Effect of Andrographis paniculata polysaccharide on human retinoblastoma Y79 cell proliferation and apoptosis
Bing Xu, Lei Li, Wei Zhang, Yue Li, Mao-Ren Wang, Jing-Chen Liu, Kai-Ye Dong,Ido Didi Fabian, Dong Qiu, Cai-Rui Li, Yi-Min Xiang
1Department of Ophthalmology, Fuling Central Hospital,Chongqing 408000, China
2Department of Ophthalmology, the First Affiliated Hospital,Dali University, Dali 671000, Yunnan Province, China
3Department of Pathology, Fuling Central Hospital, Chongqing 408000, China
4Clinical Medicine, Dali University, Dali 671000, Yunnan Province, China
5Goldschleger Eye Institute, Sheba Medical Center, Tel Hashomer, Tel-Aviv University, Tel-Aviv 52621, Israel
Abstract
● KEYWORDS: retinoblastoma; cell proliferation; apoptosis;cell cycle
IN TRODUCTION
Retinoblastoma (RB) is a malignant tumor originating from the developing retina. It usually occurs in children under 5 years old[1]and is the most common intraocular malignant tumor in infants[2-3]. At present, the global average annual incidence is approximately 1/15 000-1/20 000, and there are approximately 9000 annual new cases, among which approximately 1100 are reported in China. Although the incidence is low, it seriously affects the vision of children and even endangers their lives[4-5]. A large number of studies have shown that the pathogenesis of RB is mainly related to the aberrance of autosomal 13q14 (RB1 gene), and recent studies have shown that the alteration of the MYCN gene is also related to the occurrence of RB[1,6]. Clinically, it often causes red eyes, eye pain, strabismus, blindness, and can even be life-threatening. Due to its high degree of malignancy, the mortality rate of children patients is 100% without treatment[7].Therefore, there is need for the development of effective treatment strategies for this disease. The present treatment options are varied and highly dependent on tumor stage,pathology, and genetic background[8]. Currently, chemotherapy,radiotherapy, local photocoagulation, cryotherapy, and enucleation are often used in the treatment of RB[9]. However,most chemotherapeutic drugs have serious side effects.Therefore, there is still a need for a novel, safer, and more effective treatment.
Traditional herbs are characterized by low side effects and low prices[10]. Ligustrazine, matrine, celastrol, morin, and andrographolide and genistein have been proved to have an anti-RB effect[10-16]. However, the study of the effects of the Andrographis paniculata (A. paniculata) polysaccharide against RB has not been reported.
Cell proliferation and apoptosis are common cellular processes, the imbalance between which can lead to the occurrence and development of tumors[17]. CDK1 and cyclinB1 are key proteins regulating the G2/M phase of the cell cycle,which can promote the transition from the G2 phase to the M phase and accelerate cell division and proliferation[18-19]. At present, it is believed that there are three apoptosis pathways in cells, namely the exogenous pathway, endogenous pathway,and endoplasmic reticulum pathway. These three pathways are carried out by the activation of caspase (cysteine-aspartate specific protease) proteins, followed by the involvement of mitochondria to perform apoptosis[20-21]. The endogenous pathway is the mitochondrial pathway, primarily comprising the activation of caspase-9. The exogenous pathway, the death receptor pathway, primarily comprises caspase-8 activation.The endoplasmic reticulum pathway involves caspase-12 activation. In these three pathways, caspase-3 and caspase-7 are the key proteases and executors[22]. For our study, we selected the human RB Y79 cell line to explore the specific mechanism of the A. paniculata polysaccharide in cell apoptosis and cell cycle signaling pathways.
