Immunomodulatory Activities of Polysaccharides from Lobaria kurokawae Yoshim
SHEN Chao-qun, DONG Fang, CHEN Jian
(1.Guangdong Institute of Food Inspection (Guangdong Inspection Center of Wine and Spirits) Guangzhou 510165, China)(2.School of Food Science and Engineering, South China University of Technology, Guangzhou 510641, China)
Abstract: The aim of this study was to determine the immunomodulatory activities of the neutral polysaccharide LKY-I extracted from Lobaria kurokawae Yoshim. The polysaccharide LKY-I was isolated sequentially using ultrasonic-assisted water extraction, ethanol precipitation,diethylaminoethyl cellulose (DEAE-52) ion-exchange chromatography, and gel permeation chromatography. We investigated the immunomodulatory activities using different concentrations of LKY-I. The polysaccharide LKY-I at a concentration range of 31.25–500 µg/mL had no obvious in vitro toxic effects in macrophages (RAW 264.7). The phagocytic ability of macrophages treated with 500 μg/mL LKY-I was higher than that of the macrophages in the positive control group treated with lipopolysaccharide (LPS); absorbance, 1.39. In addition, LKY-I induced the production of nitric oxide (NO) up to 55.42 ± 1.79 μM. Compared with the control group, the LKY-I group showed an increase in the secretion of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) by 102.3% and 289.5%, respectively. Furthermore, LKY-I at a dose of 125–500 μg/mL showed a marked increase in the mRNA expression levels of inducible nitric oxide synthase (iNOS), TNF-α, and interleukin 6(IL-6). LKY-I in combination with LPS or concanavalin A (ConA) significantly promoted the proliferation of lymphocytes. Thus, our findings suggest that LKY-I has immunomodulatory effects and can be used as a health-promoting food supplement.
Key words: lobaria kurokawae yoshim; polysaccharides; immune response
Many fungi, including lichen-forming fungi, secrete active ingredients, especially polysaccharides, and they have gained much popularity because of their broad spectrum of biological and pharmacological activities,such as immunological activities[1], antitumor activity[2],hypoglycemic effects[3], and antioxidant activity[4].Polysaccharides have been thought to improve the immune system of the body by activating cells that promote the secretion of cytokines and antibodies. To date, some fungal polysaccharides such as lentinan fromLentinus edodes,schizophyllan fromSchizophyllum commune, and Krestin fromCoriolus versicolorare commercially available in Japan and Korea as anticancer or immunomodulatory agents[5,6]. Therefore, polysaccharides can be used as novel drugs or as health-promoting foods because of their favorable activities and relatively low toxic and side effects[7].Lobaria kurokawaeYoshim (LKY) belongs to theAscolichenes,Lecanorales,Stictaceae, andLobaria Hoffmgroups of lichen-forming fungi, which grow in the wild in Southeast China and are widely used as traditional herbal medicines and food. Previous studies have determined the extraction and antioxidant activity of polysaccharides,and to date, no studies have examined the immunomodulatory activity and the mechanism underlying the production of polysaccharides fromL. kurokawaeYoshim. Our results establish the potential ofL.kurokawaeYoshimas a health food and as a novel drug.To our knowledge, we have investigated for the first time the immunomodulatory of the polysaccharide obtained fromL. kurokawaeusing advanced technology and the murine macrophage cell line RAW 264.7. A previous study[8]has reported hot water extraction and subsequent purification of a polysaccharide fraction (LKY-I)obtained from the fruit body ofL. kurokawaeYoshim.
In a previous study, we optimized the process of extraction of the LKY polysaccharide and analyzed the structure of the LKY-I[8]. Our results showed that the weight-average relative molecular mass (Mw) of LKY-I was 6.16 × 104U and the polysaccharide content was 88.91%. The results of gas chromatography (GC) showed that LKY-I contains five types of monosaccharides,namely rhamnose (Rha), xylose (Xyl), mannose (Man),glucose (Glu), and galactose (Gal), with a molar ratio of 1.1:2.0:46.5:28.5:20.5, respectively. Results of Fourier-transform infrared (FT-IR) spectroscopy showed characteristic absorption peaks for LKY-I, and the monosaccharide residues were present in the form of pyran rings. The results of periodate oxidation, Smith degradation, and nuclear magnetic resonance (NMR)imaging showed 24.68% (1→6) and (1→) glycoside bonds in LKY-I; in addition, LKY-I had a D-glucopyranose ring containing mannose with α- and β-configurations. Here, we investigated the immunomodulatory activities of LKY-I to assess its potential application in the health food industry.
