Nicotinamide suppresses bevacizumab-induced epithelial-mesenchymal transition of ARPE-19 cells by attenuating oxidative stress
Li Zhou, De-Peng Shi, Wen-Juan Chu, Shan Song, Xiang-Hui Hao, Ling-Ling Yang,Hai-Feng Xu,
1Medical College, Qingdao University, Qingdao 266071,Shandong Province, China
2State Key Laboratory Cultivation Base, Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute,Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao 266071, Shandong Province,China
3Qingdao Eye Hospital, Shandong Eye Institute, Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao 266071, Shandong Province, China
Abstract
● KEYWORDS: nicotinamide; epithelial-mesenchymal transition; bevacizumab; oxidative stress; ARPE-19 cells
INTRODUCTION
Anti-vascular endothelial growth factor (VEGF) therapy has been used to treat ocular diseases for more than 10y[1-3], and it is now the first-line choice for the treatment of vascular fundus diseases, such as neovascular age-related macular degeneration (nAMD), macular edema secondary to diabetic retinopathy, and retinal vein occlusion[4-9]. Intravitreal injection of anti-VEGF agents may result in a rapid decrease in central retinal thickness and subsequently improve visual acuity. However, with the wide application of these agents and the extended follow-up periods required, some unfavorable effects have also been reported, with fibrosis of the neovascular membrane being the most prominent. Subretinal fibrosis of the choroidal neovascular membrane may lead to the loss of previously gained visual acuity in nAMD patients, while tractional detachment due to fibrosis of the retinal neovascular membrane may also seriously impair visual function[10-12].Currently, the mechanism underlying anti-VEGF-induced fibrosis is not fully understood, and no effective solution has been identified that can be employed to reduce the fibrotic process after anti-VEGF therapy.The formation of choroidal neovascularization (CNV) is the main characteristic of nAMD. When the excessive wound healing response of CNV occurs, subretinal fibrosis will happen. The formation of a fibrous membrane is characteristic of subretinal fibrosis[13-14]. The main cellular components of the fibrous membrane are myofibroblasts, which can stem from retinal pigment epithelium (RPE) cells. When RPE cells undergo epithelial-mesenchymal transition (EMT), as a result of various stimulating factors, epithelial cells are converted to myofibroblasts[14]. Therefore, RPE cells can be used as a reliable tool to investigate the mechanisms of subretinal fibrosis in vitro. Furthermore, human retinal pigment epithelial cells (ARPE-19) have been widely used in numerous studies as an alternative to native RPE cells due to their similar gene expression patterns[15]. In especial, the use of ARPE-19 cells has been widely accepted in EMT-related studies[16-18]. To sum up, the sufficient sources of supply and convenient use both contribute to the usage of ARPE-19 cells in this study.
Nicotinamide (NAM) as the water-soluble amide form of vitamin B3[19]not only exerts the therapeutic effects in fibrotic disorders including fibrosis and renal interstitial fibrosis[20-21],but also plays a role in attenuating oxidative stress by protecting cells against reactive oxygen species (ROS)[22-24].Moreover, many studies have reported that oxidative stress is a trigger that can promote fibrosis in numerous organs[25-27]. Our group had investigated the possible mechanisms underlying anti-VEGF therapy-induced fibrosis and we found that bevacizumab (BEV; an common used anti-VEGF agent) not only increased the level of fibrosis related cytokines[28-29], but also stimulated the expression of ROS (data not shown). Lin et al[30]also demonstrated that anti-VEGF agents could cause the oxidative stress damage of ARPE-19 cells by increasing ROS level. So, it is rational to hypothesis that oxidative stress might take part in BEV-induced EMT of ARPE-19 cells.Therefore, in this study, we investigated whether NAM, as an antioxidant has the ability to inhibit BEV-induced EMT by attenuating oxidative stress in ARPE-19 cells in vitro.
