Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
size distributions☆
Kailiang Zeng 1,2,Jie Zhou 1,2,Zhaoliang Cui1,2,*,Yue Zhou 1,2,Chuan Shi1,2,Xiaozu Wang 1,Liyue Zhou 3,Xiaobin Ding 3,Zhaohui Wang 1,2,*,Enrico Drioli4
1 State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 National Engineering Research Center for Special Separation Membrane,Nanjing Tech University,Nanjing 210009,China
3 Nanjing Jiuying Membrane Technologies Co.,Ltd.,Nanjing 211899,China
4 Research Institute on Membrane Technology,ITM-CNR,Via Pietro Bucci 17/C,Rende 87036,Italy
1.Introduction
Currently,environmental pollutions,such as disposal of hazardous and municipal waste,emission of industrial and municipal wastewater,have polluted most of surface and ground waters in China.Demand for clean freshwater is rising due to the increasing population,rapidly developing economy and accelerated urbanization.Therefore,the current water crisis requires sustainable recycling utilization of water resources[1-3].Micro filtration(MF)and ultra filtration(UF)with PVDF hollow fiber membranes are increasingly applied in drinking water treatment,wastewater reclamation and pre-treatment for reverse osmosis(RO)owing to their advantages of high separation efficiency,high water products,low capital cost and small footprint[4-12].However,membrane fouling remarkably deteriorates their permeability and consequently increases the operation costs[13],limiting the application wideness of membrane technology.Alleviating membrane fouling is therefore the most important target to achieve optimum operations.
Many researchers have studied the fouling mechanisms[14-16].Membrane fouling is affected by three major factors such as membrane material properties,feed characteristics and operating conditions[14].When using membrane to deal with municipal sewage and industrial wastewater,organic matters,such as proteins,polysaccharides,or humic acids,are the major foulants.It is of note that polysaccharides were major foulants in some real feed streams such as in sugar beet or sugar cane processing,in papermaking wastewater retreatment and in medical application[17].The fouling behavior of different membranes caused by polysaccharides became complicated due to their chain-like structure.Membrane material properties,such as pore size,surface hydrophobicity and roughness in fluence membrane fouling to some extent.It is generally considered that hydrophobic membrane is more subject to fouling compared to hydrophilic one[14].But the effects of the surface properties are not significant compared with the pore size according to Kotaku's[18]research.It was concluded that the degree of fouling mitigated as poly(vinylidene fluoride)(PVDF)membrane pore size increased from 0.02 μm to 0.4 μm by comparing the rate of pressure increase.In contrast,Qu[4]reported that a membrane with loose pores showed rapid flux decline resulting from the reduction of pore size and formation of cake layer.Lin et al.[19]have investigated the membrane fouling by dissolved organic matter.The result revealed that fouling of the membrane with larger pores was more serious due to pore blocking.Jin[20]observed that ceramic membrane,with the smallest pore size of 80 nm,experienced least fouling with increase of trans membrane pressure(TMP).These inconsistent findings are related to the usage of different membrane materials and properties of feed streams.The synergistic effect of pore size and pore size distribution on fouling behavior of membrane is still not clear.Many studies explained that membrane fouling is the result of pore blocking,but cannot provide accurate value of membrane pore size fouled by organic matters.It is necessary to investigate the effect of pore size and pore size distribution on fouling behavior and the fouling propensity.
Membrane fouling has been attributed to adsorption,concentration polarization,gel layer formation as well as membrane pore blocking caused by organic matters[21].The four types of fouling pattern result in responding filtration resistance.The fouling occurred on membrane surface can basically be removed by combination of relaxation and backwashing,while,internal fouling caused by pore blocking and adsorption,is hardly removed by physical cleaning methods.Therefore,it is useful to elaborate the divergence of fouling behavior for membrane with different pore sizes if different types of fouling resistance can be calculated.In the actual filtration system,choosing an optimum permeate flux is very important to long-term running of filtration.Therefore,it is practical to determine the critical flux(which means no fouling occurred below these flux values),threshold flux and limiting flux in order to optimize the procedure of filter operation and cleaning process[22-24].
