YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway
Teng Li, Hong-Bing Zhang, Jia-Min Meng, Bo Yuan, Wen-Juan Lin, Yue Feng,Xiao-Dong Chen
1First Affiliated Hospital of Northwest University, Northwest University, Xi’an 710069, Shaanxi Province, China
2College of Life Sciences, Northwest University, Xi’an 710069, Shaanxi Province, China
3Department of Ophthalmology, Xi’an No.1 Hospital, Xi’an 710002, Shaanxi Province, China
4Shaanxi Institute of Ophthalmology, Shaanxi Provincial Key Lab of Ophthalmology, Clinical Research Center for Ophthalmology Diseases of Shaanxi Province, Xi’an 710002,Shaanxi Province, China
Abstract
● KEYWORDS: YM155; retinal pigment epithelial cell;epidermal growth factor receptor; mitogen-activated protein kinase
INTRODUCTION
T he abnormal retinal pigment epithelial (RPE) cells’survival have been deemed to an important reason in the development of proliferative vitreoretinopathy (PVR), a severe blinding disease resulted by the shrink of proliferative cellular membranes within the vitreous cavity and on the retinal surface[1]. Normal RPE cells are situated between photoreceptors and choroid, and they play a crucial role in maintaining the visual cycle and metabolism[2]. When the extracellular environment changes, such as the results of retinal surgery or trauma, RPE cells in the intraocular are exposed to serum factors because of damage to the blood-retinal barriers.This process leads to abnormal migration and proliferation of RPE cells. Some approaches were used to solve the development of PVR via attenuating RPE cells’ migration and proliferation, but an effective therapy for PVR remains a challenge for ophthalmologists.
Some studies have suggested that several growth factors and cytokines are related to the development of PVR[3].The epidermal growth factor receptor (EGFR) plays an important role in proliferation and migration of cell[4-6]. RPE cells’ proliferation and migration have been induced by the epidermal growth factor (EGF) via the EGF/EGFR/MAPK signaling pathway[7]. Some scholars have also found that the development of PVR can be attenuated by inhibiting RPE cells’ proliferation via EGF down-regulation[8]. In a previous study, we also found that the EGFR/MAPK signaling pathway and EGFR/AKT signaling involved RPE cell survival[9-10].Therefore, targeting EGFR to inhibit the proliferation of RPE cells might be a way to prevent the development of PVR.
YM155 is a small molecule inhibitor that mainly inhibits cells’survival, proliferation and migration via down regulated survivin protein (a member of the apoptosis protein family)[11-14]. Some studies have shown that YM155 can be utilized to treat lung cancer[15]and inhibit tumor cell growth by down-regulating the phosphorylation of EGFR[16-17]. Other studies found that YM155 induced apoptosis in cardiomyocytes through the PI3K/Akt, P38MAPK and ERK pathways[18]. Moreover,some research groups found that YM155 inhibited RPE cells’proliferation, migration, and epithelial mesenchyme transition process through the transforming growth factor-β (TGF-β)pathway[19-20]. However, YM155’s mechanism on RPE cells is not completely understood.
In the present study, we explored YM155’s effect on the RPE cells’ survival, proliferation and migration in vitro. Our results suggested that YM155 suppresses RPE cells’ proliferation and migration, and induce cell death by impacting the EGFR/MAPK signaling pathway. These data suggest that YM155 offers some therapeutic potential to treat RPE cells’ aberrant survival in PVR.
MATERIALS AND METHODS
Reagents PreparationThe study’s YM155 and bromodeoxyuridine (BrdU) were purchased from Selleck Chemiacls LLC (Houston, TX, USA). EGF came from Peprotech Inc. (Rocky Hill, NJ, USA). The methyl thiazolyl tetrazolium (MTT) and FluoroShield™ with DAPI came from Sigma-Aldrich (St. Louis, MO, USA). The EGFR,phosphor-EGFR, JNK, phosphor-JNK, ERK, phosphor-ERK, P38MAPK, phosphor-P38MAPK, β-actin, and secondary antibodies were from Cell Signaling Technology(Danvers, MA, USA). BrdU antibody came from Proteintech Inc. (Wuhan, Hubei Province, China). The study also used fluorescence secondary antibodies were from Abcam Inc.(Cambridge, MA, USA).
