Clinical characteristics of progressive nonarteritic anterior ischemic optic neuropathy
Omer Y. Bialer, Hadas Stiebel-Kalish
1Neuro Ophthalmology Unit, Ophthalmology Department,Rabin Medical Center, Petah Tikva 4941492, Israel
2Sackler School of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel
Abstract
● KEYWORDS: nonarteritic anterior ischemic optic neuropathy; optic neuropathy; disc edema; visual field defect
INTRODUCTION
Nonarteritic anterior ischemic optic neuropathy (NAION)is the second most common optic neuropathy in adults after glaucoma[1].
After the initial visual loss, most patients remain stable. Vision improves during the first 6 mo after the event in approximately 41%-43% of patients with NAION, while in 15%-19% vision deteriorates[2-3]. This has been referred to as the progressive form of NAION.
The pathogenesis of NAION is presumed to be due to ischemic damage to the optic nerve head (ONH)[4-6]. This sets in motion a vicious cycle of increasing ischemia in which hypoxia of the ONH axons causes blockage of axoplasmic flow, leading to swelling of the axons and optic disc edema. The optic nerve serves as a relatively inflexible closed compartment and therefore the axons can expand only by compressing the surrounding tissues (compartment syndrome mechanism). The compression of capillaries and smaller vessels increases axonal hypoperfusion, which in turn leads to further axoplasmic stasis and swelling and so forth[1,4-5].
Based on the vicious cycle presumed to occur in which edema leads to further ischemia, progressive visual deterioration would have been expected in the majority of cases, yet only a minority of NAION patients exhibit progressive visual loss after presentation. We speculated that most NAION cases are progressive but that this progression is not clinically evident, since most are not evaluated early enough. This study examined whether patients with progressive NAION present sooner after symptom onset.
SUBJECTS AND METHODS
Ethical ApprovalThis is a retrospective, observational study of patients with NAION who were evaluated at the neuro ophthalmology clinic of a tertiary medical center. The study was approved by the Rabin Medical Center Institutional Review Board (IRB), and complied with the principles of the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
The medical records of all the patients seen between January 1st, 2012, and December 31st, 2018 were searched. The earliest date for chart review was chosen due to assimilation of the current electronic medical record system at our institution in 2012. Search terms used to identify patients with NAION were: ischemic optic neuropathy, NAION, visual field defect,optic atrophy, optic neuropathy and visual loss.
Similar to the criteria used by the Ischemic Optic Neuropathy Decompression Trial (IONDT)[2], NAION was confirmed clinically based on a combination of the following: acute(<21d) painless visual loss, decreased visual acuity, a relative afferent pupil defect (RAPD), color vision loss, visual field defect typical of retinal nerve fibre layer (RNFL) bundle damage, documented disc edema in the involved eye and crowded optic disc in the fellow eye.
Inclusion criteria included: age >18y; documentation of disc edema in the acute stage and optic atrophy at last clinic visit;follow up for at least 3mo since presentation. Exclusion criteria included: 1) Pain with eye movement; 2) Onset of visual loss within 2wk following a nonocular surgery[implying a perioperative anterior ischemic optic neuropathy(AION)]; 3) Onset of visual loss within 2wk after head trauma (implying a traumatic optic neuropathy); 4) Suspected arteritic AION-typical symptoms of giant cell arteritis (GCA)with elevated erythrocyte sedimentation rate and C reactive protein or a temporal artery biopsy showing of vasculitis; 5)Ophthalmologic pathology that may impair the measurement of visual acuity or visual field, including dense cataract, macular disease, visually significant retinopathy, or deep amblyopia.
For the final database of patients with NAION we collected the following data: age, sex, past medical history, risk factors associated with NAION, time (in days) from their first symptom (disease onset) to the first ophthalmological examination (time to presentation) and from presentation to last follow up (follow up period). We also recorded the corrected visual acuity, color vision and the result of visual field testing in the first ophthalmic examination (at presentation) and last follow up.
Best corrected high contrast Snellen visual acuity was measured with patients wearing prescription glasses or pinhole occluder and converted to the logarithm of minimal angle of resolution (logMAR) for statistical analysis. The following values were used for nonnumeric visual acuities: no light perception (NLP) 3.0; light perception (LP) 2.3; hand motion(HM) 2.0; finger counting (FC) 1.7[7-8]. The following values were used when visual acuity was tested from 3 feet only:3/18-3/36=1.4 logMAR; 3/54-3/72=1.54 logMAR; 3/108-3/180=1.65 logMAR.