1 Materials and methods
1.1 Materials and reagents
The fruit bodies ofL. kurokawaeYoshim were purchased from a local market in the Yunnan Province,China, and were identified by Professor Di-Yong Wang,Department of Pharmaceutical Sciences, Guangdong Pharmaceutical University. The samples were thoroughly washed with tap water, dried at 45 °C, were ground into a fine powder with a mixer, and screened through a 60-mesh sieve. The powder obtained was stored in a dryer until use. The murine macrophage RAW 246.7 cell line was obtained from Sun Yat-sen University.Dulbecco’s modified Eagle’s medium (DMEM) medium,RPMI 1640 medium, penicillin, streptomycin, and phosphate-buffered saline (PBS, pH 7.4) were purchased from Gibco Life Technologies (St. Louis, MO, USA).Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biological Technology Co. Lipopolysaccharide(LPS), 3-(4,5-dimethylthiazol-z-yl)-2,5-diphenyltetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO)were purchased from Sigma-Aldrich Chemical Co. (St.Louis, MO, USA). Enzyme-linked immunosorbent assay(ELISA) kits for interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were purchased from Neobio Science Technology Co., Ltd. (Shenzhen, China). All other chemicals and reagents were of analytical grade.
1.2 Isolation and purity of LKY-I
LKY-I was extracted as follows[8]:L. kurokawaeYoshim was screened through a 60-mesh sieve; then,ultrasonic-assisted water extraction was performed,decolorization was performed using D354FD, and deproteinization was performed using the Sevag reagent;subsequently, we performed chromatography using the diethylaminoethyl (DEAE) cellulose-52 column (elution curve) and distilled water for elution of the polysaccaride.
LKY-I was eluted using the G-100 Sephadex column and was detected using the phenol-sulfuric acid method. Preliminary results obtained from the elution curve indicated that LKY-I was a homogeneous polysaccharide with a wide range of molecular weight distribution. The results are shown in, and the elution peaks were single symmetrical peaks. Results of gel permeation chromatography (GPC) indicated that LKY-I is a homogeneous polysaccharide.
1.3 Determination of the immunological activities of LKY-I
1.3.1 Cell culture
RAW 264.7 murine macrophages were cultured in DMEM supplemented with 10% inactivated FBS, 100U/mL penicillin, and 100 μg/mL streptomycin. The cells were placed in an incubator under an atmosphere of 5%CO2at 37 °C.
1.3.2 Cytotoxicity assay
The samples were treated using a modified version of the method[9]. RAW 264.7 cells (5 × 105cells/well)were cultured in 96-well microplates overnight and then were treated with 20 μL of serial concentrations of samples (31.25, 62.5, 125, 250, 500, or 1000 μg/mL) and LPS (1 μg/mL) for 24 h. An equal volume of the culture medium was used as a blank control. Subsequently, the cells were stained with 20 μL of MTT at a final concentration of 5 mg/mL for 4 h in the dark and then the medium was discarded. The formazan crystals formed in the cells were dissolved using 150 μL of DMSO. The absorbance was measured at 570 nm on a microplate reader (1420 Multilabel Counter Victor3; Perkin-Elmer,MA). The results were expressed as the ratio of absorbance values of the treatment and blank control.
1.3.3 Macrophage phagocytosis assay
The phagocytic ability of macrophages was determined by measuring the uptake of neutral red[10].Briefly, RAW 264.7 cells (5 × 105cells/well) were seeded on 96-well plates and were incubated for 24 h. The culture medium was discarded, and the cells were treated with various concentrations of the samples (31.25–500μg/mL) for 24 h. Then, the supernatant was removed, and 0.07% neutral red solution (150 μL/well) was added and incubated for 1 h. The supernatant was discarded, and the cells in 96-well plates were washed twice with PBS to remove the neutral red that was not phagocytosed by the RAW 264.7 cells. Then, we added the cell lysis buffer(1% glacial acetic acid: ethanol = 1:1, 150 μL/well) and incubated the cells at room temperature for 24 h.Subsequently, we measured the optical density of each well at 540 nm using a microplate reader. The RPMI 1640 medium and LPS (1 μg/mL) were used as the blank and positive control, respectively.
1.3.4 Production of nitric oxide
Nitric oxide (NO) production was detected using the Griess method with some modifications[11]. RAW 264.7 cells (5 × 105cells/well) were seeded on 96-well plates and were incubated for 24 h. Then, the cells were stimulated with the culture medium (for the control group), LPS (1 μg/mL), and various concentrations of samples from 31.25–500 μg/mL for 24 h. An equal volume of the Griess reagent was added to the cell supernatants, and the absorbance was measured at 540 nm using a microplate reader.
1.3.5 Quantitative analysis of cytokines
Quantitative analysis of cytokines was performed according to a previously described method[12]. The production of IL-6 and TNF-α was determined using a commercially available ELISA kit.