MATERIALS AND METHODS
Cell Culture and TreatmentARPE-19 cells were obtained from the American Type Culture Collection (Manassas,VA, USA). Cells were cultured in DMEM/F-12 medium(Invitrogen, Carlsbad, CA, USA) supplemented with 10%fetal bovine serum (FBS; Gibco, Life Technologies, Carlsbad,CA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin(Sigma-Aldrich, St Louis, MO, USA), at 37℃ in a humidified atmosphere containing 5% CO2. When cells reached 60%-70%confluence, the medium was replaced with DMEM/F-12 containing 2% FBS. At the same time, the cells were treated with different agents as follows for various purposes:
1) Determining an optimal BEV treatment time point for establishing EMT model in ARPE-19 cells: BEV (0.25 g/L;Roche, Indianapolis, IN, USA) was added into the medium for 24, 48, and 72h, respectively. When reaching each time point, the cells were harvested, and EMT-related markers were detected at protein level. Based on the variation degree of EMT-related markers, one time point, at which the most obvious variation of EMT-related markers presented, was chosen to be the optimal time point to conduct further experiments.
2) To investigate the role of NAM in BEV-induced EMT:ARPE-19 cells were treated with NAM (10 mmol/L, Sigma-Aldrich), with or without BEV. Specifically, cells were divided into the following four groups: a control group, a BEV treatment group, a BEV and NAM treatment group, and a NAM treatment group. When reaching the optimal time point determined by previous experiment, cells were harvested and EMT-related markers, the cellular ROS and H2O2levels,as well as the cellular total antioxidant capacity (TAC) were evaluated.
Western Blotting to Examine EMT-Related MarkersARPE-19 cells were harvested after receiving various treatments and total protein samples were prepared using radio-immunoprecipitation assay (RIPA) lysis buffer mixed with the protease inhibitor, PMSF (RIPA:PMSF=100:1).The supernatants were boiled for 10min in 4×SDS sample buffer to denature the proteins. Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes, which were then blocked with 5% skim milk for 1h. The PVDF membranes were incubated with anti-fibronectin, -α-SMA, -vimentin, -ZO-1 primary antibodies (1:2000; Abcam, Cambridge, UK) or anti-GAPDH primary antibodies (1:10 000, Abcam) overnight at 4℃. They were then incubated with HRP-conjugated secondary antibodies (1:4000; Absin Bioscience Inc, Shanghai,China) for 1h at room temperature. Immune complexes were detected using a chemiluminescence kit (Millipore, Billerica,MA, USA) and visualized using a ChemiDocTMTouch system(Bio-Rad, Shanghai, China). The band intensities of the targeted proteins were normalized to GAPDH using Image Lab software (Bio-Rad).
Measurement of Cellular ROS and H2O2 LevelsAfter treating ARPE-19 cells for the optimal time point,intracellular ROS and H2O2levels were measured. Cells were incubated with the peroxide-sensitive fluorescence probe, 2,7-dichlorodihydrofluorescein diacetate, acetylester (10 μmol/L; Molecular Probes, Eugene, OR, USA)to detect the expression level of ROS and the H2O2sensor,pentafluorobenzenesulfonyl fluorescein (5 mmol/L; Santa Cruz Biotechnology, Dallas, TX, USA) to measure the expression level of H2O2for 30min at 37℃. The fluorescence intensities of these two probes were measured using a Nikon confocal laser-scanning microscope.
Total Antioxidant Capacity MeasurementWhen reaching the optimal treatment time point, the TAC was measured according to the manufacturer’s instructions (Beyotime,Shanghai, China). In brief, ARPE-19 cells were collected in PBS and intracellular antioxidants were released by ultrasonication. The samples were then incubated with 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid; ABTS)for 6min at room temperature and the absorbance was measured at 414 nm. The experimental values were calibrated to the total protein concentration, as determined using a bicinchoninic acid kit (Vazyme).