In this study,four types of PVDF hollow fiber membrane,with different pore size distributions,were selected to investigate the fouling behavior.The role of pore size distribution on the flux decline and pore reduction was systematically investigated.The adsorption interactions between four types of membrane and dextran were investigated by the relative water flux reduction.The effect of molecular weight of dextran on the flux decline and inner pore blockage was identified.Filtration apparatus equipped with four UF membranes was used to realize TMP stepping tests,in order to determine the critical flux and limiting flux.To make clear the fouling characteristics of membranes with different pore size distributions, filtration resistance caused by four types of fouling was calculated.Pilot study for surface water treatment with two membrane modules was conducted to investigate the performance of anti-fouling property of the optimized PVDF membrane in realistic application.Trans membrane pressure was recorded during the longterm operation of the UF system when the in fluent surface water had frequent variations in temperature and turbidity.
2.Materials and Methods
2.1.Materials and chemicals
Commercial PVDF hollow fiber membranes were kindly supplied by Nanjing Jiuying Membrane Technology Co.,Ltd.(Nanjing,China).Ethanol(>99.7%)was purchased from Wuxi Yasheng Chemical Co.,Ltd.Isobutanol was purchased from Lingfeng Chemical Co.,Ltd.(Shanghai,China).Kerosene was purchased from Aladdin Co.,Ltd.(Shanghai,China)and used as a liquid for porosity measurement.Dextran(MW:40000,70000,100000,150000,500000)from Sigma Life Science was used to simulate foulants.Deionized(DI)water was made by a Pure Reverse Water System from Nanjing Tech University.
2.2.Membrane characterization
Overall porosity was determined by gravity method.First,the hollow fiber membranes were blocked off both ends and immersed in kerosene for 24 h to wet the membranes.The wetted membranes were weighed using a digital microbalance.Then,the wet membranes were dried in an oven at80°C until the weight of the dry membrane keptstable.The porosity of the hollow fiber membranes(ε)was calculated using the following equation[25]:

where wwis the mass of the wet membrane(g),wdis the mass of the dry membrane(g),ρpand ρkare densities of PVDF(1.78 g·cm-3),and kerosene(0.82 g·cm-3),respectively.
The membrane hydrophobicity was characterized by measuring water contact angle using Drop Meter A-100(MAIST Measurement Co.,Ltd.,China).DI water droplets with a diameter of 0.4 μl were dropped onto membrane surface at room temperature,while the images were captured by a digital camera allowing apparent static contact angles to be measured.Each sample was measured at five different positions to obtain the average.
The pore size distribution was measured by liquid-liquid displacement porometry(LLDP)from GaoQ Functional Materials Co.,Ltd.(Nanjing,China).Isobutanol-rich phase from the mixture of waterisobutanol(50/50,v/v)was employed as displacing liquid and the water-rich phase was used as wetting liquid phase.The interfacial tension of the two immiscible phases was 1.7×10-3N·m-1.By measuring the pressure and the flow through the membrane,the corresponding pore radius can be calculated using the Cantor equation[26].

where P is the pressure,γ is the inter facial tension,r is the pore radius and θ is the contact angle.The Hagen-Poiseuille equation[27]can be used to obtain a correlation between the fluid,Q,and the number of pores,n,of pore radius,r.

where η is the viscosity of the displacing liquid,L is the pore length and the pores were assumed to be cylindrical.
The morphologies of hollow fiber membrane were captured by field emission scanning electron microscopy(FESEM,Hitachi S4800,Japan).The dried membrane samples were broken in liquid nitrogen and then dried at 80°C in a vacuum oven for one day.The outer surface and cross-section of hollow fiber membranes were coated with gold using a HITACHI E-1010 Ion Sputtering device before taking SEM images.