1.3.6 Reverse transcription-polymerase chain reaction
The mRNA expression levels were examined according to a previously described method[13]. To measure the levels of inducible nitric oxide synthase(iNOS) and cytokines, we treated the RAW 264.7 cells (1mL, 1 × 106cells/well) with 1 mL of LPS (1 μg/mL) or samples at concentrations of 125, 250, or 500 μg/mL, and then were incubated at 37 ℃ in the presence of 5% CO2for 18 h. After incubation, RNA was extracted from the cells using the TRIzol reagent (Invitrogen, Carlsbad, CA,USA). The concentration of RNA was determined using a spectrophotometer before constructing cDNA with an oligo-(dT)20primer and Superscript III RT (Invitrogen,Carlsbad, CA, USA). The resulting cDNA was amplified by PCR using GoTaq Flexi DNA Polymerase (Promega,Madison, WI, USA). Reverse transcription amplification was conducted with an initial denaturation (94℃ for 30 s), annealing (56 ℃ for 40 s), and extension (72℃ for 1 min) followed by a final extension step at 72 ℃ for 10 min. The products of reverse-transcription polymerase chain reaction (RT-PCR) were electrophoresed on 1%agarose gels and were visualized using ethidium bromide staining, followed by viewing the gels under UV transillumination (Kodak Digital Science, Kenne-saw,GA, USA). The results were expressed as the relative intensity compared to that of β-actin. The sequences of the primers used in this study were as follows:
iNOS, forward, 5′-CGGCAA ACATGACTTCAGG C-3′ and reverse, 5′-GCACATCAAAGCGGCCATAG-3′;IL-6, forward, 5′-TACTCGGCAAACCTAGTGCG-3′and reverse, 5′-GTGTCCCAACATTCATATTGTCAG T-3′; TNF-α, forward, 5′-ATGAGCACAGAAAGCATG ATC-3′ and reverse, 5′-TACAGGCTTGTCACTCGAA TT-3′. Reverse transcription amplification was performed with an initial denaturation at 94 °C for 3 min, 30 cycles of denaturation (94 °C for 30 s), annealing (56 °C for 40 s), extension (72 °C for 1 min), and a final extension step at 72 °C for 10 min. The PCR products were run on 1%agarose gels and were visualized using ethidium bromide staining.
1.3.7 Effects of LKY-I on the proliferation of splenic lymphocytes of mice
The proliferation of splenic lymphocytes was determined using the MTT assay[14,15]. The Kunming mice were killed by cervical dislocation; then, the spleens were aseptically separated and were washed three times using PBS buffer. The cell pellets were resuspended in RPMI 1640 medium, and the splenocytes (100 μL/well, 1× 106cells/mL) were seeded in 96-well plates in the presence of concanavalin A (ConA, 5 μg/mL) or LPS (1 μg/mL). Filter-sterilized samples of LKY-I (final concentrations of 0, 62.5, 125, 250, or 500 μg/mL) were added to the wells. LPS and ConA served as the negative control and the collaborative group, respectively. The cells were incubated at 37 °C in a humidified incubator with 5% CO2for 48 h; then, we added 20 μL of MTT (5 mg/mL) to each well and incubated for another 4 h.Subsequently, the cells were centrifuged at 3000 rpm for 5 min at 4 °C to separate the MTT, and the precipitate of the formazan crystals was solubilized in DMSO (150 μL/well). The proliferation of splenic lymphocytes was determined by measuring the optical density (OD) at 570 nm using a multifunctional microplate reader (Boteng,USA).
1.4 Statistical analysis
The results were expressed as the means ± standard error of mean (SEM). Statistical differences were calculated using Student’st-test, one-way analysis of variance (ANOVA), and Duncan’s multiple-range test.The criticalpvalue was set at 0.05; a probability value ofp< 0.05 was considered to be statistically significant.
2 Results and discussion
2.1 Homogeneity and the average molecular weight of LKY-I
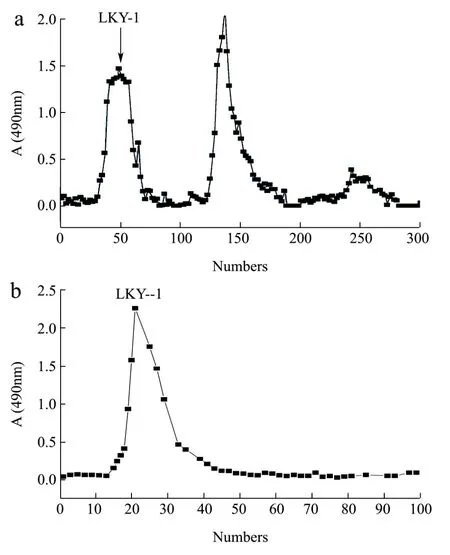
Fig.1 Elution curve of LKY-I obtained using diethylaminoethyl(DEAE)-52 column chromatography (a) and Sephadex G-100 column chromatography (b)
We obtained the crude polysaccharide fromL.kurokawaeYoshim after a series of processing steps,including hot-water extraction, ethanol precipitation, and deproteinization. The polysaccharide was purified using DEAE-52 (Fig. 1a[8]) and Sephadex G-100 column chromatography (Fig. 1b[8]), the results showed that LKY-I appeared as a single and symmetrical peak, which was detected using the phenol-sulfuric acid method.