MATERIALS AND METHODS
Extraction and Purification ofA. paniculataPolysaccharideThe extraction of A. paniculata polysaccharide was carried out as previously described[23-24]. A 4-fold increased volume of petroleum ether was used to reflux the A. paniculata powder for 4h. The filter residue was then dried at 40℃ and refluxed with 4 times the volume of anhydrous ethanol for 4h. Again,the filter residue was dried at 40℃, and 40 times deionized water was added, and the drug was extracted at 65℃ for 7.5h,with this step was repeated 4 times. The filtrate was collected and concentrated at low atmospheric pressure, and the protein was removed using the Sevage method. It was then precipitated with 75% ethanol overnight at 4℃. The precipitate was washed 3 times with anhydrous ethanol, acetone, and ether, in turn; then, freeze-dried to a constant mass and allowed to stand in the dryer. Finally, the composition and purity of the product were analyzed by infrared and ultraviolet spectra analysis.
Cell Culture and GroupingThe human RB Y79 cell line(obtained from and authenticated by Beijing Beina Chuanglian Biotechnology Research Institute) was exposed in RPMI 1640 medium (Thermo Scientific, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Scientific,USA) and 1% penicillin-streptomycin in 37℃ humidified incubator with 5% CO2. When the cell proliferation density reached approximately 80%, the cells were subcultured. The experiments were conducted on cells during logarithmic growth. Cells were divided into experimental groups(A. paniculata polysaccharide) and negative control groups(equal volume complete medium).
Cell Proliferation AssayY79 cells were made into 1×105/mL single cell suspension and inoculated on 96-well plates with 100 μL of suspension per well. RPMI 1640 complete medium was added to the control groups with 100 μL added per well.In the experimental groups, 100 μL of complete medium containing A. paniculata polysaccharide was added per well.The final concentration was 100, 200, 300, and 400 μg/mL,and for each concentration, there were three repeat wells. After 24, 48, and 72h of culture, 20 μL of enhanced Cell Counting kit-8 (CCK8) reagent (Beyotime Biotechnology Co., Ltd.) was added to each well, and the chromogenic reaction was allowed for 4h at 37℃. Absorbance of each well at 450 nm was measured with a microplate reader. The experiments were repeated three times. Cell survival rate was calculated as: cell survival rate(%)=[(As-Ab)/(Ac-Ab)]×100%, where, As: experimental well(Y79 cell+drug+CCK8); Ac: control well (Y79 cell+CCK8);Ab: blank Well (medium+CCK8).
Detection of Cell Apoptosis Rate by Flow CytometryThe Y79 cells were treated with mock or 200 μg/mL A. paniculata polysaccharide for 48h first. Then, 1×106cells were washed twice by precooled PBS and stained with 5 μL Annexin V-FITC and PI (Vazyme Biotech Co., Ltd.) at room temperature for 10min and were then analyzed using flow cytometry (ACEA NotoCyteTMflow cytometry, Essen Biosciences, USA). The experiments were repeated three times.
Detection of Cell Cycle by Flow CytometryThe Y79 cells in the experimental groups (200 μg/mL A. paniculata polysaccharide) and the control groups (equal volume complete medium) were first treated for 48h. Then, 1×106cells were washed twice by precooled PBS and fixed overnight with 75% ethanol at 4℃. After centrifugation, 100 μL of RNaseA was added, followed by placing in a water bath at 37℃ for 30min. Following this, 400 μL of PI (4A Biotech Co., Ltd.)was added and the mixture was incubated at 4℃ in the dark for 30min, and then measured on the enzyme-labeled instrument.The experiments were repeated three times.
RNA Isolation and QuantificationIn the experimental groups, the final A. paniculata polysaccharide concentration was 200 μg/mL, and in the control groups, the same volume of complete medium was added. After 48h of culture, Trizol reagent (Vazyme Biotechnology Co., Ltd.) was used to extract the total RNA, and then reverse transcription was performed with Hiscript®II Q RT Supermix for qPCR (vazyme Biotechnology Co., Ltd.). The amplification was carried out in triplicate using ChamQTM SYBR®qPCR Master MiX (Vazyme Biotech Co., Ltd.) on a 7900 H T RealTime PCR System (Applied Biosystems, Carlsbad, CA, USA) according to the instructions.The expression of mRNA was normalized to β-actin. Primers were (Table 1) purchased from GenScript (Nanjing) Co., Ltd.The reaction conditions were as follows: 95°C for 5min, and a total of 40 cycles at 95°C for 15s, 55°C for 15s, and 72°C for 60s.The relative mRNA expression was calculated using the 2-ΔΔCtmethod from three independent experiments.