Cell CultureHuman immortalized RPE cell lines (ARPE-19 cells) were came from American Type Culture Collection(ATCC; Manassas, VA, USA) and incubated with DMEM/F-12 culture medium (5% CO2, 37°C). ARPE-19 cells cultured to 80%fusion and were used for this study’s experiments. YM155 was soluble in DMSO to formulate 10, 20, 50, 100 μmol/L of stock solutions and dissolved in the culture medium at a 1:1000 ratio to treat the cells.
MTT AssayThe ARPE-19 cells [(0.5-1.0)×104/well] were seeded in 96-well plates for 12h, and added 0, 10, 20, 50 and 100 nmol/L of YM155 for 6, 12 and 24h. The ARPE-19 cells were cultured in the DMEM/F-12 medium without serum containing 50 μg/mL MTT solution for 4h. At last, the cultural medium was replaced with 150 μL DMSO in each well. The absorbance valve was collected with a micro plate absorbance reader at 570 nm.
Cell Apoptotic AssayThe ARPE-19 cells in the 12-well plates were added 0, 10, 50 and 100 nmol/L of YM155 for 12h. Next, the samples were collected and then stained using annexin V-FITC and a propidium iodide (PI) solution (Key gen Inc., Nanjing, China). In brief, the samples were rinsed with phosphate buffer saline (PBS) twice and then digested with trypsin for two minutes at 37°C. The cell pellets were washed with PBS once and then resuspended in 500 μL of binding buffer and stained with 5 μL annexin V-FITC and 5 μL PI in darkness for 15min. In the end, the cells were detected by the flow cytometer (BD Biosciences, CA, USA).
Cell Proliferation AssayThe ARPE-19 cells were cultured in 12-well plates on the glass slides contained with 0.1% gelatin incubated for 12h. After treatment with 0, 10, 20, and 50 nmol/L of YM155 or 100 ng/mL of EGF and 20 nmol/L of YM155 combined with 100 ng/mL of EGF for 24h, the cells were exposed to 30 μmol/L BrdU for 4h and then fixed with 4%paraformaldehyde (PFA). The cells were washed three times with PBS and treated with 2 mol/L of hydrochloric acid for 30min. Next, the glass slides were blocked with a blocking buffer (5% bovine serum albumin) for 1h. The samples were gently rinsed three times in PBS, and incubated for 2h with BrdU antibody (1:100). Coverslips were lightly washed with PBS three times and exposed to fluorescence secondary antibodies in the dark (1:1000) for 2h. After washing three times with PBS, the samples were stained with DAPI, and then viewed by fluorescence or confocal microscopy (Zeiss, Germany).
Measurement ofCellMigrationThe ARPE-19 cells were cultured in 12-well plates until confluence, and then they were performed for “wound injury” by a 10 μL tip. After treatment with 0, 10, 20, and 50 nmol/L of YM155 or 100 ng/mL of EGF and 20 nmol/L of YM155 combined with 100 ng/mL of EGF, the cells were cultured for 24h. Images of the ARPE-19 cells were captured, and their relative migration distance was estimated by measuring gap sizes.

Figure 1 YM155’s effect on the ARPE-19 cells morphology and viability A: Micrographs of YM155 treated ARPE-19 cells at 0-100 nmol/L for 6, 12, and 24h. Scale bar: 50 μm. B: ARPE-19 cell viability was tested by MTT. C: YM155 treated ARPE-19 cells for 12h and cells were detected using flow cytometry. D: Quantitative data of ARPE-19 cell apoptosis in the untreated group and YM155 treatment group for 12h. E:Quantitative data of cell necrosis in the untreated group and YM155 treatment group for 12h. aP<0.05, bP<0.01.
Immunoblotting AnalysisImmunoblotting was executed in the past described[21]. After treatment with 0, 10, 20, 50 and 100 nmol/L of YM155, the ARPE-19 cells were lysed by in 1×SDS (sodium dodecyl sulfonate) buffer. The samples were heated at 100°C for 15min, isolated in polyacrylamidetricine gels with 10% polyacrylamide. The proteins transferred from gels to polyvinylidene fluoride (PVDF) membranes,and blocked with 5% skim milk about one hour. The PVDF membranes were added primary antibodies (1:1000) overnight and rinsed with PBST three times. Secondary antibodies conjugated with horseradish peroxidase (1:1000) were combined for 2h. Finally, the PVDF membranes were rinsed three times and submerged in chemiluminescence solution,and then analyzed by the Chemi Doc XRS system (Bio-Rad,Hercules, CA, USA). The relative densities of the different bands of protein were tested and β-actin was served as a control.