Reverse Transcription-Quantitative Polymerase Chain Reaction to Detect EMT-Related MarkersAfter treatment for the optimal time point, ARPE-19 cells were harvested, and total RNA was extracted using TRIzol reagent (Invitrogen).cDNA was then synthesized using reverse transcriptase(Vazyme, Nanjing, China), according to the manufacturer’s protocol. Target genes were amplified in a total volume of 10 μL (5 μL of SYBR Mix, 0.2 μL of sense primer, 0.2 μL of antisense primer, 0.5 μL of diluted cDNA, and 4.1 μL of nuclease-free water), using a SYBR qPCR Master Mix(Vazyme) and a Rotor-Gene 6000 system (Qiagen, Hilden,Germany). Gene expression levels were normalized relative to GAPDH mRNA and reported as the fold change compared to controls. The following primer sequences were used:fibronectin forward, 5’-GGGACCGTCAGGGAGAAAA-3’and reverse, 5’-CGAGATATTCCTTCTGCCACTGTT-3’;α-SMA forward, 5’-GGTGACGAAGCACAGAGCAA-3’ and reverse, 5’-CAGGGTGGGATGCTCTTCAG-3’; vimentin forward, 5’-GCAGGAGGCAGAAGAATGGTA-3’ and reverse, 5’-GGGACTCATTGGTTCCTTTAAGG-3’; and ZO-1 forward, 5’-AGGATCCATATCCCGAGGAAA-3’ and reverse, 5’-CGAGGTCTCTGCTGGCTTGT-3’. GAPDH,forward 5’-CATGTTCGTCATGGGTGTGAA-3’ and reverse 5’-GGCATGGACTGTGGTCATGAG-3’.
Immunofluorescent Staining to Detect the Expression of α-SMAAfter treating ARPE-19 cells in 96-well plates for the optimal time point, they were fixed with 4% paraformaldehyde for 15min at room temperature, which obtained from the second portion of grouping. Then cells were permeabilized with 0.1% Triton X-100 for 5min. After washing three times with PBS, cells were blocked with 5% normal goat BSA for 1h at room temperature and then incubated with primary anti-α-SMA antibodies (1:100) overnight at 4℃. The cells were then incubated with FITC-conjugated secondary antibody (1:500;Bioss, Beijing, China) in blocking solution for 2h at room temperature. The nuclei were subsequently counterstained with DAPI for 10min. Images were visualized under a confocal laser-scanning microscope (Nikon, Tokyo, Japan).
Statistical AnalysisThe experimental results were repeated for three times. Statistical analyses were performed using SPSS 22.0 software (IBM, Armonk, NY, USA). Quantitative data are expressed as the means±standard deviations. One-way analysis of variance with a least significant difference test for pairwise comparison was used for multiple sample analysis.P<0.05 was considered statistically significant.
RESULTS
BEV Promoted the EMT of ARPE-19 CellsIn Western blotting, BEV produced time-dependent effects on the upregulation of mesenchymal markers (fibronectin, α-SMA,and vimentin) and the downregulation of the epithelial marker,ZO-1, in ARPE-19 cells (Figure 1). The most obvious changes in EMT-related markers occurred when the cells were treated with BEV for 72h. At 72h, the comparative expression levels of fibronectin, α-SMA, and vimentin were approximately 2.17- (P<0.001), 2.61- (P<0.001), and 2.06-fold (P<0.001)higher, respectively, than those in control cells. Moreover,BEV decreased the expression of ZO-1 by approximately 72%at 72h, when compared with that of control group (P<0.001).Therefore, 72h was chosen to be the optimal time point and was employed in further experiments.
BEV Induced the Oxidative Stress and Reduced the TAC of ARPE-19 CellsThe expression levels of ROS and H2O2were used to evaluate the condition of oxidative stress in ARPE-19 cells (Figure 2). The results showed that there were low accumulation levels of ROS and H2O2, which exhibited hypofluorescence, in ARPE-19 cells. Whereas, the fluorescence intensities of ROS and H2O2significantly enhanced when the cells were treated BEV. Besides, the TAC decreased by approximately 77% (P<0.001) in the BEV-treated cells when compared with the control group (Figure 3). Thus, these results indicated that BEV enhanced the level of oxidative stress and decreased the TAC in ARPE-19 cells.