2.3.Static adsorption and filtration experiments
For static adsorption experiments,dextran solution with different molecular weights(40000:70000:100000:150000:500000=1:1:1:1:1)was added to a cell and the outer surface of membrane was exposed to the solutions at the total concentration of 1.0 g·L-1for 24 h withoutany flux.The dextran solution was then removed and the membrane was rinsed for 2 min using DI water.Pure water permeate flux was measured before and after exposure.Membrane performance was evaluated by calculating the relative pure water flux reduction according to the following equation:

where RFR is the relative water flux reduction.J0and Jaare pure water permeate fluxes before and after exposure,respectively.
Membrane fouling experiments were conducted at constant TMP.The main phenomenon of fouling at constant TMP was flux decline caused by pore blocking or gel-layer forming.The decline rate and extent symbolized the fouling propensity.By filtrating dextran mixture solutions at a constant pressure of 0.1 MPa for 2 h,the constant TMP experiment was operated,using hollow fiber membrane module with an area of approximately 36.0 cm2.Fig.1 shows the simplified schematic of the cross- flow experimental set-up.The hollow fiber membrane was initially immersed in ethanol for one hour to remove residues and then flushed with DI water for one hour before the filtration process.The feed cross flow velocity was 0.82 m·s-1(feed flow rate=1.2 L·min-1,Re=2930)and the feed concentration was 1.0 g·L-1.Organic solute was dextran with the molecular weight range from 40000 to 500000(40000:70000:1000000:150000:500000=1:1:1:1:1)for Experiment I and single molecular weight like 40000,150000 or 500000 for Experiment II,respectively.The flux profiles with time were recorded gravimetric ally.

Fig.1.Schematic diagram of the fouling experimental apparatus(1-feed tank,2-septum pump,3-membrane module,4-permeate tank,5-pressure regulators,6-electromagnetic valve).
2.4.Critical flux and limiting flux tests
TMP stepping filtration was adopted to determine the critical and limiting fluxes,using the same set-up(as shown in Fig.1).Mixture solution of dextran with molecular weight of 40000,70000,100000,150000 and 500000(total concentration of 1.0 g·L-1)was used for experiments as feed solution.TMP was increased from 0.02 MPa with an interval of 0.005-0.010 MPa,and each TMP step lasted for 10 min.The permeate flux was continuously monitored,and the TMP increased until the flux did not increase with TMP.Then continued filtration experiment was conducted at the maximum TMP.Various studies have concluded that below the critical flux there is no TMP increase ordecline in flux with time,while limiting flux corresponds to the maximum values of flux when TPM increased[15].
2.5.Resistance calculations

where J is the filtration flux,ΔP is the TMP,μ is the solution viscosity,Rmis the intrinsic resistance of membrane and Rfis the fouling resistance.According to different types of membrane fouling caused by organics,Rfcan be divided into Ra,Rb,Rgand Rcwhich means adsorption,pore blocking,gel-layer and concentration polarization,respectively.