ImmunofluorescenceThe ARPE-19 cells were cultured in 12-well plates on the glass slides contained with 0.1% gelatin incubated for 12h. The cells were processed with 50 nmol/L of YM155 and added 4% PFA, and then were washed with PBS for three times. Next, the cells were permeated by 0.1% Triton X-100 for 2min and then washed three times in PBS. The samples were blocked with 5% bovine serum albumin about 2h. The cells were incubated with first antibodies at room temperature for 2h. After washing with PBS three times, the cells were labeled with fluorescent secondary antibodies for 1h. Finally, the cells were washed with PBS three times and were stained with DAPI, and then viewed by fluorescence microscopy (Zeiss, Germany).
Statistical AnalysisAll data were presented as mean and standard error of mean (SEM) of 3 or more times experiments.Date analysis was executed by using Graph Pad Prism 7.Student’s t-tests and one-way ANOVA analysis were used to compare control and YM155 treated groups. Significant differences between mean values were indicated as P<0.05.
RESULTS
YM155’s Effect on the Morphology and Viability of ARPE-19 CellsAs Figure 1A shows, treatment with YM155 above 20 nmol/L induced a rounded cell morphology and caused a gap increase. As Figure 1B illustrates, the MTT showed that YM155 inhibited cell viability in a time- and concentration-dependent manner. Compared to the un-treatment group, treatments with 10, 20, 50 and 100 nmol/L of YM155 for 6h decreased cell viability by 13.3%, 33.5%, 47.8% and 61.9%, respectively. The corresponding treatments for 12h decreased by 42.3%, 55.6%, 73.0% and 74.7%, respectively.The corresponding treatments for 24h decreased by 64.6%,78.5%, 82.1% and 82.6%, respectively.
YM155 Induces Apoptosis and Necrosis of ARPE-19 CellsAs Figure 1C-1E shows, compared with the untreated group(apoptosis, 1.3%±0.4%; necrosis, 0.8%±0.2%), treatment with 10 or 50 nmol/L of YM155 for 12h did not significantly induce apoptosis (1.5%±0.3%, 1.6%±0.3%) or necrosis (0.5%±0.1%,2.1%±0.2%). However, treatment with 100 nmol/L of YM155 for 12h significantly induced apoptosis (2.5%±0.1%) and necrosis (5.4%±0.1%). These results showed that a high dose of YM155 induces a small amount of cell apoptosis and necrosis.
YM155 Inhibited ARPE-19 Cell Proliferation and MigrationYM155’s effect on ARPE-19 cells’ proliferative capacity was assessed by BrdU assay. The percentage of BrdU+cells in the untreated group was 45%. Compared to the untreated group, treatment with 10 nmol/L YM155 group reduced by 20%. Meanwhile, the 20 and 50 nmol/L YM155 treatment groups had no BrdU+ARPE-19 cells (Figure 2A-2B).In the wound-healing assay, compared to the untreated group,wound healing in the 10, 20, and 50 nmol/L YM155 decreased by 12%, 24% and 29%, respectively (Figure 2C-2D).