Color vision was tested with the Ishihara pseudoisochromatic plates and the result was recorded as the percent of plates correct out of 12. Visual fields were done with the Humphrey Field Analyzer (HFA) II-750/III-860 (Carl Zeiss Meditec,Dublin, CA, USA) and either the 24-2 or 30-2 format.Strategies used were either: FastPAC, SITA standard and SITA fast. The printout was reviewed for the type of visual field defect (altitudinal defect, etc.). Information about the mean deviation was incomplete; therefore, it was not included in the analysis.
Patients with progressive NAION were identified if they worsened in 2 out of 3 parameters: 1) Best corrected visual acuity ≥3 Snellen lines; 2) Color vision ≥4 Ishihara plates out of 12; 3) A new visual field defect during standard automated perimetry in a previously uninvolved quadrant (e.g.,superonasal, inferotemporal).
The date when progression was diagnosed was documented and the time period from first onset was calculated. We compared the demographic data, risk factors, visual outcome,follow up period and “time to presentation” between patients with progressive NAION and patients with stable NAION.Last observation carried forward was used to deal with missing data.
Dealing with Duplicate DataNineteen patients with bilateral NAION during the study period were included in the statistical analysis. Their demographical data, followup period and risk factors were recorded twice (once for each eye). Twelve patients had the same clinical course bilaterally (one had progressive NAION and 11 had stable course). Their data was recorded twice in the same study group, potentially introducing bias to the results. We repeated the statistical analysis with every patient recorded only once. The results and statistical significance were the same as the original computation (results not shown), confirming that the duplicated data did not affect the statistical analysis.
Statistical AnalysisMedian and interquartile range (IQR)were calculated for numerical variables and proportion (%) for categorical variables. The Mann-Whitney U test and Fisher Exact test were used for numerical and categorical variables,respectively. Two tailed P values <0.05 were considered statistically significant. Statistical analysis was performed using R: a language and environment for statistical computing(R Foundation for Statistical Computing, http://www.R-project.org).
RESULTS
One hundred and eighteen patients with overall 137 NAION events were seen at our Neuro Ophthalmology Unit in the7y of the study period. All the patients were Caucasians.Nineteen patients (13.8%) had bilateral NAION. Mean age was 61.8±12.9y, 18 (13.1%) were younger than 50 years old.Eighty-five patients (62%) were men. Mean presenting visual acuity was 0.5±0.5 logMAR (~20/63) and 42/108 (39%) had altitudinal visual field defect.

Table 1 Demographics and visual characteristics of progressive NAION
Nine NAION cases were excluded because their clinical course was unknown, being lost to followup shortly after their initial examination. Another 6 NAION cases (five with stable course)were excluded because they did not complete at least 3mo of follow-up.
Overall, the study group included 122 NAION events: 20(16.4%) had progressive NAION and 102 had NAION with stable clinical course (stable NAION). The median time interval between the first symptom and the date when progression was first documented was 20d (range 6-66d, mean 26d), after excluding 4 outliers (range 71-362d): two patients missed their scheduled appointments and came for followup a few months later. Two patients entered an interventional clinical trial (the Quark/NORDIC/PAREXEL study) and their clinical data during the study period was unavailable for review.
Table 1 presents the demographical data, visual data, and followup period. There was no statistically significant difference between groups in age, sex, the proportion of bilateral involvement or the proportion of onset at young age (<50 years old). Both groups had similar visual acuity impairment and dyschromatopsia at presentation. Expectantly,at their last followup, patients with progressive NAION had significantly worse visual outcome compared to stable NAION(Table 1), confirming the diagnosis of progression.
Table 2 presents the prevalence of risk factors associated with NAION in each group. The most common risk factors overall were dyslipidemia and hypertension (57.3% and 59.8% of the whole cohort, respectively). The groups did not differ in the prevalence of risk factors. Several risk factors were rare and therefore did not allow statistical comparison.
The time from onset of symptoms to first ophthalmological examination was 7d or less in 82 NAION events (73.9%)of 111 cases with available data. Patients with progressive NAION presented to medical care significantly earlier than patients with stable NAION (Figure 1). The time from onset to first examination was median 2d (IQR 1-5.2, mean 4d) in patients with progressive NAION compared to 5d (IQR 3-8.7,mean 7d) in patients with stable NAION (P=0.011). The relative risk for having progressive NAION if a patient was first seen within 2d from onset was 2.25 (confidence interval 1.31-3.85, P=0.01).
DISCUSSION
In a cohort of patients with NAION seen in a tertiary medical center, 16.4% had severely progressive clinical course which resulted in median visual acuity of 20/200. Except for timing of presentation, no other identifiable clinical or demographical risk factor was found to be associated with NAION progression. Patients with progressive visual loss presented earlier than those with stable visual loss (median 2d vs 5d respectively).
