Review on current concepts of myopia and its control strategies
Raju Kaiti, Ranjila Shyangbo, Indra Prasad Sharma, Manish Dahal
1Nepal Eye Hospital, Kathmandu 44600, Nepal
2National Academy of Medical Sciences, Kathmandu 44600,Nepal
3Gyalyum Kesang Choeden Wangchuck National Eye Center,JDW National Referral Hospital, Thimpu 11001, Bhutan
Abstract
● KEYWORDS: atropine; myopia; myopia control; myopic maculopathy; public health
INTRODUCTION
Considered a simple “refractive anomaly” that can be corrected by conventional optical or surgical interventions in the past, myopia is now an emerging global public health concern. Myopia, an error of visual focusing that makes distant objects appear blurred, not just imposes refractive ramifications but poses a serious risk for visionthreatening ocular complications resulting in irreversible vision loss later in life. The World Health Organization (WHO)defines high myopia as -5.00 diopter (D) or greater, and is associated with increased risk of irreversible blindness[1].Owing to its high prevalence, contribution to visual morbidity with increased risk for vision-threatening conditions (retinal breaks and detachment, glaucoma), myopia has gained priorities in global initiative for the elimination of avoidable blindness[1]. Myopia has become a global public health and socio-economic problems[2-6]predominantly in East Asia[7-10].Halting the onset and progression of myopia is a major therapeutic goal. The global efforts to study myopia and the race to find the most effective methods to control myopia is ongoing. The epidemiology and pathophysiology of myopia and high myopia largely remains undiscovered and evidences continues to evolve. Optical interventions, pharmaceutical and environmental and behavioural modification methods are studied as measures to retard myopia onset and progression.Pharmaceutical management with atropine has demonstrated consistent and promising effect in slowing myopia progression[2].
MYOPIA PREVALENCE
Prevalence of myopia varies globally; developed countries,particularly in East Asia, have high prevalence of myopia and it continues to increase. Similar trends are reported in other parts of the world but with a lesser extent[11-12]. The prevalence of myopia is highest in East Asia with China, Japan, the Republic of Korea and Singapore reporting a prevalence of approximately 50%. A relatively lower prevalence is reported in Australia, Europe and North and South America[13]. While myopia was prevalent in 36.4% children of 8 years old in Taiwan, China in 2016, the prevalence rate of myopia was only 2.4% in the Netherlands among 6 years old children in 2018[14-15]. The prevalence of high myopia is estimated to range between 2%-5% in white populations and between 5%-10%in Asian populations[16], while the prevalence of pathologic myopia is estimated to be about 1% in white populations[17]and about 1%-3% in Asians[18-21]. The annual incidence of blindness due to pathologic myopia was found to be 1-5 per 100 000 in white population[22-23]and 5-10 per 100 000 in Asian population[24]. These statistics show the predominance of myopia among Asian race in comparison to the non-Asian race. A hospital-based study in a tertiary hospital in Nepal (South Asia), reported a myopia prevalence of 47.16% in children[25].Evidences show that, globally 22.9% of the population had myopia and 2.7% had high myopia in 2000, and projects that these figures will increase to 49.7% and 9.8%, respectively by 2050[26]. It is estimated that in 2020 2.6 billion live with myopia and is estimated to increase to 4.7 billion by 2050;almost half the global population[27-28]. Myopia prevalance has significantly increased from 79.5% to 87.7%; moderate myopia(38.8% to 45.7%), severe myopia (7.9% to 16.6%), and terminal myopia (0.08% to 0.92%)[29]. The statistics suggest an alarming increase of myopia prevalence globally, rendering it a burden in public health.