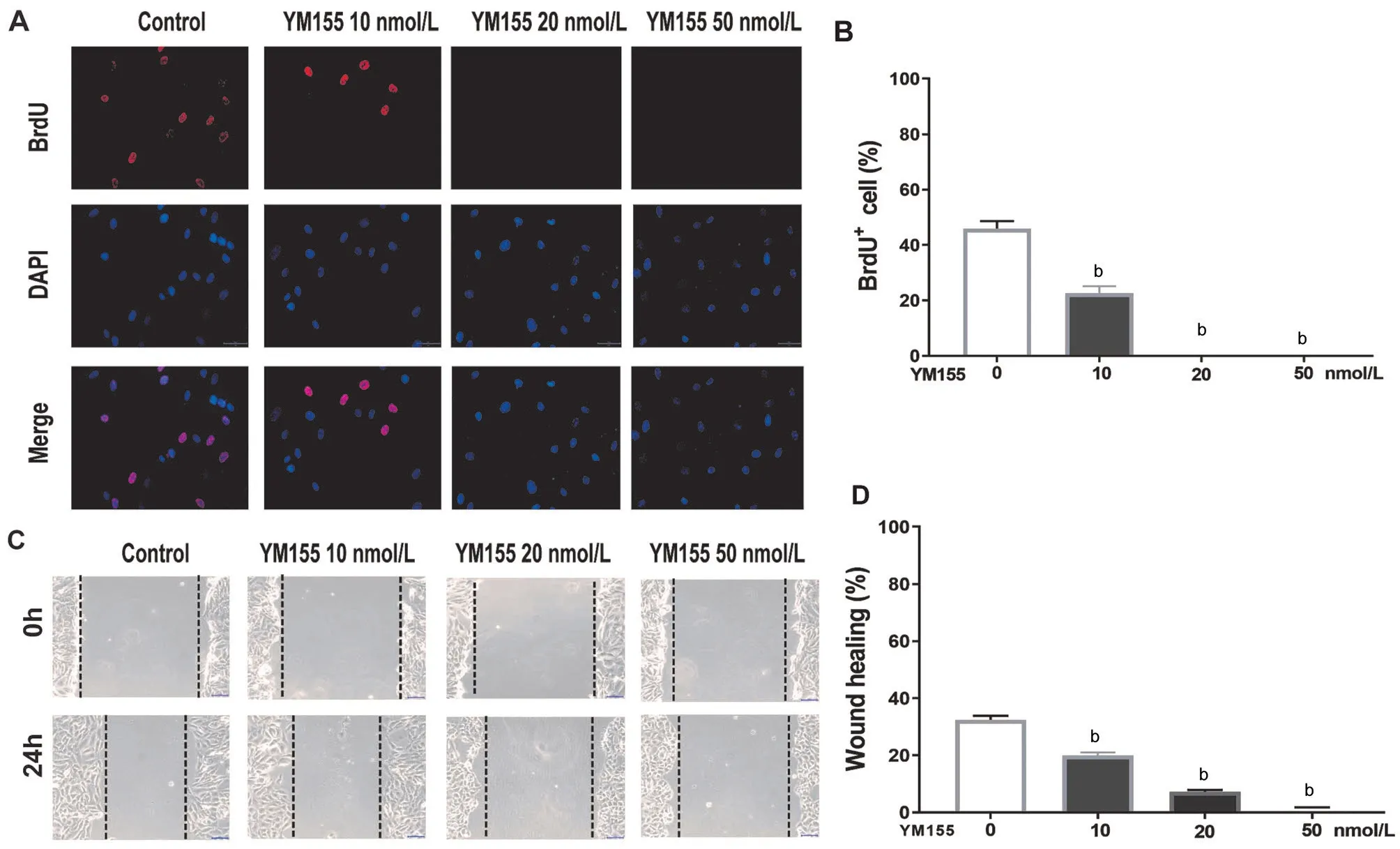
Figure 2 YM155 inhibits ARPE-19 cells’ proliferative and migrative capacity A: Immunofluorescent pictures of a proliferative ARPE-19 cell were labeled with BrdU, and after treated with 0, 10, 20 and 50 nmol/L of YM155 for 24h. Scale bar: 50 μm. B: Quantitative data of a BrdU+ARPE-19 cell. C: ARPE-19 cells’ migrative capacity assays were presented in three independent experiments, and then were treated with 0, 10,20 and 50 nmol/L YM155 for 24h. Scale bar: 100 μm. D: Quantitative data of the migration results. bP<0.01.
YM155’s Effect on the EGFR/MAPK Signal Pathway in ARPE-19 CellsAs Figure 3A and 3B show, YM155 above 20 nmol/L significantly down-regulated the expression of total EGFR and phosphorylated ERK proteins while increasing the expression of phosphorylated P38MAPK and phosphorylated JNK.
Immunofluorescence detection showed that, in the control group ARPE-19 cells, EGFR was evenly expressed in the cell membrane, and treatment with 50 nmol/L of YM155 for 12h induced endocytosis and transferred EGFR from the membrane to the cytoplasm, where it was expressed in granular form around the cell nucleus. Moreover, phalloidin and DAPI staining showed that YM155 induced cytoskeleton damage,and the cell nuclei became larger (Figure 3C).