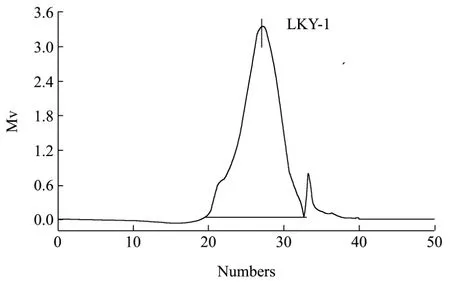
Fig.2 Results of gel permeation chromatography of LKY-I
Homogeneous polysaccharide, in this study, refers to a series of polysaccharide mixtures with uniform molecular weight distribution within a certain range.Results of GPC analysis indicated that LKY-I exhibited a single and symmetrical peak with an elution time of 26.55 min (Fig. 2[8]), which suggests the presence of a single type of polysaccharide in the sample. The calibration equation derived from linear regression indicated that the Mw was approximately 6.16 × 104u,the Mn was 2.91 × 104u, and the coefficient is 2.12,which implied that LKY-I was one of the homogeneous polysaccharides with a wide range of molecular weight distributions, according to the retention time.
2.2 Cytotoxic effects of LKY-I in RAW 264.7 cells
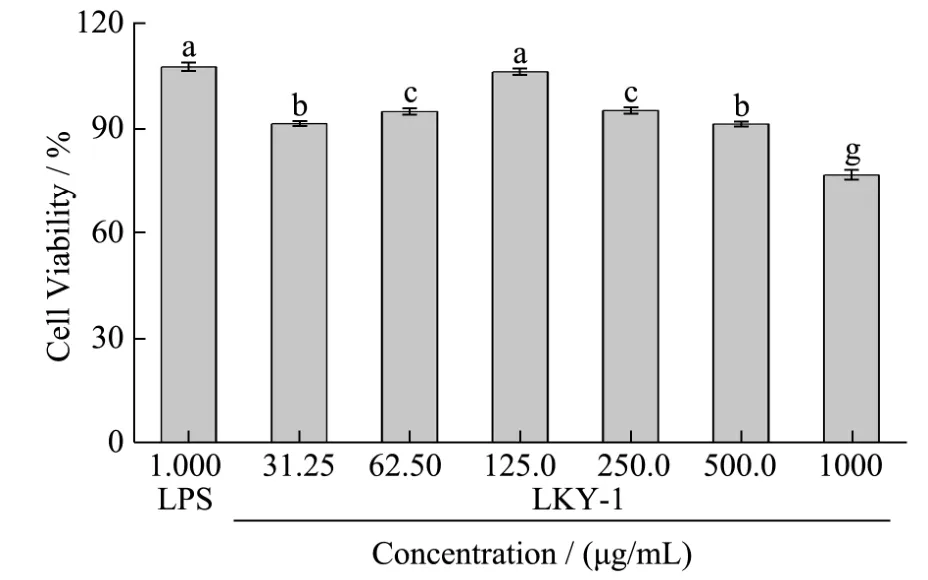
Fig.3 Effects of LKY-I on the viability of RAW 264.7 cells
Macrophages play an indispensable role in the innate and adaptive immune response to pathogens and in-tissue homeostasis[16]. RAW 264.7 cells are a macrophage-like cell line, and studies performed using this cell line may provide insights about the immunobiological activity of LKY-I. Therefore, we examined the immunomodulatory activity of LKY-I in RAW 264.7 cells. We determined the cytotoxic effects of LKY-I on RAW 264.7 cells by using MTT assay. Cells treated with 31.25, 62.5,125, 250, 500, or 1000 μg/mL of LKY-I for 24 h showed cell viability values of 91.23%,94.68%, 105.97%, 94.92%, 91.17%, and 76.59%,respectively (Fig. 3), which indicated that LKY-I at a concentration less than 500 μg/mL was not toxic to RAW 264.7 cells. However, LKY-I at a concentration greater than 1000 μg/mL showed marked cytotoxic effects.Therefore, we used LKY-I at a concentration of 31.25–500 μg/mL to determine phagocytosis and production of NO and cytokines in RAW 264.7 cells.
2.3 Effect of LKY-I on the phagocytic activity of RAW 264.7 cells
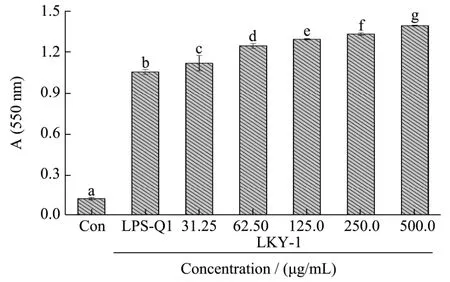
Fig.4 Effects of LKY-I on phagocytosis of murine peritoneal macrophages
Macrophage activation is one of the most important events in the immune response, and it indicates the upregulation of the innate immune response[17]. One of the most distinguished features of macrophage activation is an increase in phagocytosis, including pinocytic and phagocytic activity, and is determined in vitro by measuring the cellular update of dyes such as neutral red and malachite green[18]. We used the neutral red assay to determine the phagocytic activity of RAW 264.7 cells.The absorbance increased in a dose-dependent manner with increasing concentrations of LKY-I, which indicated that LKY-I increased the phagocytic activity of RAW 264.7 cells (Fig. 4). Compared with the negative control and positive control (LPS, 1 μg/mL), LKY-I significantly increased phagocytosis. Compared to the positive control group, the LKY-I group treated at a dose range of 31.25–500 μg/mL showed a significant increase in phagocytosis (p< 0.01). The absorbance value of cells treated with LKY-I at a concentration of 500 μg/mL (1.39)was higher than that of the positive control group (LPS group, 1.05) (Fig. 4). Our results showed that LKY-I effectively increases the phagocytic ability of the macrophages.