CLASSIFICATION
Myopia has been classified based on degree, anatomical features, inheritance, age of onset, progression rate, pathological presence and theory of myopia development. Donders[30]classified myopia on the rate of progression as stationary,temporarily progressive and permanently progressive. The stationary myopia is generally of low degree and does not progress. The temporary myopia may progress to late 20s after which the rate of progression becomes zero. Contrary to which the permanently progressive myopia ascends rapidly until mid-1920s to mid-1930s and then progresses more slowly. The progressive varieties are known to be associated with sight threatening ocular conditions. Borish[31]classified myopia anatomically as axial myopia in which the axial length of the eye is too long for its refractive components and refractive myopia in which the refractive components of the eye are much stronger than expected. Depending upon degree, myopia is classified as low (<3.00 D), medium (3.00 D-5.00 D) and high myopia (>5.00 D). Physiological myopia results from failure of correlation between the refractive components of the eye. Pathologic myopia (atlernatively, malignant myopia or degenerative myopia) is determined by the presence of optical system of the eye of an element which lies outside the limit of the normal biological variations[32]. High myopia with degenerative changes in the macula, optic nerve and peripheral retina are pathologic myopia. Eyes with pathological myopia are at the highest risk of developing potentially sight-threatening conditions like retinal detachments,myopic choroidal neovascularization (CNV), myopic macular degeneration (MMD), foveoschisis, glaucoma, and cataract[33-34]. Grosvenor[35]classified myopia based on age into four categories: congenital myopia (present at birth), youth onset myopia (onset between 6 years of age to early teens),early adult-onset myopia (onset between 20 to 40 years of age)and late adult-onset myopia (onset after 40 years of age).
ETIOLOGY AND RISK FACTORS
Evidence on etiology and risk factors of myopia onset and progression continue to evolve. The established association risk factors are as follows:
1) Age: Age is an important determinant of myopia as only a small portion of infants are myopic at birth; much of this neonatal myopia is associated with prematurity[36]. Myopia exhibits low prevalence in babies and toddlers[37], and even after formal schooling begins the prevalence rate appears to be low. Myopia usually onsets at the age of 6 to 8y and the rate of progression and axial length elongation is faster in this age group[38-40]. Myopia was found to stabilize at 15.61y with the average -4.87 D of myopia and for every year of delayed stabilization, there was an increase of 0.27 D more myopia per year delayed[41].
2) Gender: Gender-wise predilection of myopia is conflicting and is thought to be confounded by age. Numerous studies[42],including COMET Group demonstrated no significant differences in myopia between males and females[43-44].However, Hirsch[45]found more myopic mean refraction among boys of age group from 5 to 6y but more myopia among girls by age of 14. This finding was consistent with that of adult study conducted by Albirk[46]. The COMET study also found that males have a slower rate of progression of myopia than females[41]. This has been attributed to the influence of age and earlier maturation typically found in females[31].
3) Race: Predominance of myopia among Asian population[13,16,18-21]in comparison to the non-Asian renders race/ethnicity as a paramount risk factors for prevalence and progression of myopia. In NHANES Survey[47], prevalence of myopia across all age groups in whites (26%) was twice that in African American (13%). In a literature-based prevalence of myopia among college students, the prevalence was found to be 48.5% in Chinese[48], 34.7% in Eurasian[48], 30.45%in Indian[48], 24.5% in Malay[48], 15.8% in Israeli[49], 14.5%in Swedish[50]and 11.05 in British[51]. The COMET Group also found that myopia stabilized at a younger age (13.82y)with less absolute myopia (-4.36 D) in African Americans.Caucasians took the longest to stabilize (16.32y) and Asians had the highest amount of absolute myopia at the point of stabilization (-5.45 D)[41].
4) Heredity: Sorsby and Leary[52]concluded that all refractive errors including myopia are genetically determined which can occur in autosomal dominant[53], autosomal recessive[53]and X-linked inheritance[53]form. The wide variability of the prevalence amongst different ethnicity supports the genetic disposition of myopia[54]. There is an increased risk for development of juvenile myopia even if one parent were myopic[55]. It has also been reported that there is over sixtimes increased risk of juvenile-onset myopia if both parents are myopic[56]. Parental myopia is an important risk factor for both development and progression of pathologic myopia[57].The genetic influence in development of myopia has been supported by studies among monozygotic and dizygotic twins in which heritability of myopia was greater in monozygotic twins compared to dizygotic twins (95% vs 29%)[58]. The findings were consistent with several other studies[59-62].