It's achievable to calculate fouling resistance by measuring pure water fluxes(PWFs)before and after adsorption,after ultra filtration and steady flux of filtration.PWF(J0)of the fresh membrane corresponds to the intrinsic resistance(Rm)according to Darcy's law;PWF(J1)after adsorption corresponds to the intrinsic and adsorption resistance;rinsing the membrane for 5.0 min and backwash it for 2.0 min at the pressure of 0.1 MPa after ultra filtration procedure,and the PWF was set as J2.The flux reduction from J1to J2can be attribute to pore blocking;the steady flux(J4)corresponds to the total filtration resistance,Rf; filtration was stopped for 1.0 min when permeate flux reached the steady state and then the filtration was restarted.The initial flux after restarted filtration was set as J3,and the increase of flux from J4to J3can be attribute to the elimination of concentration polarization.The different types of membrane fouling can be written as:
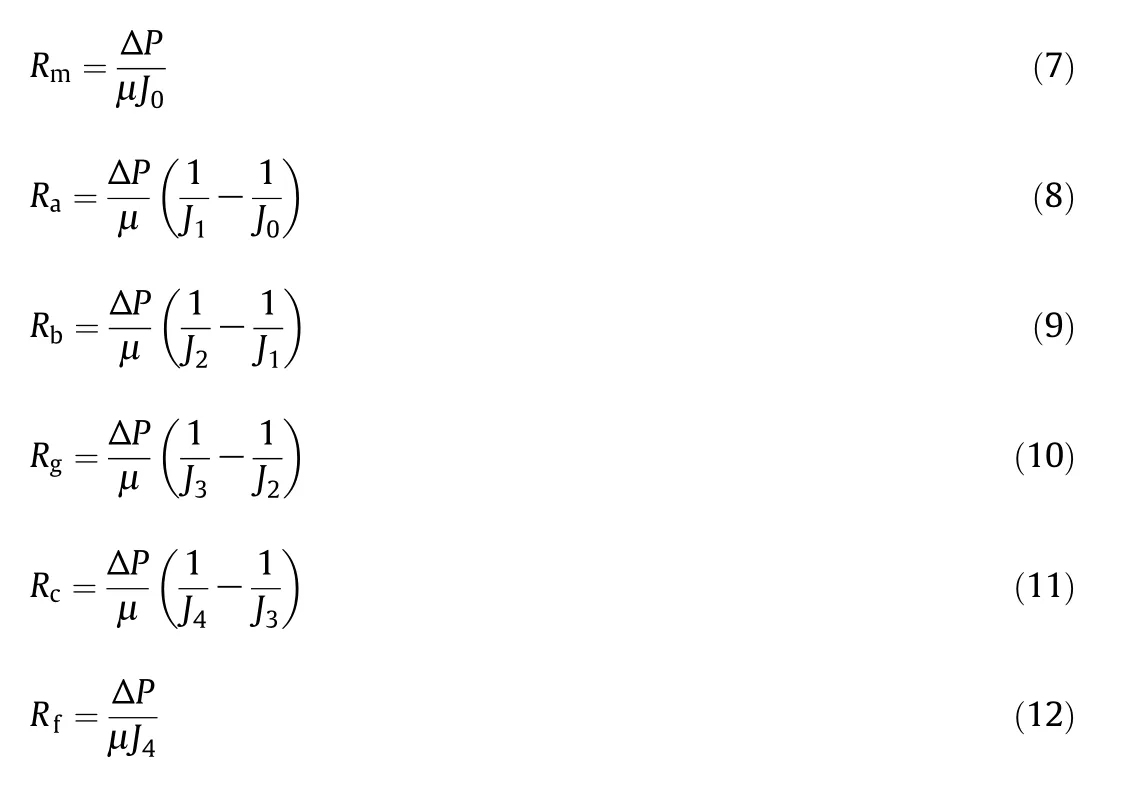
2.6.Pilot study for surface water treatment
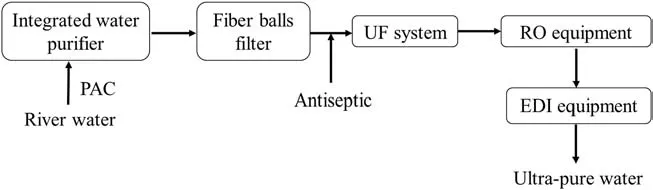
Fig.2.The process flow diagram of ultra-pure water preparation technology.
A pilot-scale setup was installed in a thermal power plant.The raw surface water was pumped from the Grand Canal at Changzhou,China.Ultra filtration was used in the process for ultra-pure water production as pre-treatment for reverse osmosis(RO)system.The process flow diagram of water treatment technology is shown in Fig.2.Two membrane modules,with an effective area of 50 m2and length of 1.8 m,were employed and denoted as M25 and M60,which referred to PVDF hollow fiber membranes with mean pore sizes of 25 and 60 nm,respectively.The diameter of module is 8 in.and the effective length of hollow fiber inside the module is 60 in.