Macrophages can phagocytose aging bacteria,damaged cells, and necrotic tissues invading the body.Phagocytic capacity is one of the most important indicators of the non-specific immune system of the body[19]. Our results showed that LKY-I significantly increased the phagocytic ability of the macrophages,which may be due to the binding of the polysaccharide with a specific receptor on the surface of macrophages[20–22]. The bioactivities of polysaccharides were mostly dependent on their chemical structure, and the polysaccharides containing mannose and fucose residues bound to mannose receptors to mediate the phagocytosis of macrophages. A previous study[8]showed that LKY-I contains five types of monosaccharides,namely Rha, Xyl, Man, Glu, and Gal, with a molar ratio of 1.1:2.0:46.5:28.5:20.5, respectively. Therefore, the presence of mannose in LKY-I may play a role in increasing the phagocytosis of the macrophages.
2.4 Measurement of NO production from macrophages
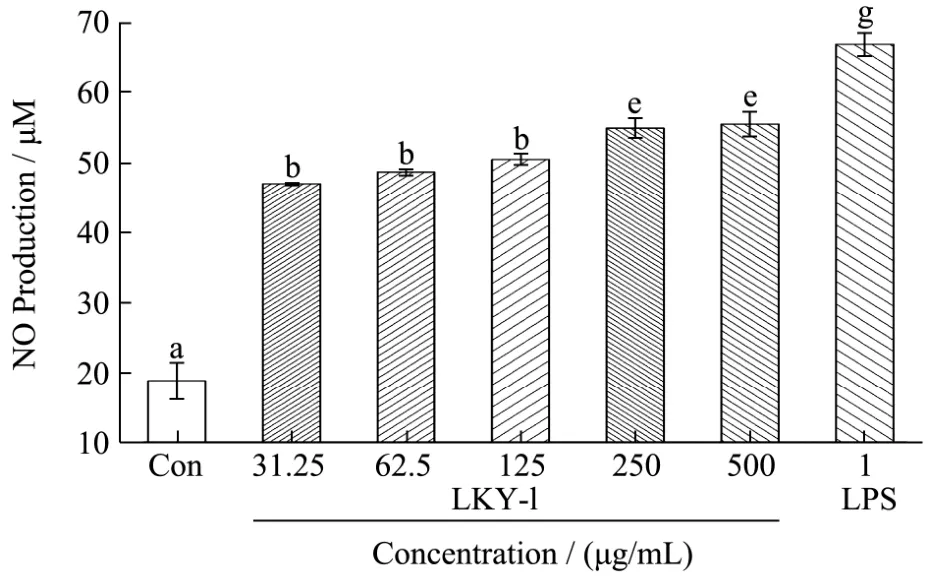
Fig.5 Nitric oxide (NO) release from mouse macrophages stimulated by LKY-I
NO is an important signaling molecule governs a diverse range of physiological processes[23]. NO is a non-specific molecule secreted by activated macrophages that can kill or inhibit the growth of various pathogenic microorganisms. The production of NO is closely related to inflammation. We determined the nitrite concentrations in the supernatant of polysaccharide-stimulated macrophages as an indicator of NO production. To investigate the macrophage-stimulating activities of the exopolysaccharides, we examined the effect of LKY-I obtained from gel filtration on the release of NO from mouse macrophages, by measuring the level of its stable breakdown product nitrite. The tests were performed in triplicate, and the results were presented as the mean values (Fig. 5). The positive control, LPS at a concentration of 1 μg/mL, had a strong effect on stimulating NO production in macrophages. Compared to the control, LKY-I induced a dose-dependent increase in the production of NO (p< 0.01). LKY-I at a concentration of 500 μg/mL had the highest effect on the production of NO; the concentration of NO was 55.42 ±1.79 μM. A significant difference was not observed in the NO production between the groups treated with 250μg/mL (54.88 ± 1.47 μM) and 500 µg/mL of LKY-I. NO is one of the most important mediators of the regulation of immunologic functions. Our results confirmed that LKY-I acts as stimulator of NO release in the macrophages and plays a role in activating the macrophages. Further, we examined whether the LKY-I-induced production of NO in RAW 264.7 cells was because of the increase in the mRNA expression levels of iNOS.