YM155 Inhibited ARPE-19 Cell Proliferation and Migration Induced by EGFAs Figure 4A and 4B show,the number of BrdU+cells in the control group were 40%.Compared with the untreated group, no BrdU+ARPE-19 cells were in the 20 nmol/L YM155 treatment group, while the percentage of BrdU+cells in the EGF treatment group increased by 25%. Compared with EGF treated group, the percentage of BrdU+cells in the EGF+YM155 group decreased to zero.
Moreover, we also detected YM155’s effect on ARPE-19 cells’ migration after induced by EGF. In the wound injury experiment, compared with untreated group, the percentage of ARPE-19 cells’ migration rate in the EGF treated group increased by 21%. Compared with the EGF treated group, the ARPE-19 cells’ migration rate in the EGF+YM155 combined treatment group decreased by 42% (Figure 4C-4D).
YM155 Affects EGFR/MAPK Signaling Induced by EGF in ARPE-19 CellsFinally, YM155’s effect on the EGF-induced EGFR/MAPK signaling pathway was tested. The results presented that EGF significantly increased the protein level of phosphor-EGFR, phosphor-P38MAPK, phosphor-ERK, and phosphor-JNK. In contrast, EGF induced the phosphor-EGFR and phosphor-ERK was significantly inhibited by 20 nmol/L of YM155 pretreatment for 12h (Figure 5).
DISCUSSION
RPE cells constitute the outer retinal barrier, which has an important anatomical and physiological function and is considered to be a crucial trigger in the formation and development of PVR[22]. When the blood-retinal barriers are disrupted, RPE cells are exposed to serum factors, which can stimulate RPE cells’ migration and proliferation[23]. EGFR has been reported to be able to mediate EGF-induced RPE cell migration and proliferation, and it has also been found to be expressed in the epiretinal membrane, which may have a facilitative effect on PVR[24-25].
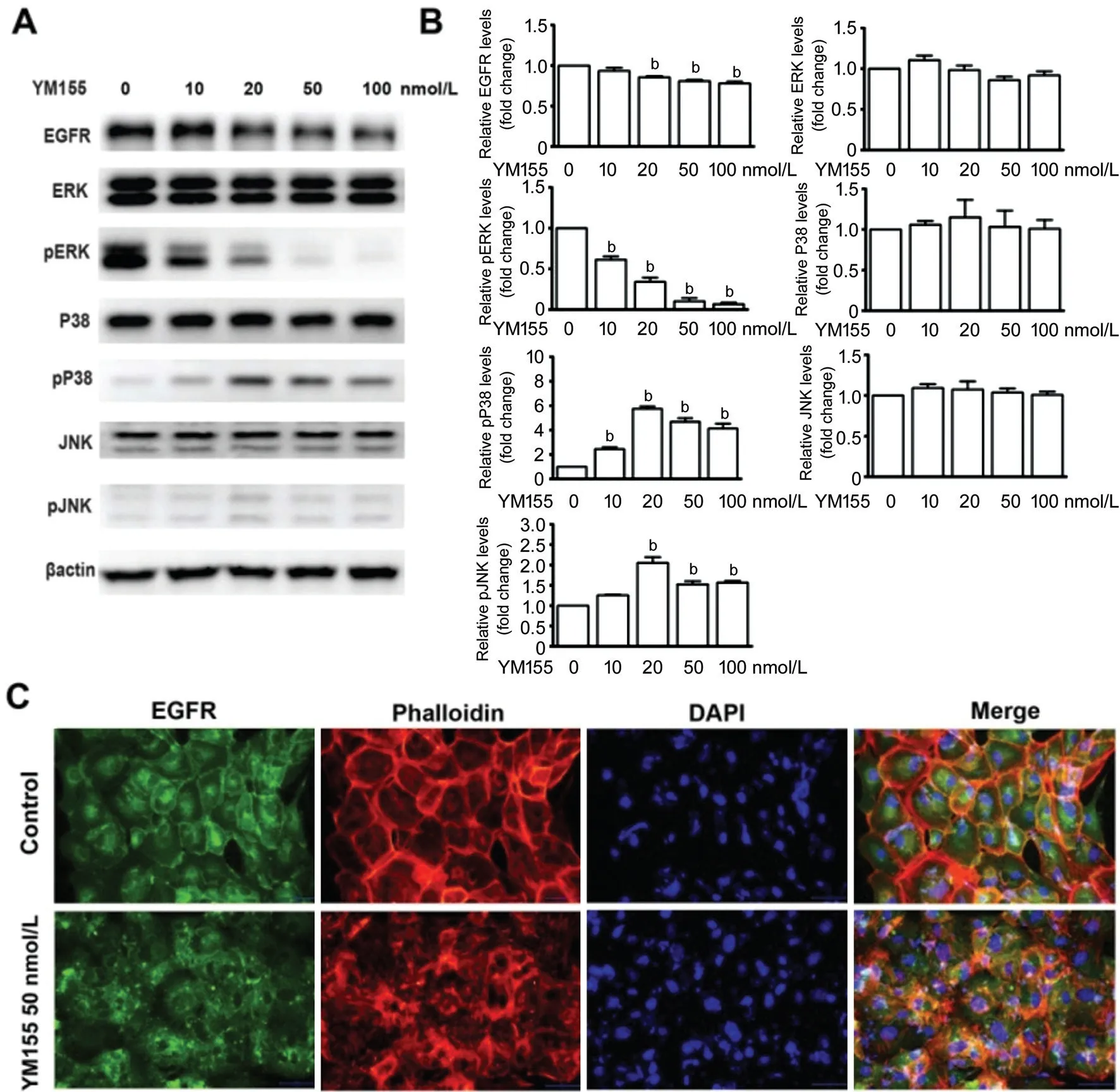
Figure 3 YM155’s effect on the EGFR/MAPK signal pathway in ARPE-19 cells A: ARPE-19 cells were treated with 0, 10, 20, 50, and 100 nmol/L YM155 for 12h. Total EGFR, total and phosphor ERK, total and phosphor P38MAPK, total and phosphor JNK were tested by immunoblotting. B: Quantitative data of the immunoblotting from triplicate experiments. bP<0.01. C: Immunofluorescent pictures of ARPE-19 cells that were treated with 50 nmol/L YM155 for 12h, and cells staining with total EGFR, phalloidin and DAPI. Scale bar: 50 μm.