The pilot experiment was conducted with dead-end filtration mode.Schematic diagram of the experimental apparatus is shown in Fig.3.Membrane fouling studies are often conducted at constant TMP in laboratory scale,while industrial operations usually performed at constant permeate flux in order to meet the actual demand for water products.The whole filtration cycle operation includes permeation,aeration,emission,backwash and rinse.The time setting of each operation and related parameters were listed in Table 1.The first 30 s during the process of backwash was chemical cleaning and the pH of backwash water was 2.6-3.0.Each module was initial kept at a constant membrane permeate flux of 40.5 LMH and then the target flux increased to 48 or 60 L·m-2·h-1if the TMP remains constant with the extension of filtration time.Cleaning technology included physical cleaning(aeration and backwash)and chemically enhanced backwash(CEB).Turbidity wasmeasured using a HACH 2100N Turbidimeter(USA).The profile of TMP- flux as a function oftime was monitored automatically to compare the fouling behavior between M25 and M60.

Fig.3.Schematic diagram of the experimental apparatus in membrane module ultra filtration(1-feed tank,2-pre- filter,3-membrane module,4-sewage tank,5-detergent,6-backwash tank,7-aeration).

Table 1 Operation parameters of pilot study
3.Results and Discussion
3.1.Characterization of the pristine PVDF membrane
Table 2 lists the basic parameters of the pristine PVDF membranes including mean pore size,pure water flux(PWF),overall porosity,and contact angle.M1,M2,M3 and M4,possessed mean pore sizes of 24,41,52 and 94 nm,respectively.The pure water flux increases with thepore size from M2 to M4.SEM images of the outer surface revealed the differences in surface morphologies and pore size according to Fig.4.The pore size distributions are shown in Fig.5.M1 presented the minimum pore size and M4 showed the maximum pore size.Compared with M3 and M4,M1 and M2 had smaller pore size and narrow pore size distribution.The contact angle ranged from 62.1°to 66.9°,indicating hydrophilic surface without obvious difference.
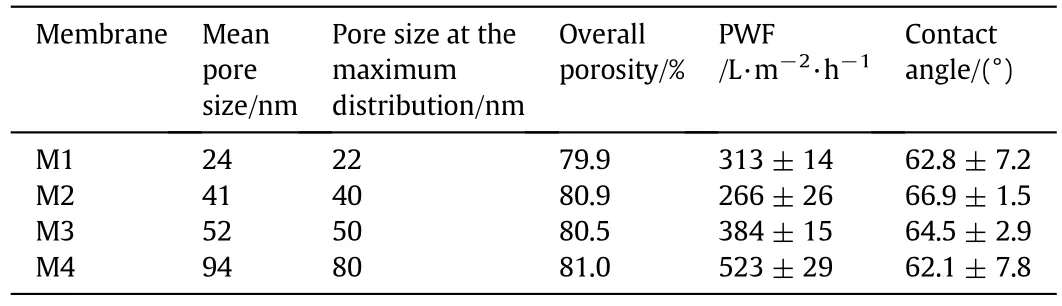
Table 2 Basic parameters of the pristine PVDF hollow fiber membranes
3.2.Effects of dextran fouling on membrane micro structure and permeability
Filtration experiment I was conducted at a constant pressure using four membranes to study membrane fouling behavior.A typical permeate flux evolution of the four membranes with time is presented in Fig.6(a).The initial fluxes were dependent on membrane pore size,and M4 had the highest initial flux due to its largest mean pore size.However,membranes with smaller pore size showed higher steady flux values.M1,with the smallest pore size,was the system that experienced the lowest fouling extent among the four membranes.The flux decline rate increased with the increase of membrane pore size from M1 to M4 according to Fig.6(b).