2.5 Effects of LKY-I on the secretion of TNF-α and IL-6
Macrophages are important delivery cells that can defend against the invasion of pathogens and improve the immune capabilities by secreting inflammatory mediators[24]. Cytokines such as TNF-α and IL-6 play important roles in the signal transduction in the immune process[25]. We investigated the effect of LKY-I on the production of TNF-α and IL-6 from RAW 264.7 macrophages (Fig. 6A-B). Our results indicate that compared to the normal control group, the group treated with 31.25, 62.5, 125, 250, or 500 μg/mL of LKY-I showed a significant increase in the production of cytokines (p< 0.01). LKY-I at a concentration of 500µg/mL showed a marked increase in the production of TNF-α and IL-6 by 102.3% and 289.5%, respectively,compared to that using the positive control using LPS.The secretion of these cytokines was increased by 92.7%and 235.9%, respectively, in the cells treated with 1 μg/mL LPS. Our results showed that LKY-I induced a dose-dependent increase in the secretion of cytokines IL-6 and TNF-α in RAW 264.7 cells, and thus, LKY-I has marked immunomodulatory activities.
Macrophage activation by polysaccharides is thought to be mediated primarily through the recognition of polysaccharides by specific receptors. These receptors,known as pattern recognition molecules, play important roles in recognizing pathogen-associated molecular patterns[26]. Therefore, further studies are necessary to determine the immune receptors ofL. kurokawaeYoshim polysaccharides to understand the mechanism underlying the immunomodulatory properties of these polysaccharides.
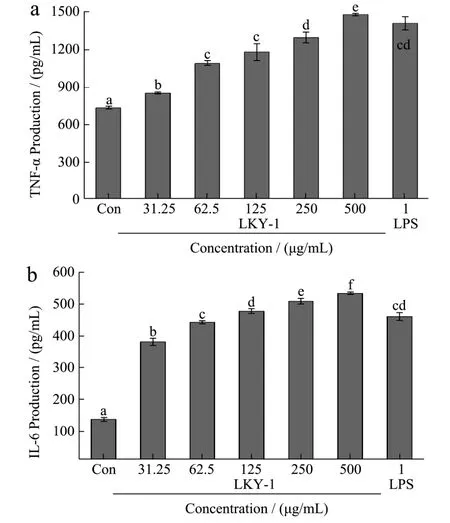
Fig.6 Effects of LKY-I on the production of tumor necrosis factor α (TNF-α) (A) and interleukin 6 (IL-6) (B) in RAW 264.7 cells
2.6 Effects of LKY-I on the mRNA expression levels of iNOS, TNF-α, and IL-6 in RAW 264.7 cells
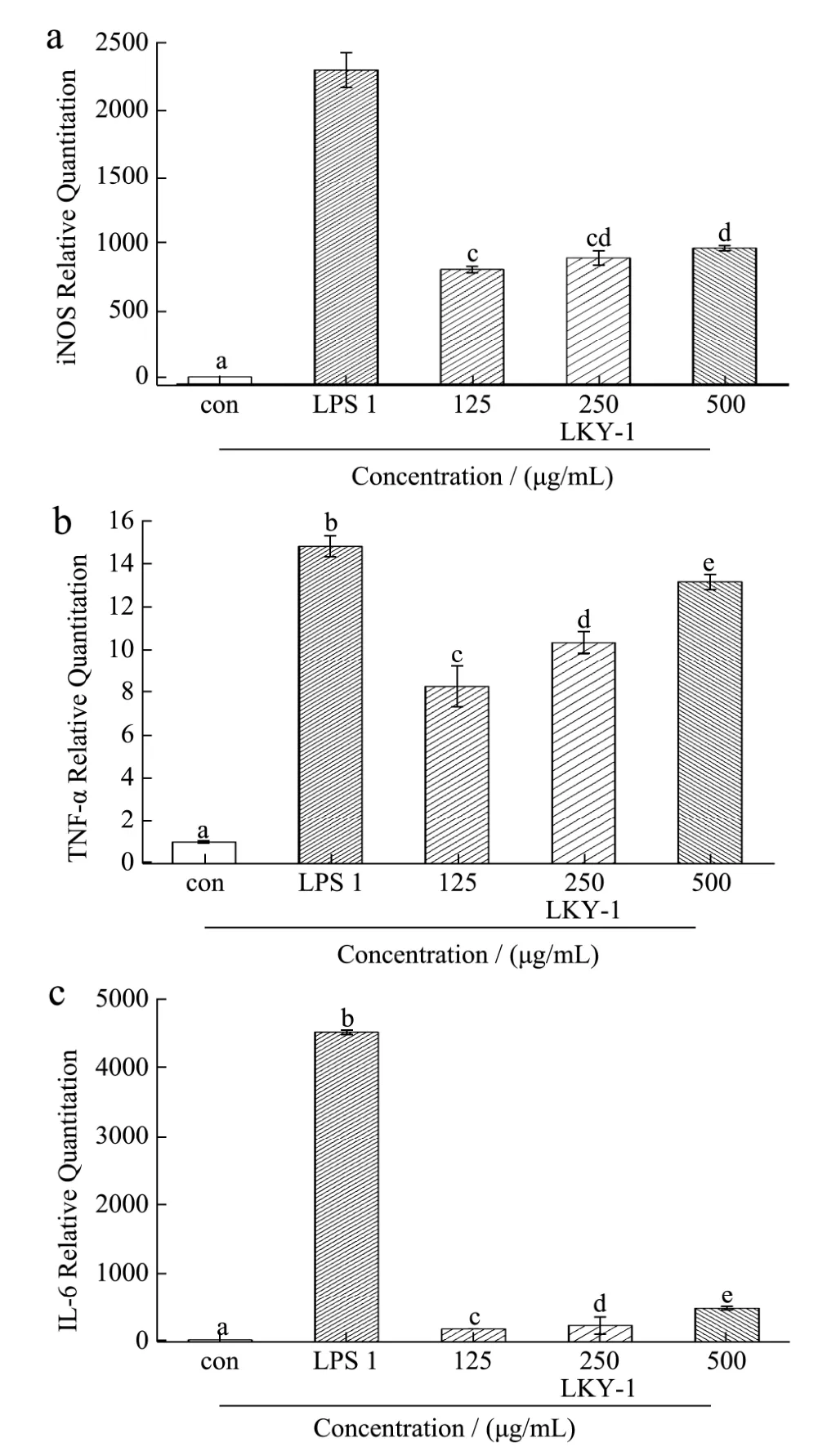
Fig.7 The mRNA expression levels of inducible nitric oxide synthase (iNOS) (A), tumor necrosis factor α (TNF-α) (B), and interleukin 6 (IL-6) (C) in RAW 264.7cells treated with LKY-I