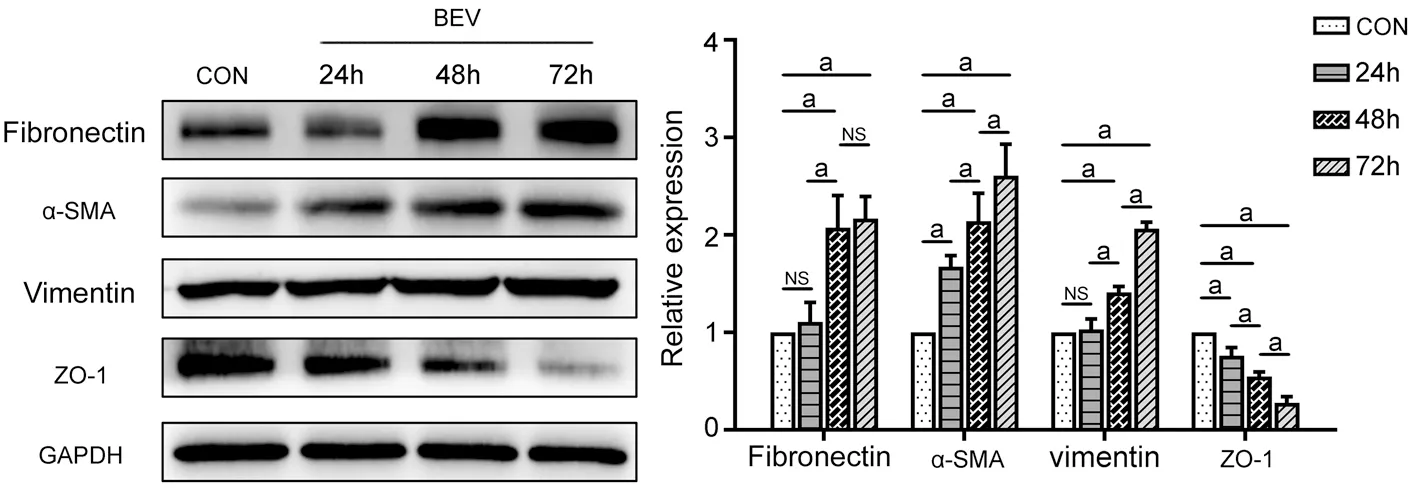
Figure 1 BEV promoted the EMT of ARPE-19 cells ARPE-19 cells were treated with BEV for 24, 48, and 72h, respectively. Western blotting indicated that EMT-related markers (fibronectin, α-SMA, vimentin, and ZO-1) changed in a time-dependent manner when the cells were treated with BEV. The most obvious changes in EMT-related markers occurred when the cells were treated with BEV for 72h, which exhibited the higher comparative expression levels of fibronectin, α-SMA and vimentin, as well as the lower expression of ZO-1. aP<0.05; NS: Not significant.
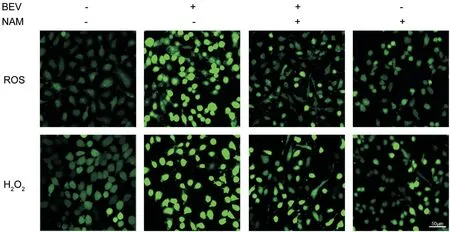
Figure 2 NAM attenuated BEV-induced oxidative stress in ARPE-19 cells BEV markedly increased the expression levels of ROS and H2O2 when compared with the control group, which exhibited hyperfluorescence in BEV-treated ARPE-19 cells. However, the accumulations of ROS and H2O2 were decreased when the cells were treated with BEV and NAM, which showed the hypofluorescence in ARPE-19 cells.