Western Blot AnalysisThe Y79 cells in the experimental groups (the final concentration of A. paniculata polysaccharide was 200 μg/mL) and the control groups (the same volume of complete medium was added) were collected after 48h of culture and then protein was extracted on ice. Gel electrophoresis was performed after protein quantification using the bicinchonininc acid (BCA) method and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in skimmed milk for 2h and then incubated overnight at 4℃ with primary antibodies (1:1000, Cell Signaling Technology, USA). Afterward, the membranes were incubated with secondary antibodies (1:1000, Beyotime Biotechnology Co., Ltd.) at room temperature for 2h. The protein bands were observed by miniChemi K4 (Beijing Sage Creation Technology Co., Ltd.) and analyzed by Image J. The data were derived from three independent experiments.
Statistical AnalysisThe data were analyzed by SPSS 21.0 and expressed as the mean±standard deviation. The one-way ANOVA method was used for the multiple-group comparison and the t test was used for the pairwise comparison. P<0.05 was considered to be statistically significant.
RESULTS
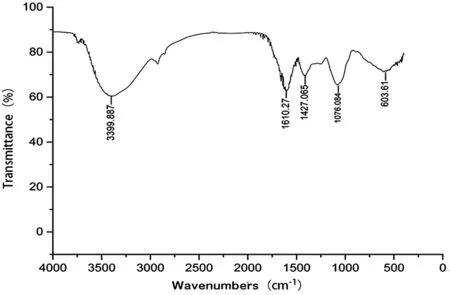
Figure 1 Infrared spectrum of A. paniculata polysaccharide.
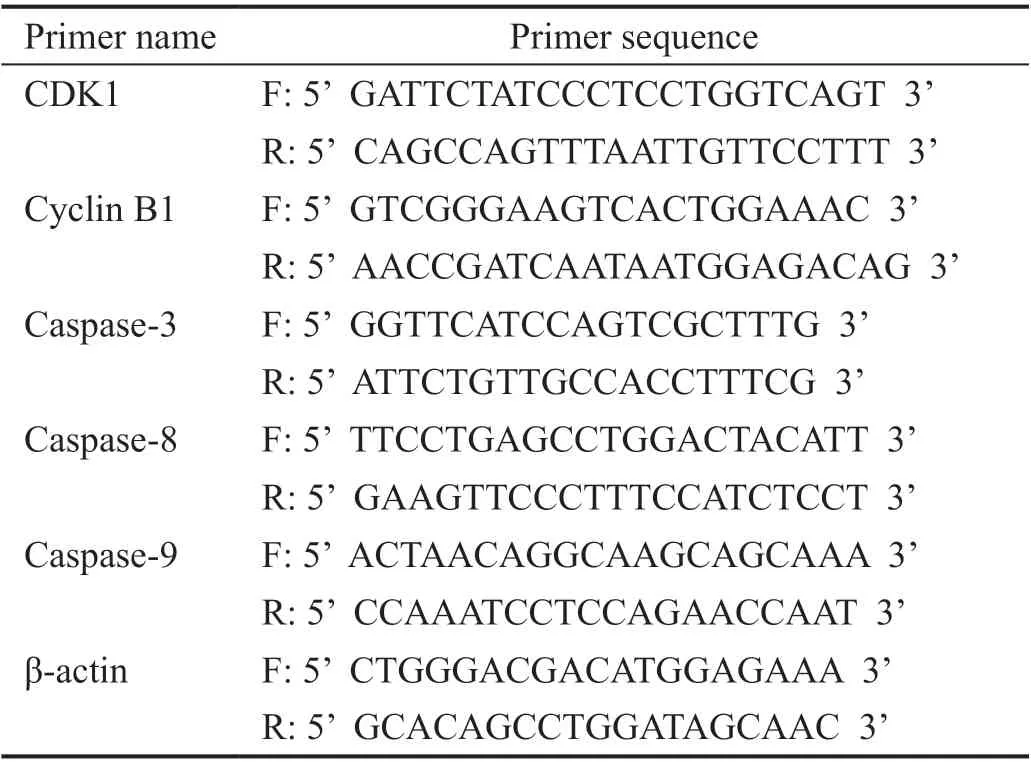
Table 1 Quantitative polymerase chain reaction primer sequences