The COMET group also revealed that parental myopia was positively corelatted to myopic progression and increases in axial length[57].
5) Environmental factors: The roles of non-genetic or environmental factors in development of myopia have been extensively studied. Extensive near work during childhood have been known to cause abnormal eye growth[63-64].Experimental and epidemiological studies have indicated that schooling, study, reading and other near work are associated with increased axial length and myopia progression[49,65-71].Literature also suggest that outdoor time has a negative, or protective, association with myopia[44,72-73].
Beside the aforementioned factors, diet[74-75], socioeconomic status[47,68], intelligence[49,76-77], and geography[78-79]are known to be an important risk factor for myopia. Myopia has been associated with introversion personality[80], greater intelligence and cognitive abilities[49,76-77], and higher socioeconomic standards[47,68]. Various systemic conditions[81-82]including albinism, Down syndrome, Marfans Syndrome, Sticklers Syndrome, dental caries and diabetes have been reported to be an essential risk factor. Deprivation-like myopia have been induced in corneal opacifications[83], eyelid closure[84],vitreous hemorrhage[85]and congenital cataract[86]. In a research conducted by Nathan et al[87]among low vision pediatric patients, myopia was common in diseases like aniridia, cerebral palsy, coloboma, optic atrophy, nystagmus,optic nerve hypoplasia, retinitis pigmentosa, retinopathy of prematurity (ROP), and toxoplasmosis. Glaucoma have been connected with myopic refractive errors[88]. Currently, role of inflammation has been proposed in myopia progression[88-89].There is also a correlation between the development and progression of myopia and activation of the complement system. Apart from inflammatory, profile oxidative stress[90-91]has also been reported as a contributory factor for myopia progression.
PATHOLOGIC MYOPIA AND PATHOPHYSIOLOGY
Pathologic myopia is generally high myopia accompanied by visual dysfunctions[92]. The term “pathologic myopia”describes the situation of pathologic consequences of a myopic axial elongation. These myopias are rapidly progressive, with early onset (between 5 and 10 years of age) resulting in a high myopia in early adulthood. Etiologically contributing factors have been mostly reckoned to be that of inheritance, along with the general growth process of the ocular structures.
The pathophysiology of myopia is multifactorial and is still poorly understood[93]. Evidence from animal studies show that myopia may be induced when quality of image formed on the retina is degraded known as form-deprivation (FD)[94],or the focal point of the image is altered with respect to the retinal plane known as lens induced defocus[94]. Both FD and lens induced defocus result in abnormal eye growth and development of refractive errors[94]. Myopization is the result of overshooting of emmetropization process. Axial myopia is consistently resulted when the retina is deprived of form or pattern stimulus through eyelid sutures[95-96]or translucent diffusers[97-98]. Animal experiments emanate the strongest evidence of visual regulation of ocular growth; shows that eyes can actively compensate for artificially induced myopic and hyperopic defocus by adjusting the axial length to the altered focal plane (i.e., emmetropization through the treatment lenses)[99]. Hyperopic defocus with minus lenses induces artificial hypermetropia that leads to a thinning of the choroid(moving the retina backward) and an increase in axial length,which results in myopia to re-establish the optimal refractive state[94]. Low degrees of scleral thinning have also been reported. Choroidal and scleral thinning is most prominent at the posterior pole and less marked at the equator[100]. The axial elongation is also associated with thinning of the retina and reduced density of the retinal pigment epithelium cells(RPE) in the retro-equatorial region, while retinal thickness and RPE cell density in the macular region and the thickness of Bruch’s membrane (BM) in any region are independent of axial length[101-102]. The axial elongation-associated increase in the fovea-optic disc distance is mainly due to the development and enlargement of parapapillary gamma zone defined as the BM free region around the optic disc[103-104]. Subsequently, the length of BM in the macular region is not increased in axially elongated eyes, unless defects in BM in the macular region have developed[105]. The independence of the RPE cell density,retinal thickness, and length of the BM in the macular region fit with the observation that the best corrected visual acuity is independent of the axial length in axially elongated eyes without myopic maculopathy[106].