Progressive visual loss in NAION has been previously reported[9-15], but no study specifically addressed progressive NAION. These retrospective reports mostly included a small number of patients or did not include the results of visual field testing and color vision. The two main studies that reported the natural history of NAION were the IONDT[2]and a study by Hayreh and Zimmerman[3].The IONDT was a multicenter, randomized clinical trial that compared the safety and efficacy of optic nerve decompression surgery with careful followup in 258 patients with NAION recruited between 1992 and 1994. The initial suggestion for optic nerve decompression that led to the IONDT was particularly made for progressive NAION[16]. The study used a decline in visual acuity as an indicator of progressive status[2].Their definition of progressive status was defined in three separate ways: patients whose vision was better than 20/64 at 14d from onset of symptoms but deteriorated to 20/64 or worse within 30d; patients whose vision was better than 20/64 at 14d but lost ≥3 lines of vision between their baseline visit and randomization visit; all patients who reported a subjective worsening of vision since onset. In the careful followup arm,7.3% had progressive NAION at 3mo after randomization and 12.4% after 6mo. However, 16.8% of patients that initially did not meet the visual inclusion criteria deteriorated 3 lines or more within 30d from onset.
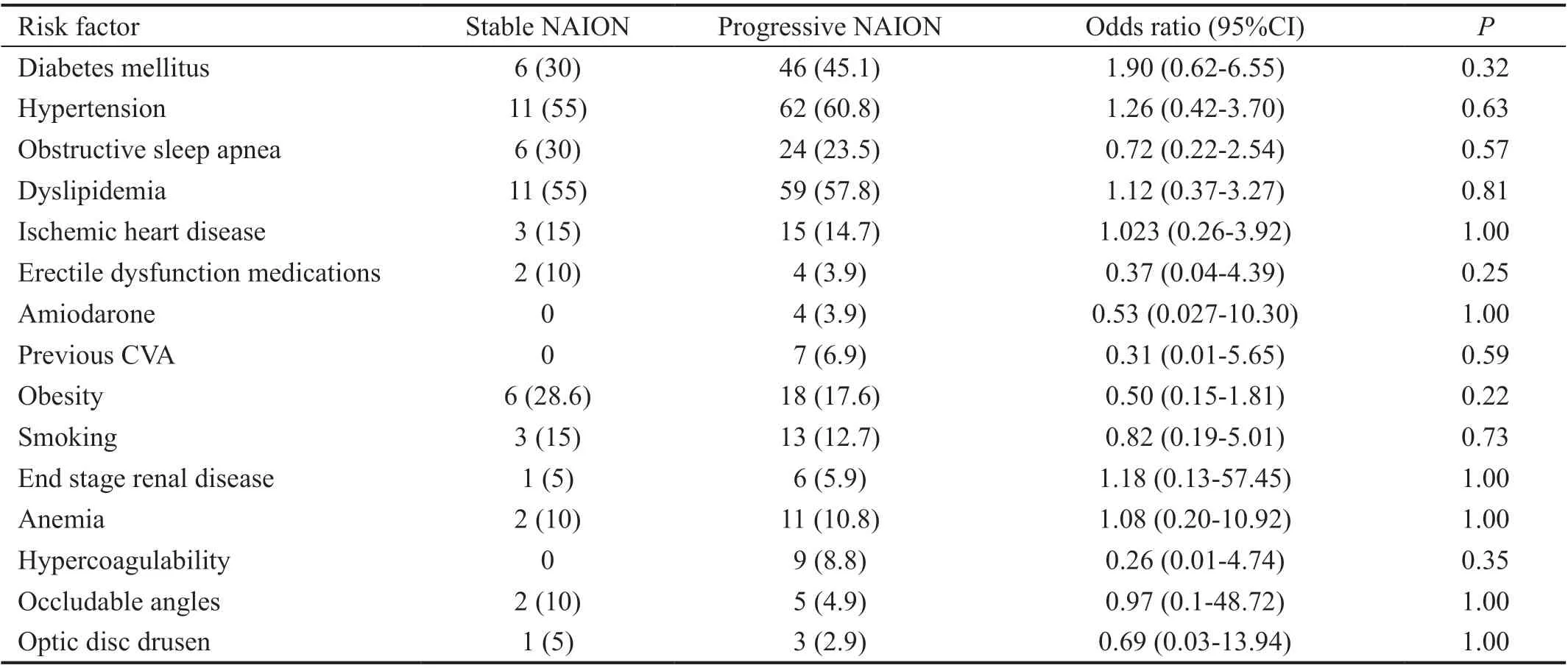
Table 2 Risk factors associated with NAION n (%)

Figure 1 The time from symptom onset to presentation Box plot of the time in days from onset to presentation. Data was available for 20/20 patients with progressive NAION and 90/102 patients with stable NAION. The median time to presentation was 2d (IQR 1-5.2)in the progressive NAION group and 5d (IQR 3.0-8.7) in the stable NAION group. P=0.011 (Mann-Whitney U test). A center line of the box indicates the median value of data. Lower and upper boundary lines of the box are the 25% and 75% quartile, and marginal lines represent 95%CI of the data.