Components and Purity Identification ofA. paniculataPolysaccharideSome studies have shown that the wave numbers of 3670-3220 cm-1, 3000-2800 cm-1, 1700-1400 cm-1, and 1200-1000 cm-1are typical absorption peaks of polysaccharides in the infrared spectrum analysis[25-26]. The infrared spectrum of the extracted compound is shown in Figure 1. It can be seen that there are obvious absorption peaks within the abovementioned spectral bands; thus, confirming the identification of the compound as a polysaccharide. The ultraviolet spectra showed no obvious absorption peak at 260 nm and 280 nm.The results showed that the nucleic acid and protein from the A. paniculata polysaccharide extract had been substantially removed, resulting in a very high purity (Figure 2).
Effects ofA. paniculataPolysaccharide on the Proliferation of Y79 CellsA. paniculata polysaccharide can significantly inhibit the proliferation of Y79 cells. The cell survival rates of the experimental groups were lower than that of the control groups, and the survival rates decreased with the increase in time and the concentration of A. paniculata polysaccharide(P<0.01; Figure 3 and Table 2).
Detection of Cell Apoptosis Rate by Flow CytometryAs can be seen from Figure 4, cell apoptosis rates increased after 200 μg/mL of A. paniculata polysaccharide treatment compared with the control groups. The cell apoptotic rates of control groups and experimental groups were 2.80%±0.30%and 10.10%±1.01%, respectively (P<0.05). This result showed that the A. paniculata polysaccharide can induce the apoptosis of Y79 cells.Compared with the control groups,aP<0.01.
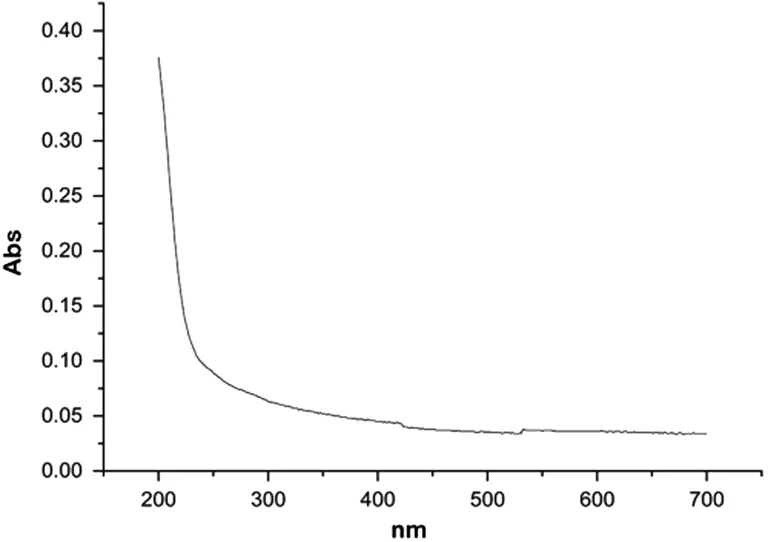
Figure 2 Ultraviolet scanning curve of A. paniculata polysaccharide.