Survivin, an inhibitor of the apoptotic protein family, inhibits apoptosis and regulates the cell cycle[26]. YM155 is an inhibitor of survivin, which inhibits cell proliferation and induces apoptosis and autophagy[18-20]. In this study, we explored YM155’s effects on RPE cells. Our results showed that YM155 significantly inhibits RPE cell’ survival in a timeand concentration-dependent manner. Some investigators found that 2 nmol/L of YM155 inhibited RPE cells and retinoblastoma cells’ viability, inducing a small amount of apoptosis cells[19]. Similarly, to determine whether YM155 induces apoptosis in RPE cells, we treated ARPE-19 cells with different concentrations of YM155. Our results showed that 100 nmol/L of YM155 induces a small amount of apoptotic and necrotic cells. Recent evidence suggested that YM155 inhibited multiple tumor cells’ growth via down regulation of EGFR[14]. Similarly, our data showed that YM155 can down regulate EGFR protein levels. Together, these data suggest that YM155 inhibits RPE cells’ survival by down regulating EGFR.
Some studies have reported that EGF induces RPE cells’proliferative capacity and promotes RPE cells’ migration through the EGFR signaling pathway[27-28]. Our results suggest that YM155 not only inhibits ARPE-19 cells’ proliferative and migrative capacity, but also suppresses the EGF-stimulated ARPE-19 cells’ proliferative and migrative capacity. These data indicate that YM155’s effects on RPE cells’ migration and proliferation may be associated with the EGF/EGFR signaling pathway.
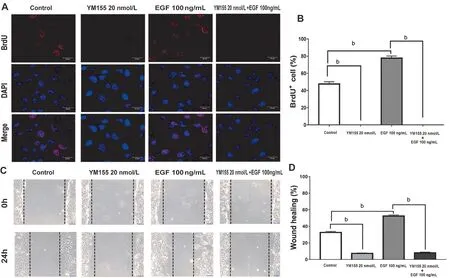
Figure 4 YM155 inhibits ARPE-19 cells’ proliferative and migrative capacity induced by EGF A: Immunofluorescent pictures of a proliferative ARPE-19 cell were labeled with BrdU. After ARPE-19 cells were presented in three independent experiments and then treated with or without EGF (100 ng/mL) and in the absence or presence of YM155 (20 nmol/L) for 24h. Scale bar : 20 μm. B: Quantitative data of the BrdU+ARPE-19 cells. C: ARPE-19 cells’ migrative capacity were presented in three independent experiments and then treated with or without EGF(100 ng/mL) and in the absence or presence of YM155 (20 nmol/L) for 24h. Scale bar : 100 μm. D: Quantitative data of the cell migration results.aP<0.05, bP<0.01.