The study by Hayreh and Zimmerman[3]systematically documented visual acuity and visual field loss in a large cohort of patients with NAION between 1973 and 2003. Visual field loss was measured with the Goldmann manual perimetry. The results were subjectively graded from 0 to 4 in steps of 0.5.Progression was defined as either worsening of 3 or more lines in visual acuity (0.3 logMAR) or a difference in visual field grade of 0.5 or more. The study found that 23/317 (7.25%)patients had progressed at 3mo (±6wk) after initial examination(but 38.8% presented more than 2wk after onset). Most of them progressed before the disc edema resolved (within an average of 7.9wk, range 5.8-11.4wk). Fifty out of 281 (17.7%)had progressed at the six-month followup examination. Hayreh and Zimmerman[3]did not look at the color vision deficiency.Furthermore, they used Goldmann perimetry instead of the Humphrey automated perimetry. Naturally, at the beginning of the study's period, Humphrey perimetry was not available,but overall, automated perimetry is more sensitive and reproducible than Goldmann perimetry[17-18]. They also used an internal method of grading visual field results that cannot be reproduced based on their manuscript alone. In comparison,the use of color vision testing strengthens our definition of progression and we used a simpler reproducible way of defining visual field progression.
Similar to our results, the IONDT[2]and Hayreh and Zimmerman[3]did not find an association between progression and demographics or systemic risk factors (hypertension,diabetes, hyperlipidemia, smoking, ischemic heart disease and migraine). We compared a more comprehensive list of risk factors between progressors and nonprogressors(e.g. obstructive sleep apnea[19-20], erectile dysfunction medication[21]). None of them were more common in the group with progressive NAION. However, overall low prevalence limited the statistical analysis.
The only difference between patients with progressive NAION and patients with stable NAION was that progressors arrived earlier for medical evaluation than patients with stable NAION.Similarly, Hayreh and Zimmerman[3]concluded that the change in visual acuity and visual field loss mostly depended on the time when a patient was first seen after the onset of visual loss. This conclusion fits the presumed pathogenesis of NAION. The pathogenesis involves a positive feedback loop of edema and axonal compression, which results in a progressive degree of ischemia of the ONH[4-5]. It is reasonable that an increasing level of ischemia would be paralleled by an increasing degree of visual loss. Therefore, if ophthalmologists could theoretically have examined all patients with NAION on “Day 1” and then serially over the course of several weeks,we hypothesize that most, if not all of the patients would show some degree of progressive visual loss.
The demographics and prevalence of risk factors in our study resemble those of previous studies[3,11,22-29]and therefore our results are generalizable to other medical centers. However,our patients presented for neuro ophthalmological evaluation earlier than in previous reports[2-3,27].
This study has a few limitations beyond the inherent flaws of a retrospective observational study. Not all patients with stable NAION seen during the time period of the study could recollect the timing of their first symptom (data was available for 111/122 events or 90.1%). Hence, this could have introduced sampling bias. We included a qualitative change in the visual field as criteria for progression. Using a change in the mean deviation of the visual field instead would have been a more objective and reproducible mean of documenting progression, but this data was unavailable to us. Although the cutoff values for progression were arbitrary, they are in line with previous publications[2-3]. The color vision and visual field criteria were intended to overcome inter-examination variability, often seen when different neuro ophthalmological evaluations are performed[3].
In conclusion, progressive visual loss is not an atypical clinical course of NAION but rather may be quite common. Diagnosis of progression (to any extent) is mostly associated with the timing of medical evaluation and not with the patient’s characteristics or clinical presentation. Our results indicate that the "time window" to prevent progressive visual loss is much shorter than previously believed[2-3]. Currently there are no proven therapies to prevent progressive visual loss. We would recommend focusing future therapeutic clinical trials on patients presenting with NAION within the first 4d since onset.This may increase the probability of preventing progression.
ACKNOWLEDGEMENTS
Conflicts of Interest:Bialer OY,None;Stiebel-Kalish H,None.
 International Journal of Ophthalmology2021年4期
International Journal of Ophthalmology2021年4期
- International Journal of Ophthalmology的其它文章
- Prevalence and risk factors of dry eye disease in young and middle-aged office employee: a Xi’an Study
- YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway
- Clinical features and treatment outcomes of intraocular lymphoma: a single-center experience in China
- Trends in research related to high myopia from 2010 to 2019: a bibliometric and knowledge mapping analysis
- A simple new technique for the induction of residual posterior vitreous cortex removal and membrane peeling
- Differential degeneration of rod/cone bipolar cells during retinal degeneration in Royal College of Surgeons rats