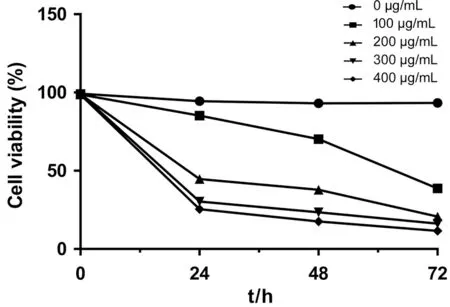
Figure 3 Effects of A. paniculata polysaccharide on the proliferation of Y79 cells.
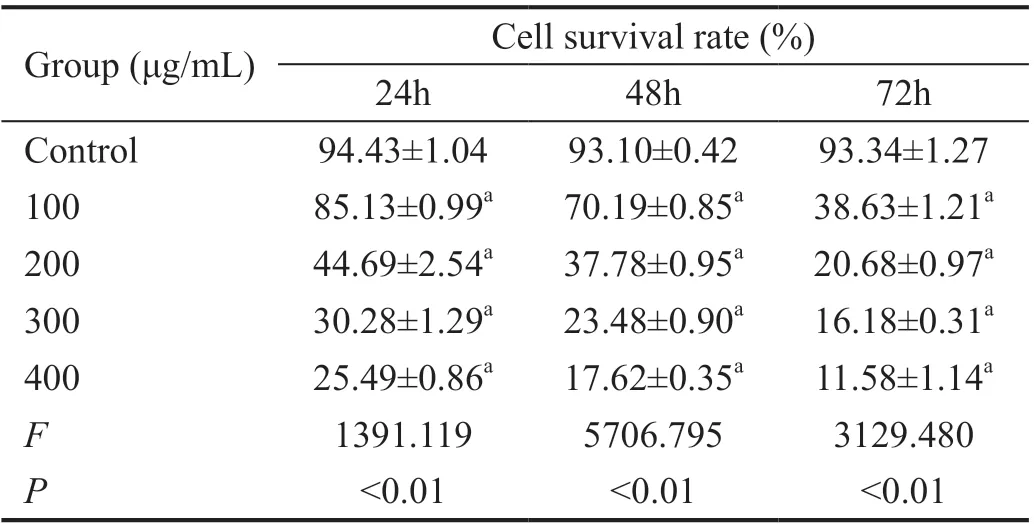
Table 2 Survival rates of Y79 cells at different A. paniculata polysaccharide concentrations and time points n=3, mean±SD
Effect ofA. paniculataPolysaccharide on Caspase-3,Caspase-8, and Caspase-9 mRNA Expression in Y79 CellsAccording to RT qPCR, the mRNA expression of caspase-3,caspase-8, and caspase-9 in the experimental groups was higher than that in the control groups (P<0.05 or 0.01).These results suggested that A. paniculata polysaccharide can upregulate the expression of caspase-3, caspase-8, and caspase-9 in Y79 cells at the transcriptional level (Figure 5).
Effect ofA. paniculataPolysaccharide on Caspase-3,Caspase-8, and Caspase-9 Protein Expression in Y79 CellsThe Western blot analysis results showed that the protein expression of caspase-3, caspase-8, and caspase-9 in the experimental groups was significantly higher than that in the control group (P<0.05). The results were consistent with the results of RT qPCR. These results meant that A. paniculata polysaccharide can upregulate the expression of caspase-3,caspase-8, and caspase-9 in Y79 cells at the translational level(Figure 6).

Figure 4 Detection of cell apoptosis rate by flow cytometry.

Figure 5 Effect of A. paniculata polysaccharide on caspase-3,caspase-8, and caspase-9 mRNA expression in Y79 cells Compared with the control groups, aP<0.05, bP<0.01.