iNOS is a significant isoform of the enzyme NOS,which induces the synthesis of NO. In addition,polysaccharides promote the production of NO from RAW 264.7 cells by upregulating the expression of iNOS mRNA[27]. Thus, we investigated the mechanism underlying the upregulation of NO production by examining the mRNA expression of iNOS in RAW 264.7 cells treated with polysaccharides. The expression of mRNA in the blank control group was set as 1 unit, and the quantities of mRNA relative to those in the sample group are shown in Fig. 7 (A). Results of RT-PCR analysis showed that the mRNA expression level of iNOS was higher in the group treated with 125–500 μg/mL of LKY-I than in the normal control group;however, the increase in the mRNA expression was not dose dependent. The mRNA expression levels relative to the control group in the cells treated with different concentrations of LKY-I were 809, 895, and 968 units.Furthermore, LPS (1 μg/mL) markedly upregulated the expression of iNOS mRNA (2,297 units). Our results indicated that possible mechanism underlying the upregulation of NO production in RAW 264.7 cells could be increased protein and mRNA expressions of iNOS.
We observed an increase in the mRNA expression level of TNF-α Fig. 7(B). Compared to the normal control group, the groups treated with various doses of LKY-I (125, 250, or 500 μg/mL) and LPS (1 μg/mL) for 24 h showed a significant increase in the mRNA expression levels of TNF-α(p< 0.01); meanwhile, the expression levels were similar between the control and LPS treatments (1 μg/mL), when the concentration of LKY-I polysaccharide was 500 μg/mL. Our results indicated that LKY-I could increase TNF-α production by upregulating theTNF-αgene expression. Since gene expressions are regulated by mediators, our results indicate that LKY-I increased the TNF-α level by a mechanism involving transcriptional regulators.
RAW 264.7 cells treated with LKY-I at concentrations of 125–500 μg/mL showed a similar increase in IL-6 mRNA as that observed with iNOS and TNF-α mRNA. Compared to the blank control group, the LKY-I-treated group induced a marked increase in the mRNA expression levels of IL-6 (Fig. 7C). RAW 264.7 cells treated with different concentrations of LKY-I showed relative IL-6 mRNA expression levels of 160, 213, and 465 units. However, LPS (1 μg/mL) had the highest effect on the expression of theIL-6gene, and relative expression mRNA expression level was 4,509 units. Our results showed that LKY-I induced a considerable increase in the mRNA expression levels of IL-6, which suggests that polysaccharides fromL. kurokawaeYoshim might enhance cellular immune activity. Further studies are required to understand the role of polysaccharides in immunoregulation.