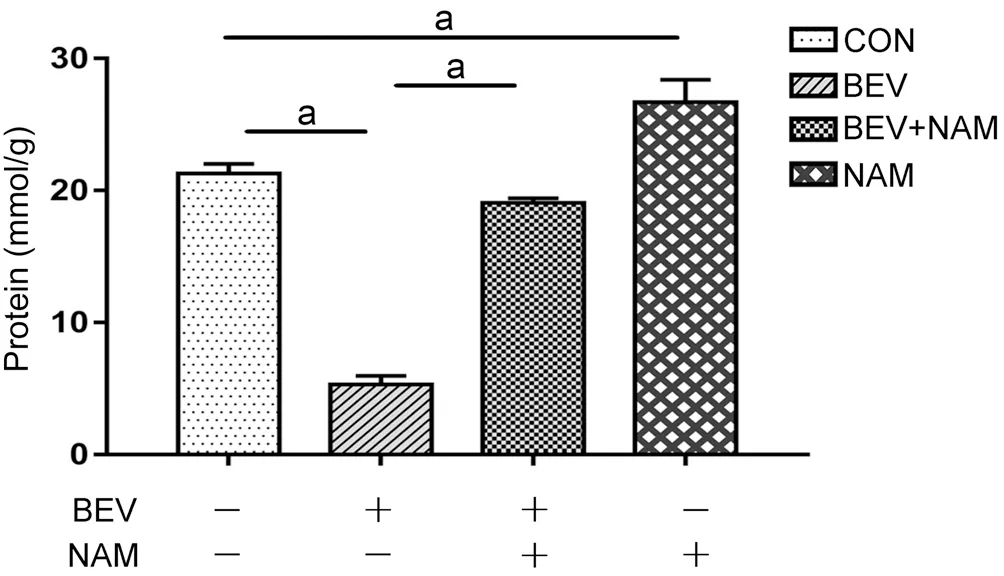
Figure 3 NAM enhanced the TAC of ARPE-19 Cells In the BEV treatment group, the TAC of ARPE-19 cells decreased by approximately 77% when compared with the control group. Whereas,NAM effectively reversed the impairment of cellular TAC caused by BEV, which showed the TAC of ARPE-19 cells increased by approximately 72% compared with the BEV treatment group. aP<0.05.

Figure 4 Reverse transcription quantitative PCR showed that NAM inhibited BEV-induced EMT in ARPE-19 cells In the BEV and NAM treatment group, the mRNA expression levels of fibronectin, α-SMA, and vimentin were lower than their levels in the BEV treatment group. Meanwhile, ZO-1 mRNA expression level increased in the BEV and NAM treatment group compared with the BEV treatment group. aP<0.05; NS:Not significant.

Figure 5 Western blotting indicated that NAM suppressed BEV-induced EMT of ARPE-19 cells The protein expression levels of fibronectin, α-SMA, and vimentin decreased, and ZO-1 protein expression levels increased in the BEV and NAM treatment group compared with those in the BEV treatment group. aP<0.05; NS: Not significant.
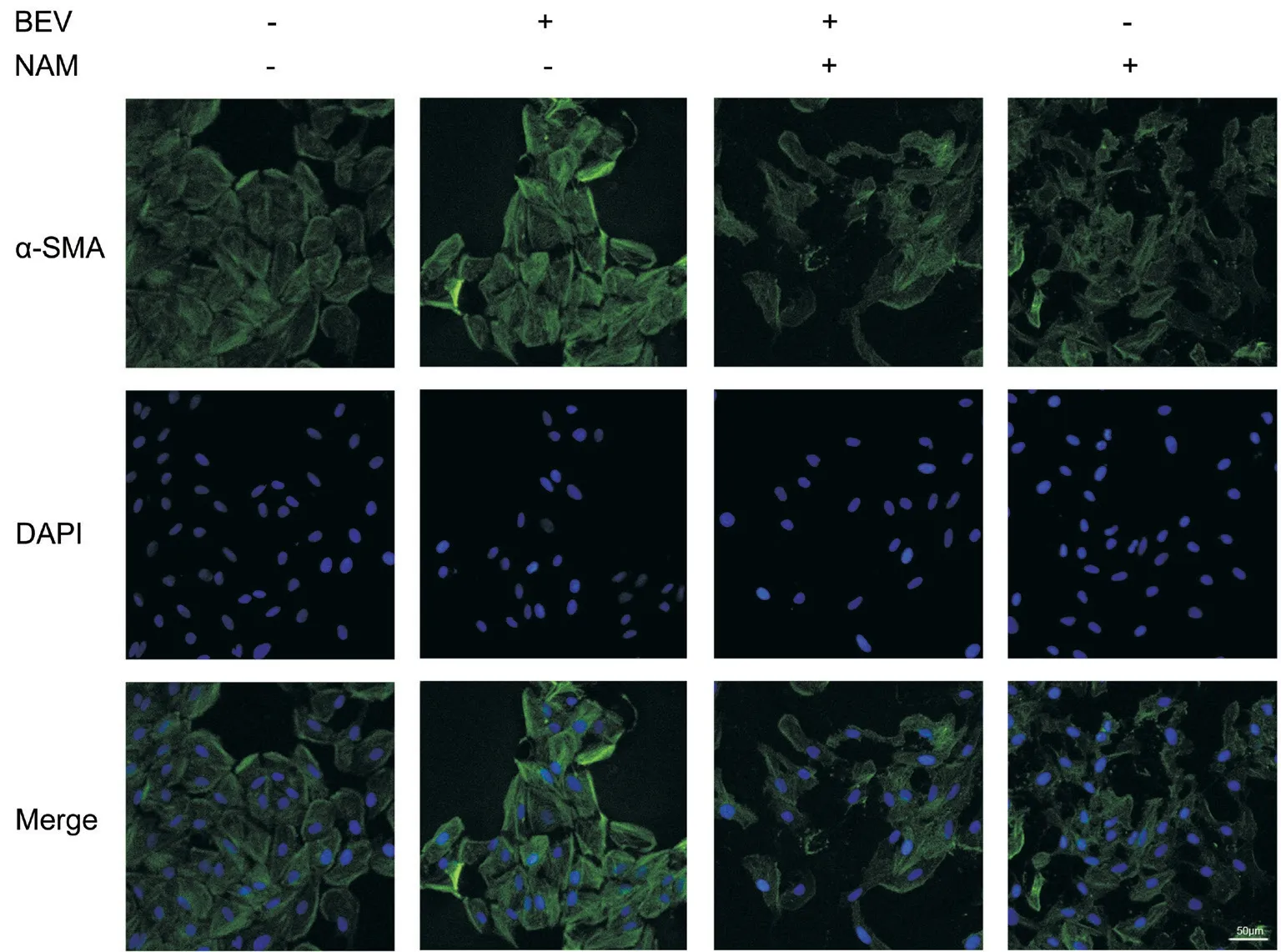
Figure 6 Immunofluorescent staining manifested that NAM weakened BEV-induced overexpression of α-SMA in ARPE-19 cells The high expression level of α-SMA was presented in BEV treatment group when compared with the control cells. Whereas this phenomenon was reversed in the BEV and NAM treatment group, which exhibited the hypofluorescence of α-SMA.
NAM Inhibited BEV-Induced EMT of ARPE-19 CellsReverse transcription quantitative PCR and Western blotting showed that the BEV-induced upregulation of fibronectin,α-SMA, and vimentin and downregulation of ZO-1 were reversed at both the mRNA (Figure 4) and protein (Figure 5) level by NAM treatment. In the BEV and NAM treatment group,the mRNA expression levels of