MYOPIC MACULOPATHY
There are disagreement on definition for pathological myopia,and reseachers have recently reached to a consensus. The acceptable definition of pathologic myopia is an eye having chorioretinal atrophy equal to or more serious than diffuse atrophy by META-PM study group classification[107-108]and/or the presence of posterior staphylomas. Myopic maculopathy[108-110],or more commonly known as MMD or myopic retinopathy is the most common cause of impaired vision in high myopia.The signs of myopic maculopathy include: 1) posterior staphyloma[111]; 2) myopic chorio-retinal atrophy[108]; 3) diffuse chorioretinal atrophy[112]; 4) patchy chorioretinal atrophy[113];5) lacquer cracks[114-116]; 6) choroidal neovascularization and related macular atrophy[117]; 7) myopic macular retinoschisis[118]; 8) dome-shaped macula (DSM)[119-120].
According to the META-PM classification[108], myopic maculopathy lesions have been categorized into five categories as shown in Table 1.
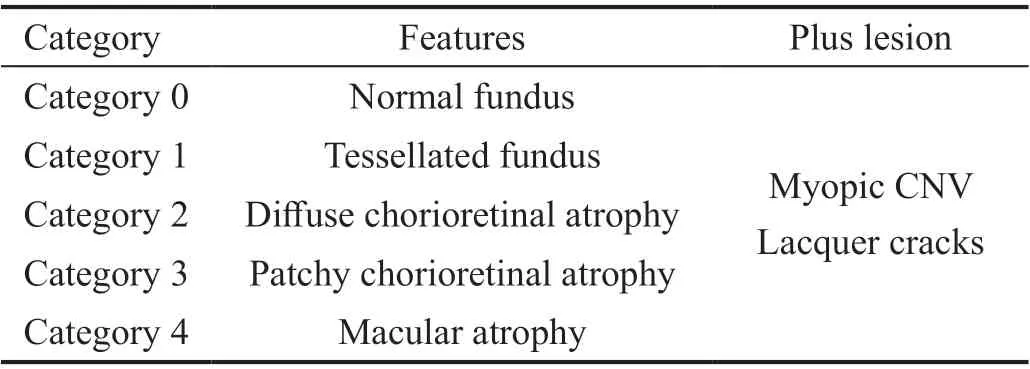
Table 1 META-PM classification of myopic maculopathy
High Myopia and Cataract SurgeryHigh myopia poses a significant challenge during cataract surgery. During cataract surgery for high myopia biometry becomes extremely important. Inaccuracy of axial length measurements and the incorrect choice of lens formula can lead to unpredicted refractive outcomes; often errorneous hyperopic outcome[121].Additional difficulty intraperatively is caused by a relatively deeper and often unstable anterior chamber, a floppy and larger capsular bag and frequently weaker zonules[122]. Postoperatively the incidence of retinal detachment ranges between 0.4% to 5.0%, significantly higher than in low myopia[123-124].For patients with high myopia, adequate counseling,preoperative planning and appropriate intraocular lens (IOL)choice are important. To meet the patient’s expectation following cataract surgery. Use of newer lens formulas and adjustments/transformations and using using manufacturer’s lens constants are beneficial.
METHODS OF MYOPIA CONTROL
Considering the significantly increased risks of pathologic myopia in those with high myopia, control of myopia progression has become an important clinical goal[125].Numerous methods have been studied and applied to achieve myopia control. Current modalities for slowing/halting myopia progression include progressive addition lenses (PALs)[126-128],peripheral defocusing lenses[129-130], contact lenses[131], overnight orthokeratology (Ortho-K)[132-133], multifocal soft contact lenses[134], outdoor activities[135-137], and pharmacological agents[138-140]. Pharmaceutical intervention for myopia control includes several drugs but the daily application of atropine has been the successful in myopia progression[141-142]. Each myopia control strategies are described below.