Figure 5 YM155 affects EGFR/MAPK signaling stimulated by EGF in ARPE-19 cells A: ARPE-19 cells were treated with or without YM155 20 nmol/L for 12h, and EGF (100 ng/mL) induced for 0-60min in a medium without serum. Phosphor-EGFR, phosphor-P38MAPK,phosphor-ERK, and phosphor-JNK were tested by immunoblotting. B: Quantitative data of the immunoblotting results. aP<0.05, bP<0.01.
Some scholars have reported that YM155 down regulates the expression of EGFR protein, induces the location of EGFR from the cytoplasmic membrane to the nucleus, and induces cell autophagy[29-30]. In this study, to explore YM155’s mechanism on RPE cells through the EGFR signaling pathway, we used different concentration of YM155 to treat ARPE-19 cells for 12h. Immunoblotting results performed that the total levels of EGFR and phosphorylated ERK declined significantly; however, the expression levels of phosphorylated P38MAPK and phosphorylated JNK increased significantly.In the present study, changes in ARPE-19 cells’ morphology were also observed using a microscope. Cells gradually showed a rounded morphology, the nuclei became larger, and the intercellular gaps became smaller. Immunofluorescence staining results showed that YM155 promoted the endocytosis of EGFR, cytoskeleton disarray, and the nuclei becoming much larger. Based on these Western blot, immunofluorescent and migration results, we speculate that YM155 suppresses RPE cells’ proliferation and migration via affecting EGFR/MAPK signaling pathway and disrupting cytoskeletal formation.
Finally, we detected YM155 treatment’s effects on EGFinduced signal pathways. Our results demonstrated that EGF induced increase of phosphorylated EGFR, phosphorylated ERK, phosphorylated P38MAPK, phosphorylated JNK,whereas YM155 pretreatment down regulates the level of phosphor-EGFR and phosphor ERK induced by EGF.Moreover, interestingly, EGF induces below band of phosphorylated JNK, however YM155 induces the other two bands of phosphorylated JNK, which indicates that EGF and YM155 might activate different subunit of JNK. These data suggest that YM155 affects RPE cells’ survival via regulating EGF/EGFR/MAPK signal pathways.
In summary, our results suggest that YM155 inhibits RPE cells’ viability, migration, and proliferation while inducing a small amount of RPE cells death via EGFR/MAPK signaling pathway. YM155 might, therefore, be an agent to treat or prevent abnormal RPE cell proliferation and migration in PVR.
ACKNOWLEDGEMENTS
Foundations:Supported by the Natural Science Foundation of Shaanxi Province, China (No.2018JM7040); the Science and Technology Planned Projects of Xi’an City, China[No.20YXXJ008(4)]; the Health Research Personnel Training Project of Xi’an Health Commission, China (No.J201901009).
Conflicts of Interest: Li T,None;Zhang HB,None;Meng JM,None;Yuan B,None;Lin WJ,None;Feng Y,None;Chen XD,None.
 International Journal of Ophthalmology2021年4期
International Journal of Ophthalmology2021年4期
- International Journal of Ophthalmology的其它文章
- Prevalence and risk factors of dry eye disease in young and middle-aged office employee: a Xi’an Study
- Clinical features and treatment outcomes of intraocular lymphoma: a single-center experience in China
- Trends in research related to high myopia from 2010 to 2019: a bibliometric and knowledge mapping analysis
- A simple new technique for the induction of residual posterior vitreous cortex removal and membrane peeling
- Differential degeneration of rod/cone bipolar cells during retinal degeneration in Royal College of Surgeons rats
- Bilateral choroidal detachment and exudative retinal detachment following laser peripheral iridotomy in a case of ocular Vogt-Koyanagi-Harada’s disease