fibronectin, α-SMA, and vimentin were approximately 35% (P<0.001), 74% (P<0.001),and 41% (P<0.001) lower, respectively, than their levels in the BEV treatment group. Meanwhile, ZO-1 mRNA expression level increased by approximately 56% in the BEV and NAM treatment group compared with the BEV treatment group(P<0.001). More obvious changes in EMT-related marker levels between the above two groups were observed by Western blotting. The protein expression levels of fibronectin, α-SMA,and vimentin decreased by approximately 80% (P<0.001), 68%(P<0.001), and 50% (P<0.001), respectively, and ZO-1 protein expression level increased approximately 60% (P=0.004) in the BEV and NAM treatment group compared with those in the BEV treatment group. The expression of α-SMA also was detected by immunofluorescent staining (Figure 6). The fluorescence intensity of α-SMA increased when the cells were treated with BEV compared with the control group. However,there was a decreased fluorescence intensity of α-SMA in the BEV and NAM treatment group, when compared with that of BEV treatment group. Additionally, when NAM was used in ARPE-19 cells alone, the mRNA expressions of α-SMA and vimentin decreased by approximately 52% (P<0.001)and 63% (P<0.001) respectively, and ZO-1 expression increased by about 73% (P<0.001) compared with control group (Figure 4). Besides, when compared with control group, the 40% reduction of fibronectin protein also reached significant difference (P<0.001) in NAM-treated cells, and the protein level of α-SMA also decreased by approximately 44%(P<0.001; Figure 5). Moreover, there was a slight reduction of fluorescence intensity of α-SMA in NAM-treated cells, when compared with control group (Figure 6). Hence, these results indicated that NAM protected ARPE-19 cells from BEVinduced EMT, and NAM also played the role in inhibiting the tendency of EMT in ARPE-19 cells.