Optical Interventions
Bifocals and multifocal lensesIn the past decade, several studies have been conducted to assess the effect of bifocal,multifocal, and PALs spectacles on myopia progression. The near prescription of these lenses is considered to halt myopia progression by reducing accommodative effort and lag at near.Masked randomized controlled trial (RCT) showed that with bifocals myopia progression during the study period of 2.5y slowed by 20%[128]. PALs were initially considered insignificant in halting myopia progression. However, COMET-2[127]study sought that PALs of 2.0 D resulted in reduction of myopia progression by 24% in a 3y study. PALs are a preferable option for myopic children with accommodative lag and esophoria.
Peripheral defocusing lensesPeripheral defocusing lenses are designed on the hypothesis that reduction of relative peripheral hyperopia effects myopia progression. Peripheral defocusing is available in both spectacle and contact lens,and have been tested with encouraging results as myopia control treatments[143-144]. Spectacle lenses designed to reduce peripheral hyperopia attenuate the rate of myopia progression in children with parental myopia[142]. Peripheral defocus modifying contact lenses are more effective than tested spectacle designs at controlling myopia progression in children[145].
Defocus incorporated multiple segmentsDefocus Incorporated Multiple Segments (DIMS) spectacle lens[also known as multi-segment of myopic defocus (MSMD)spectacle lens], a specially designed bifocal spectacle lens is under clinical trials[130]. DIMS comprises a central optical zone for correcting refractive error and multiple segments of constant myopic defocus (+3.50 D) surrounding the central zone and is based on the principle of simultaneous vision with myopia defocus for myopia control. DIMS provides clear vision and myopic defocus simultaneously for wearers at distance, intermediate or near objects simultaneously. RCT comparing efficacy of single vision lenses to that of DIMS,showed 52% lesser myopia progression and 62% lesser axial elongation with DIMS compared to children wearing single vision spectacle lenses over 2y[130].
OrthokeratologyOrtho-K lenses are specially designed rigid gas permeable contact lenses worn overnight and designed to reshape the cornea, and temporarily correct low to moderate myopia. It is based on the hypothesis of myopic defocus on the peripheral retina. Ortho-K changes the corneal shape to an oblate shape, which results in a peripheral refraction that has less hyperopic defocus. Several clinical studies on the use of Ortho-K for myopia control have been conducted and effectiveness of inhibiting myopic progression with Ortho-K with the effect of slowing axial length elongation ranging from 32% to 63%[132-133,146]and the overall treatment effect around 50% have been established.
Outdoor ActivitiesDecreased outdoor time also appears to reduce the risk of myopia development, especially in school children. With increased outdoor activities, more myopic defocus results due to relaxed accommodation for viewing distance. Moreover, the high lightening condition outdoors along with sun rays inhibits myopic shifts. Evidences suggest that regardless of the amount of near work duration and parental history of myopia, children spending more duration outdoors during daytime are less likely to develop myopia and have less myopia progression[136-137].
Pharmaceutical InterventionPharmaceutical technique for myopia control includes daily application of low-dose atropine. Shreds of evidence suggested that the drug acted by was via a non-accommodative mechanism, although atropine causes accommodation blocks[147]. The plausible mechanism is thought to be through modulation of dopamine release[148],which has been correlated with a reduction in the rate of axial eye growth[149]. Atropine is so far the safest and most successful drug identified to halt myopia progression. Newer drugs like 7- methylxanthine (7-Mx) and pirenzepine have shown promising results following successful human trials.