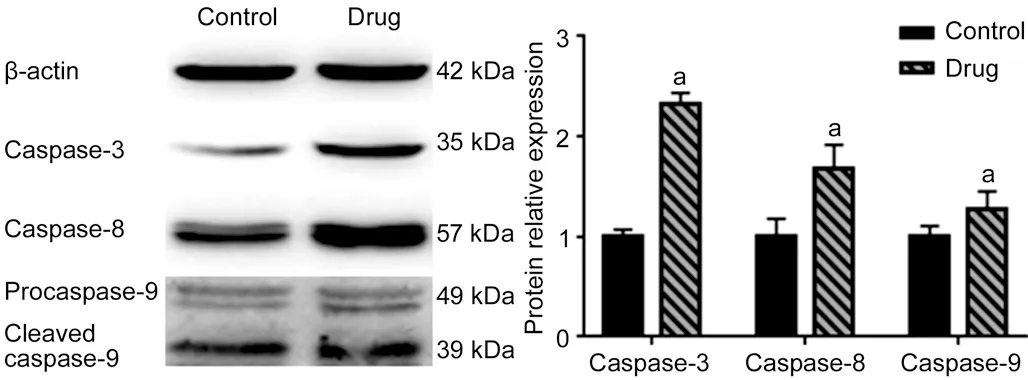
Figure 6 Effect of A. paniculata polysaccharide on caspase-3,caspase-8, and caspase-9 protein expression in Y79 cells Compared with the control groups, aP<0.05.
Detection of Cell Cycle by Flow CytometryAs depicted in Figure 7 and Table 3, compared with the control groups, the G2/M phase distribution proportion was increased after 200 μg/mL A. paniculata polysaccharide treatment, with values of 1.81%±0.54% in the control groups and 6.30%±0.75% in the experimental groups. These differences were statistically significant (P<0.05). The results showed that A. paniculata polysaccharide can induce Y79 cells to be blocked in the G2/M phase.
Effect ofA. paniculataPolysaccharide on CDK1 and CyclinB1 mRNA Expression in Y79 CellsCompared with the control groups, the mRNA expression of CDK1 and cyclinB1 in the experimental groups was significantly lower than that in the control groups as determined by RT qPCR (P<0.05). The results suggested that A. paniculata polysaccharide can downregulate the expression of CDK1 and cyclinB1 at the transcriptional level in Y79 cells (Figure 8).
Effect ofA. paniculataPolysaccharide on CDK1 and CyclinB1 Protein Expression in Y79 CellsWestern blotting results showed that the expression of CDK1 and cyclinB1 in the experimental groups was lower than that in the control groups (P<0.05). The results were consistent with the results of RT qPCR. These results meant that A. paniculata polysaccharide can downregulate the expression of CDK1 and cyclinB1 in Y79 cells (Figure 9).