NAM Attenuated Oxidative Stress and Enhanced the TAC of ARPE-19 CellsThe level of oxidative stress was evaluated by detecting ROS and H2O2levels in different groups (Figure 2). The results showed that BEV markedly increased the production of ROS and H2O2, which exhibited the hyperfluorescence in ARPE-19 cells. However, NAM effectively decreased the accumulations of ROS and H2O2caused by BEV, which showed the hypofluorescence in ARPE-19 cells. There was a slight difference of ROS and H2O2between NAM treatment group and control group. In addition, ABTS assay results showed that NAM effectively reversed the impairment of cellular TAC by BEV (Figure 3).In the BEV and NAM treatment group, the TAC of ARPE-19 cells increased by approximately 72% compared with the BEV treatment group (P<0.001). Besides, when compared with control group, the TAC increased by about 20% (P=0.001) in NAM-treated cells, which exhibited that NAM could enhance the internal TAC of ARPE-19 cells. Thus, these results showed that NAM had the powerful antioxidant ability to attenuate oxidative stress and increase TAC in BEV-treated ARPE-19 cells.
DISCUSSION
Anti-VEGF agents have revealed favorable curative effects in treating neovascular fundus diseases for more than a decade[1-5]. However, the emergence of neovascular fibrosis as a side-effect of anti-VEGF therapy has serious effects on the prognosis of patients[10-12,31]. At present, there are no specific drugs to suppress or treat the neovascular fibrosis. Therefore,it is particularly important to explore the mechanisms of neovascular fibrosis after anti-VEGF therapy. The application of anti-fibrotic agents may be effective in preventing anti-VEGF agent-induced neovascular fibrosis. In this study, we proved that NAM, as an antioxidant, could effectively suppress BEV-induced EMT of ARPE-19 cells, which might be realized by attenuating oxidative stress.
Our study focused on the effects of NAM in inhibiting BEVinduced EMT via ARPE-19 cells. The results showed that BEV effectively promoted the EMT of ARPE-19 cells, which manifested as higher expression levels of fibronectin, α-SMA,and vimentin and lower expression levels of ZO-1, at both the mRNA and protein levels. However, NAM reduced the high levels of these mesenchymal markers and increased the level of ZO-1. Therefore, it appears that NAM attenuated the BEV-induced EMT of ARPE-19 cells. NAM had been proved to have potential anti-fibrosis effects in other ocular cells. Li et al[32]had shown that NAM significantly inhibits the endothelial-mesenchymal transition of human corneal endothelial cells. Moreover, other disciplines also focus on the therapeutic effects or protective roles of NAM. For instance,in the field of dermatology, NAM not only has been accepted in prevent aging process, but also has been applied in the treatment of pellagra in clinical practice[33-34]. Besides, in the aspect of inhibiting fibrosis, such as liver fibrosis or renal interstitial fibrosis, the effect of NAM cannot be ignored from relevant experimental studies[20-22]. Thus, NAM may be an effective agent in the treatment of fibrosis-related disorders,including anti-VEGF agents-induced subretinal fibrosis.
