 3 Recoverin positive type II and type VIII CBs in control(A) and RCS rats during degeneration (B-E, scale bar=20 μm) In control P30 rats, the layers in the outer and inner parts of the IPL were made by axon terminals of type II and type VIII CBs, respectively(A). Axon terminals of type II CBs in sublamina-a remain unchanged while axon terminals in sublamina-b appeared more sparsely distributed in RCS P30 rats. Axon terminals in these two layers appeared to change very little between P30 and P45 (C). At P60, the plexus in sublamina-a became discontinuous, with vacancies created by the loss of type II CB axon terminals (D, arrowheads) and the density of axon terminals of type VIII CBs decreased further. By P90,the decrease in axon and axon terminals of type VIII CBs became more evident (E).
The diseased gene in RCS rats primarily alters the function of the RPE and rods are initially affected. Earlier studies revealed that obvious rod degeneration begin around P25 and almost all the rods disappeared by P90. Dendritic processes of RBs were gradually lost as the input signals from rods decreases, and this initiates a process of abnormal growth from the RBs. In the present study, we found that RBs sprouted new dendrites after losing all the normal processes during retinal degeneration in RCS rats. The dendrites of bipolar cells have also been shown to remodel after photoreceptor degeneration, suggesting that the maintenance of dendritic morphology of RBs is dependent on afferent input. In great contrast to RBs, even at late stage of degeneration, recoverin positive type II and type VIII CBs do not sprout new dendrites as RBs. Early studies revealed that type III and IV CBs sprout to ectopic sites in another genetically dystrophic rat retina. The ability of dendrite sprouting of subtypes of CBs differs, and need detailed investigation. Further observation of the newly sprouted dendrites of RBs revealed that they embrace the enlarged cone pedicles at P60 in RCS rats, and overlap each other by P90. The relationship between the abnormal dendrites of RBs and the residual cone pedicles became closer, indicating that synaptic connections might be formed between RBs and cones in dystrophic retina. Therefore,we infer that RBs might be able to synapse with reintroduced photoreceptors and they might be better candidates compared to type II and type VIII CBs.
In the outer plexiform layer (OPL), bipolar cell dendrites receive light-evoked signals from photoreceptors, integrate and process the signals at the cell body, and then transferred via axons terminals to amacrines and RGCs in the IPL.Axon terminal varicosities of bipolar cells are specialized presynaptic structures that contain neurotransmitter. In RCS rats, we found that axon terminal varicosities of RBs decrease while new dendrites increase. The differential changes to the dendritic and axonal terminals of RBs suggest that this visual pathway was severely compromised and the input signal from the newly sprouted dendrites was insufficient. To maintain the morphology and function of bipolar cells in dystrophic retina,additional input signals from cell or tissue transplant should be introduced at this time window.
Similar to the situation with RBs, the axon terminals of type VIII CBs showed an early reduction by P30. In contrast,the axon terminals of type II CB in sublamina-a remained unchanged until a minor decrease was seen by P60. The axon terminals of ON and OFF bipolar cells ramify in distinct IPL layers, where they are presynaptic to RGCs. The early reduction of axon terminals of RBs can be attributed to the early degeneration of rod pathway in RCS rats. The differential changes of the axon terminals of type II CB and type VIII CB might be attributed to different degeneration of presynaptic subtypes of cones and their ability to resist oxidative stress in dystrophic retina.
ACKNOWLEDGEMENTS
Foundation:Supported by the National Natural Science Foundation of China (No.81600758).
Conflicts of Interest:Huang YM,None;Yang M,None;Yuan RD,None.
 International Journal of Ophthalmology2021年4期
International Journal of Ophthalmology2021年4期
- International Journal of Ophthalmology的其它文章
- Prevalence and risk factors of dry eye disease in young and middle-aged office employee: a Xi’an Study
- YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway
- Clinical features and treatment outcomes of intraocular lymphoma: a single-center experience in China
- Trends in research related to high myopia from 2010 to 2019: a bibliometric and knowledge mapping analysis
- A simple new technique for the induction of residual posterior vitreous cortex removal and membrane peeling
- Bilateral choroidal detachment and exudative retinal detachment following laser peripheral iridotomy in a case of ocular Vogt-Koyanagi-Harada’s disease