DISCUSSION
In recent years, with the development of individualized comprehensive treatment and fundus screening, more children with RB can receive early diagnosis and treatment, greatly improving the survival rate among children. However, in many underdeveloped countries and regions, the survival rate is still low[27-28]. Currently, chemotherapy is still widely used in clinical practice, and most chemotherapeutic drugs, such as carboplatin, can cause gastrointestinal intolerance, temporary spinal cord inhibition, secondary leukemia, and other side effects[29]. Concurrent tumor resistance also limits its clinical application. Since the majority of the children with RB are less than 5 years old, this requires that chemotherapy drugs must be safe and effective. As a traditional herbal medicine,A. paniculata has been used in clinical treatment for many diseases for a long time, with the appealing characteristics of safety and affordability[30]. Many studies have confirmed the anti-tumor effect of polysaccharide drugs[31-34]. A. paniculata polysaccharide has been proven to have antioxidant activity and is capable of scavenging DPPH-, O2-, OH-, etc[23-35].Increased levels of oxidative stress in the body can lead to the uncontrolled growth and division of cells, invasion of adjacent tissues, and carcinogenesis[36]. Therefore, we explored the anti-RB activity and mechanism of A. paniculata polysaccharide.Following the treatment with A. paniculata polysaccharide,the survival rate of Y79 cells was tested and it was found that A. paniculata polysaccharide can inhibit cell proliferation. From low to high concentrations of A. paniculata polysaccharide, the cell proliferation inhibition increased gradually; it also increased with an increase in time, showing evident dose- and time-dependent response. The apoptosis rates of Y79 cells in the experimental groups were significantly higher than those in the control groups and cell cycle detection showed that the G2/M phase ratios of the experimental groups were higher than those of the control groups. Therefore, the decrease of cell survival rate after A. paniculata polysaccharide treatment may be related to its promotion of cell apoptosis and cell cycle arrest. These prompted us to further explore the cell apoptotic signaling pathway and cell cycle signaling pathway.Ohata et al[37]suggested that abnormal cell apoptosis regulation plays a very important role in the development of malignant tumors, and the key to tumor prevention and treatment is the inhibition of cell proliferation and/or promotion of cell apoptosis. The caspase family is one of the important proteins inducing cell apoptosis, among which caspase-8 is an important factor to initiate apoptosis. The activated caspase-8 can cause caspase-mediated apoptosis cascade reaction, destroy the death structural proteins and related functional proteins in cells, and cause apoptosis[38-39]. Caspase-9 is an important member of the cysteine protease family. Activated caspase-9 can lyse cell growth proteins and play an important role in cell apoptosis[40-41]. Caspase-3, known as the “killer protein”, is the most important executor of cell apoptosis and is involved in almost all apoptosis processes. It is usually activated and cut into P17 and P12 fragments, causing apoptosis[42-44].CyclinB1 is a cell cycle regulatory protein and an important member of the cyclin family and can induce phosphorylation of downstream RB proteins by forming complexes with the corresponding kinase CDK1. The phosphorylated RB protein loses its inhibitory effect on the transcription factor E2F, thus enabling E2F to resume transcriptional activity and participate in the regulation of cell G2/M phase transformation,accelerating cell cycle from G2 phase to M phase and continuously proliferating and dividing[16-17].
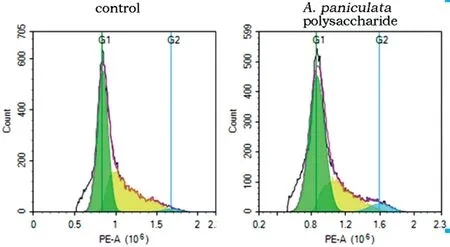
Figure 7 Detection of cell cycle by flow cytometry.
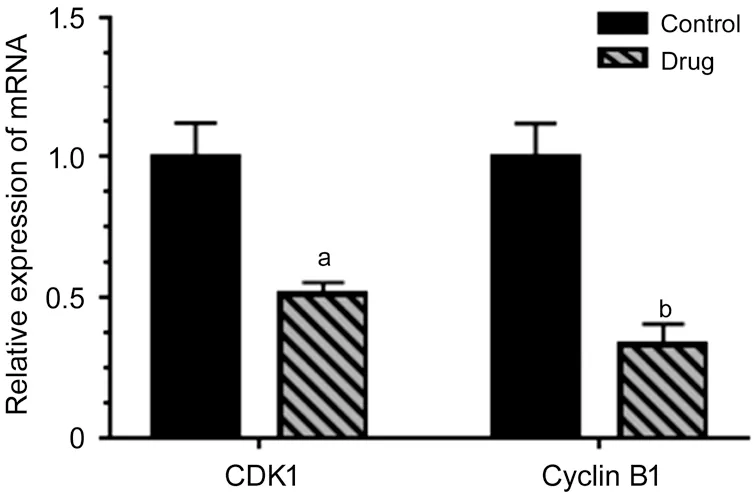
Figure 8 Effect of A. paniculata polysaccharide on CDK1,cyclinB1 mRNA expression in Y79 cells Compared with the control groups, aP<0.05, bP<0.01.