A significant feature of NAM is antioxidation, which due to NAM is a part of the coenzyme NAM adenine dinucleotide(NADH/NAD+)[19,24]. NAM can resist the attack from ROS and then reduce the level of oxidative stress[22-23]. Our study indicated that BEV promoted the productions of ROS and H2O2and reduced the TAC in ARPE-19 cells. As an antioxidant, NAM effectively suppressed the level of oxidative stress by reducing the accumulations of ROS and H2O2and by increasing the TAC in ARPE-19 cells. Therefore, NAM effectively alleviated BEV-induced oxidative stress in ARPE-19 cells. Putting all the results together, our study demonstrated a close connection between oxidative stress and EMT. NAM not only suppressed BEV-induced EMT, but also attenuated the high-level of oxidative stress caused by BEV in ARPE-19 cells. The results of previous research further confirm the causal relationship between oxidative stress and the EMT of RPE cells, which illustrate the reliability of our results. For example, it has been reported that oxidative stress induces the dissociation of intercellular junctions in a diverse range of epithelial cells, especially RPE cells[35-36]. Due to their high consumption of oxygen, RPE cells are vulnerable to oxidative stress, thereby leading to the disruption of intercellular junctions[37]. Therefore, it was reasonable to believe that BEVinduced oxidative stress might result in the EMT of ARPE-19 cells and NAM could effectively reverse this phenomenon by suppressing oxidative stress.
It has been reported that there are two major sources of NAM in human body: endogenous synthesis and exogenous intake[34].The quantity of NAM synthesized by endogenous tryptophan is insufficient to satisfy the requirements of physiological functions. Therefore, diet or specific supplements, such as meats and nuts, are critical to provide additional NAM[19,34,38].Our study also revealed the beneficial effects of NAM in preventing the tendency of EMT in ARPE-19 cells by down regulating the expressions of fibronectin, α-SMA, and vimentin, as well as up regulating ZO-1 expression at mRNA or protein levels, and further increasing the internal TAC of ARPE-19 cells. In recognition of its importance, NAM has been included in the World Health Organization’s List of Essential Medicines[39]. Thus it can be seen that NAM has broad application prospect in the future due to its advantages of sufficient source, easy access, security and effectiveness. In especial, there is reason to believe that NAM has the potential to be used in attenuating neovascular fibrosis of the ocular fundus because of its beneficial effects in humans.
Although the function of NAM in attenuating BEV-induced EMT was verified in ARPE-19 cells, a limitation of this study was that relevant in vivo experiments were lacking.Further investigation involving animal experiments will more comprehensively demonstrate these effects of NAM.
In summary, this study demonstrated that NAM suppressed the BEV-induced EMT of ARPE-19 cells by attenuating oxidative stress. Thus, NAM might be a potential therapeutic agent to reduce subretinal fibrosis caused by anti-VEGF agents. The combined application of NAM and an anti-VEGF agent might improve the prognosis of patients with angiogenesis disorders of the ocular fundus.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81670828); the Shandong Provincial Key Research and Development Program(No.2017GSF18141); the Innovation Project of Shandong Academy of Medical Sciences and the National Science and Technology Major Project of China (No.2017ZX09304-010).Yang LL is partially supported by the Taishan Scholar Youth Professional Program (No.tspd20150215; No.tsqn20161059).
Conflicts of Interest:Zhou L,None;Shi DP,None;Chu WJ,None;Song S,None;Hao XH,None;Yang LL,None;Xu HF,None.
 International Journal of Ophthalmology2021年4期
International Journal of Ophthalmology2021年4期
- International Journal of Ophthalmology的其它文章
- Prevalence and risk factors of dry eye disease in young and middle-aged office employee: a Xi’an Study
- YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway
- Clinical features and treatment outcomes of intraocular lymphoma: a single-center experience in China
- Trends in research related to high myopia from 2010 to 2019: a bibliometric and knowledge mapping analysis
- A simple new technique for the induction of residual posterior vitreous cortex removal and membrane peeling
- Differential degeneration of rod/cone bipolar cells during retinal degeneration in Royal College of Surgeons rats