Chua et al[150]conducted ATOM-1 study in which he studied the efficacy of 1% atropine drops with placebo, with the result of the mean reduction of myopia progression following 2-year treatment to be approximately 77% in the treatment group compared with the placebo group. Though the efficacy of myopia progression was successfully established, the side effects of atropine led to various concerns on drug safety.Moreover, a rebound phenomenon was observed following the cessation of atropine eye drops administration. The ATOM-2[137]evaluated the lower-concentration for myopia progression to determine the lower optimal concentration for anti-myopia effect of atropine. The efficacy of 0.5%, 0.1%, and 0.01%concentration of atropine was studied with mean myopia progression over 2y treatment to be -0.30 (0.60) D in 0.5%group, -0.38 (0.60) D in 0.1% group, and -0.49 (0.63) D in 0.01% group. The axial elongation was 0.27 (0.25) mm,0.28 (0.28) mm, and 0.41 (0.32) mm in the 0.5%, 0.1%, and 0.01% atropine groups, respectively. Rebound of myopia was observed in 0.5% and 0.1% atropine group. However, it was relatively less in 0.01% groups[138]. ATOM2 Study concluded that 0.01% atropine was better in treatment-to-side effect balance considering a fewer side effects and rebound following atropine cessation. More recently the LAMP[140]study also evaluated the efficacy and safety of low concentration atropine 0.05%, 0.025%, and 0.01% daily instillilation. It found that after one year, the mean spherical equivalent change was-0.27 (0.61) D, -0.46 (0.45) D, -0.59 (0.61) D, and -0.81 (0.53) D,respectively. The mean axial length change after 1y was 0.20 (0.25) mm, 0.29 (0.20) mm, 0.36 (0.29) mm, and 0.41 (0.22) mm, respectively. The side effects were minimal,and the drug was well tolerated. The LAMP Study provides the strongest evidence in favor of low concentration of atropine to halt myopia progression.
Apart from atropine, 7-Mx[149]also has entered human trials following successful results in experimental animal models. In a clinical study, the treatment group who received 400 mg of 7-Mx daily had mean axial length elongation of 0.192 mm per year with mean standard deviation of 0.100 which in contrast to the control group was found to be 0.247 mm per year with mean standard deviation of 0.099. Compared to atropine,no serious side effects were noted but following the drug cessation, the axial length started to lengthen normally i.e. the drug effect vanished. Human trials of 2% pirenzepine gel to halt myopia progression also have been conducted[149-153].In a study conducted by Siatkowski et al[151]and team,the group that received 2% pirenzepine group had myopic progression to be half that of the placebo group with mean myopic progression at 12mo to be -0.26 D with pirenzepine and -0.53 D with placebo. Currently, pirenzepine as antimyopia agent control studies have been halted owing to its reduced efficacy and frequency of instillment.
Surgical InterventionSurgical interventions like laser corneal refractive surgery, intraocular lens implantation and implantable collamer lens can are established to effectively treat myopia; however, do not halt its progression. Latest surgical strategies to halt progression of high myopia include subscleral injection of mesenchymal stem cells and dopamine injection[154].
Effective surgical method to halt axial elongation is the posterior scleral reinforcement (PSR)[155]. PSR involves modifying the sclera remodeling which causes direct mechanical reinforcement of the wall of the eyeball. PSR is found to be safe, effectively stabilizes the vision, prevents the axial elongation, delays the chorioretinal degeneration and ultimately halt the progression of myopia[156].
CONCLUSION
Myopia is an emerging global public health issue with high prevalence. It is necessary to note that myopia can render an otherwise fully healthy person visually challenged.Epidemiological studies are conducted in large scale worldwide and the concepts and knowledge on myopia are changing. Despite global efforts the most effective treatment
strategy is yet to be identified. Researches and clinicians needs
to remain updated with recent advancements in myopia and its control methods. Huge research gaps exist and hundreds of questions remains mysterious. There is a need to scaleup researches, engage public health experts and enhance multidisciplinary collaboration to tackle the ever increasing prevalences of myopia.
ACKNOWLEDGEMENTS
Conflicts of Interest:Kaiti R,None;Shyangbo R,None;Sharma IP,None;Dahal M,None.
 International Journal of Ophthalmology2021年4期
International Journal of Ophthalmology2021年4期
- International Journal of Ophthalmology的其它文章
- Prevalence and risk factors of dry eye disease in young and middle-aged office employee: a Xi’an Study
- YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway
- Clinical features and treatment outcomes of intraocular lymphoma: a single-center experience in China
- Trends in research related to high myopia from 2010 to 2019: a bibliometric and knowledge mapping analysis
- A simple new technique for the induction of residual posterior vitreous cortex removal and membrane peeling
- Differential degeneration of rod/cone bipolar cells during retinal degeneration in Royal College of Surgeons rats