2.7 Effects of LKY-I on the proliferation of splenic lymphocytes
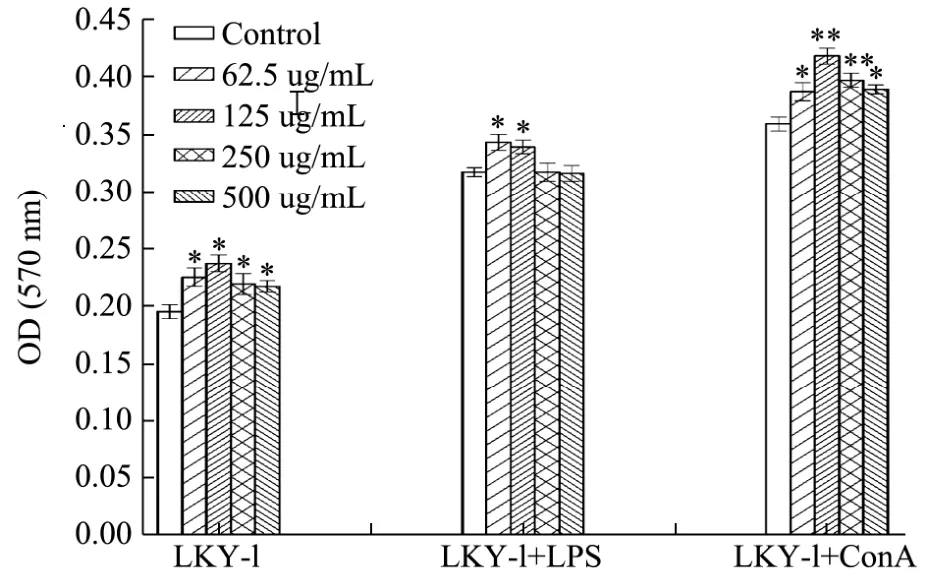
Fig.8 Effects of LKY-I on the proliferation of mouse spleen lymphocytes in vitro
The spleen plays a role in innate and adaptive immunity, and it has become the most important organ for antibacterial and antifungal immune reactivity.Therefore, the spleen is an ideal tissue in immune research[28]. The proliferation of splenocytes is an indicator of immune activation, which is related to the improvement in the immune function of T-lymphocytes or B-lymphocytes[29]. We evaluated the immunomo-dulatory activities of LKY-I by investigating its effect on the proliferation of splenocytes (Fig. 6). Results of the MTT assay showed that addition of different concentrations of LKY-I to the spleen cells significantly increased the OD value at 570 nm (p< 0.05), which indicated that compared to the control group, the LKY-I group significantly increased the proliferation of splenocytes although not in a dose-dependent manner. In particular, LKY-I in combination with LPS had a greater effect on the proliferation of splenocytes than LKY-I in combination with ConA. Compared to the LPS (1 μg/mL)group, the LKY-I + LPS group showed an increase in the OD value, which indicated that LKY-I could promote B-lymphocyte proliferation induced by LPS. LKY-I at a concentration of 62.5 and 125 μg/mL showed a significant increase in the proliferation of splenocytes (p< 0.05). However, LKY-I at a concentration greater than 250 μg/mL showed a decrease or inhibition of the proliferation of splenocytes. Treatment with the high concentration of LKY-I + LPS inhibited the proliferation of lymphocytes and showed that the polysaccharide had the function of bidirectional regulation. Compared to the ConA (5 μg/mL) control group, the LKY-I (125 or 250 μg/mL) and ConA group showed a significant increase in the proliferation (p< 0.01); in particular, the synergistic effect was the strongest when the concentration was of LKY-I was 125 μg/mL. Our results showed that LKY-I increased the activation of T and B cells. Therefore, it could be concluded that the LKY-I polysaccharide could activate T-lymphocytes and B-lymphocytes to produce cytokines and induce the proliferation of T and B cells to improve the immune system. Consequently, LKY-I showed significant immunostimulatory effects on mouse spleen lymphocyte proliferation compared to that observed in the control group. The bioactivity of the polysaccharide closely depends on its water solubility and conformation in solution. A previous study[8]showed 24.68% (1→6) and (1→) glycoside bonds in LKY-I;additionally, LKY-I had a D-glucopyranose ring containing mannose with α- and β-configurations.Therefore, the exposure of hydroxyl groups in LKY-I could improve the interaction between the polysaccharide and specific receptors and stimulate the immunostimulatory activity. This immunostimulatory activity of theL. kurokawaeYoshim polysaccharides could be used clinically for the modulation of immune response. Further studies are required to determine the mechanisms and structure-activity relationship of these polysaccharides.
3 Conclusion
We extracted polysaccharides fromL. kurokawaeYoshimusing hot water, which were then fractionated using DEAE cellulose-52 column chromatography to obtain the eluent LKY-I, which was eluted by distilled water. Our results indicated that the novel polysaccharide LKY-I significantly increased the phagocytic ability of macrophages and induced a dose-dependent increase in the production of NO, TNF-α, and IL-6in vitro.Furthermore, LKY-I significantly increased the mRNA expression levels of iNOS, TNF-α, and IL-6. In addition,the combination of LKY-I with LPS and ConA significantly increased the proliferation of splenic lymphocytes. Thus, our findings suggest that the polysaccharides isolated by hot-extraction in our study have immunomodulatory effects on the macrophages and lymphocytes, and these effects may be related to the purity and structural characteristics of the polysaccharides; the LKY-I consists of (1→6)-β-D glucan in a molecular weight range. Therefore, the potential behavior of the LKY-I as a biological response modifier implies that its potent biological activities such as antitumor activity might be due to the stimulation of the host immune system. Identifying new bioactive compounds that can stimulate the immune function has become an important area in immunopharmacological studies. Further studies are required to understand the role of the active polysaccharide LKY-I in immune regulation.Our results indicate the possible applications of these polysaccharides as medicinal, pharmacological, and functional food ingredients. Further studies concerning the structure and bioactivities of the polysaccharide LKY-I are currently underway to determine the correlation between the molecular structure and biological activities.