Both mean pore sizes and the maximum pore sizes decreased after fouling.From M1 to M4,the reductions of average pore size were 4,16,16,and 33 nm,respectively.The larger the pore size was,the higher the reduction of pore sizes fouled by dextran as presented in Fig.7.As can be seen from the reduction of pore size of M3 and M4,the membrane pore size distributions were narrowed due to the dextran absorption and pore blocking effects.Dextran entered into membrane pores during the filtration process,leading to the pore reduction.Larger membrane pores were more subject to decline compared with smaller pores from Fig.7.Membrane(M1)with smaller pore size experienced the lowest fouling extent and reduction of pores after the fouling by dextran,which indicated relatively minor contaminants into membrane pores.On the other hand,larger membrane pores were easier to be blocked,which contributed to the rapid decline of flux and higher fouling extent.
SEM images of the cross-sections and outer surface of the pristine and fouled membranes(gained after experiments I)are presented in Fig.8.As shown in Fig.8(b),there was no obvious difference between pristine and fouled membranes of M1,M2 and M3,while,largeramount of dextran entered into the inner pores of M4 and blocked its transition area of cross-sections.For the outer surface,compared with the pristine membrane(Fig.4),the fouled membranes were covered by dextran,but there was no obvious difference in surface morphology after ultra filtration experiments.It can be clearly seen that the deposited dextran foulants increased with the membrane pore size.Lots of deposited dextran adsorbed more dextran from solution and coalesced to form gellayer on the membrane surface with lager pore size.The transition area became compacted and considerable foulants deposited on the surface of M4 after ultra filtration filtration.It means that membranes with loosen surface pore structures suffered from more serious membrane fouling at both inside and outside of the surface.Both Figs.7 and 8,from different ways of characterization,indicate that membrane fouling inside the pores is more serious.
通过专家对指标体系进行重要性评估,获得判断矩阵,其中包含一维指标权重和二维指标权重两部分。专家确定第i个指标与第j个指标相对重要程度记为aij,则第j个指标与第i个指标相对重要程度记为≤aij≤9,数值越大说明i比j越重要。判断矩阵形成后需进行一致性检验,然后在一致性检验通过的基础上计算指标权数:

Fig.4.SEM images of outside of fresh membrane from M1-M4(20k×).
3.3.Membrane–solute interactions during filtration

Fig.5.Pore size distribution of the four pristine PVDF hollow fiber membranes.
Membrane-solute interaction was studied by exposing the membrane outer surface to dextran mixture solution for 24 h without pressure.The adsorption interactions can be expressed by the relative water flux reduction(RFR).As presented in Fig.9,the RFRs from M1 to M4 were 2.6%,4.9%,11.2%and 15.9%,respectively,indicating more dextran adsorption occurred on the membranes with larger pore size.The significant difference in RFR from M1 to M4 was caused by the membrane pore size,since the surface chemical properties for four membranes were similar.Solutes with higher molecular weight were excluded from smaller pores during adsorption process.Therefore,the accessible pore volume for adsorption of dextran on the membranes with smaller pore sizes was less than for larger pores.Gan[29]also observed that the solute adsorption occurs mainly in the larger pores,which is consistent with the current experiment results.There was a clear effect that more serious pore blocking occurs on the larger pores.
In order to obtain more comprehensive information on the membrane-solute interactions,ultra filtration experiments II were conducted using dextran solution with three molecular weights(40000:150000:500000=1:1:1).The purpose of high TMP was to accelerate the fouling rate to observe the flux decline.Membrane with smaller pores(M1)exhibited higher steady flux[Fig.10(a)].On the contrary,the membranes with larger pores exhibited more significant flux decline compared with smaller pores as shown in Fig.10(b).This is because larger pores were subject to be blocked as discussed above.The sharp decline of permeate flux resulted in compacted gel-layer and higher resistance when membrane with larger pore size was used.In addition,the steady flux was affected by the dextran molecular weight.The higher the dextran molecular weight was,the lower the steady flux presented.With the increase of molecular weight,the mass transfer coefficient became smaller and thus the gel-layer and concentration polarization resistances became more severe[14].