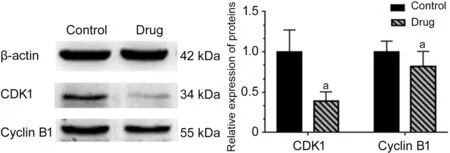
Figure 9 Effect of A. paniculata polysaccharide on CDK1 and cyclinB1 protein expression in Y79 cells Compared with the control groups, aP<0.05.
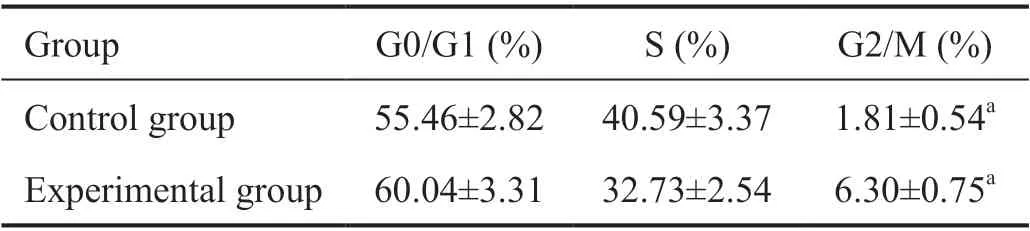
Table 3 Distribution of Y79 cell cycle detected by flow cytometry n=3, mean±SD
In our study, the expression levels of caspase-3, caspase-8,and caspase-9 in Y79 cells in A. paniculata polysaccharide treatment groups were higher than those in the control groups at the transcriptional and translational levels. These proteins are the key proteins in the apoptotic signaling pathway.This suggests that A. paniculata polysaccharide may induce Y79 cell apoptosis by activating the cell apoptotic signaling pathway. Further analysis showed that the mRNA and protein expression of CDK1 and cyclinB1 in Y79 cells treated with the A. paniculata polysaccharide was significantly downregulated compared with the control groups. CDK1 and cyclinB1 are key proteins regulating the G2/M phase, and we also found that the Y79 cells were blocked in the G2/M phase after the treatment of A. paniculata polysaccharide as determined by flow cytometry. Therefore, we speculated that the A. paniculata polysaccharide can induce apoptosis of Y79 cells by regulating the cell cycle signaling pathway.
This study has certain shortcomings. In the future, animal studies should also be conducted, using the RB xenograft model to evaluate the anti-tumor effect of A. paniculata polysaccharide and further explore other possible anti-tumor mechanisms. We will also invest in the further development of this drug. In conclusion, the A. paniculata polysaccharide has anti-RB activity. The possible mechanism is the regulation of cell apoptosis and of the cell cycle signaling pathway. As a new drug, A. paniculata polysaccharide is expected to be an effective drug or adjuvant drug in the treatment of RB.
ACKNOWLEDGEMENTS
Conflicts of Interest:Xu B,None;Li L,None;Zhang W,None;Li Y,None;Wang MR,None;Liu JC,None;Dong KY,None;FabianID,None;Qiu D,None;Li CR,None;Xiang YM,None.
 International Journal of Ophthalmology2021年4期
International Journal of Ophthalmology2021年4期
- International Journal of Ophthalmology的其它文章
- Prevalence and risk factors of dry eye disease in young and middle-aged office employee: a Xi’an Study
- YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway
- Clinical features and treatment outcomes of intraocular lymphoma: a single-center experience in China
- Trends in research related to high myopia from 2010 to 2019: a bibliometric and knowledge mapping analysis
- A simple new technique for the induction of residual posterior vitreous cortex removal and membrane peeling
- Differential degeneration of rod/cone bipolar cells during retinal degeneration in Royal College of Surgeons rats