The pore size distributions of M1 and M4 fouled by dextran with different molecular weights are shown in Fig.11.The reduction extent of pore size after ultra filtration for M1 increased with the solute molecular weight.The result of pore reduction was in accordance with flux decline rate,which indicated that the permeate flux decline was largely caused by pore blocking.The opposite result of M4 compared to M1 in terms of the effect of molecular weight on the pore reduction can be attributed to the different ways of pore blocking.The locations on which pore reduction occurred were mainly nearby the pore entrances for M1 due to the reject effect of smaller pores on dextran.However,dextran entered into the inner pores and blocked the transition area of cross-sections(Fig.8)for M4.Therefore,low molecular weight(40000)of dextran brought more serious pore reduction because the poor effect of retention for low molecular weight.
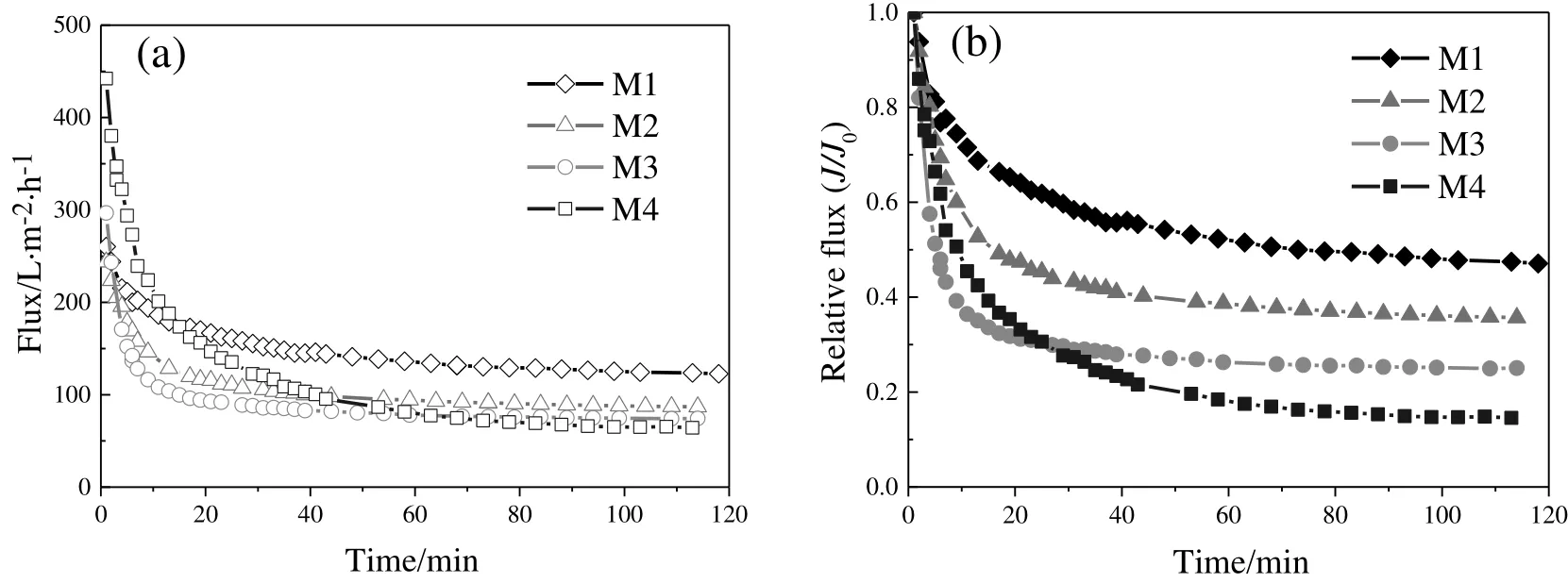
Fig.6.Flux profiles as a function of time for the four membranes.(a)Permeate flux,(b)relative flux;dextran mixture(40000:70000:100000:150000:500000=1:1:1:1:1).
3.4.Critical flux and limiting flux
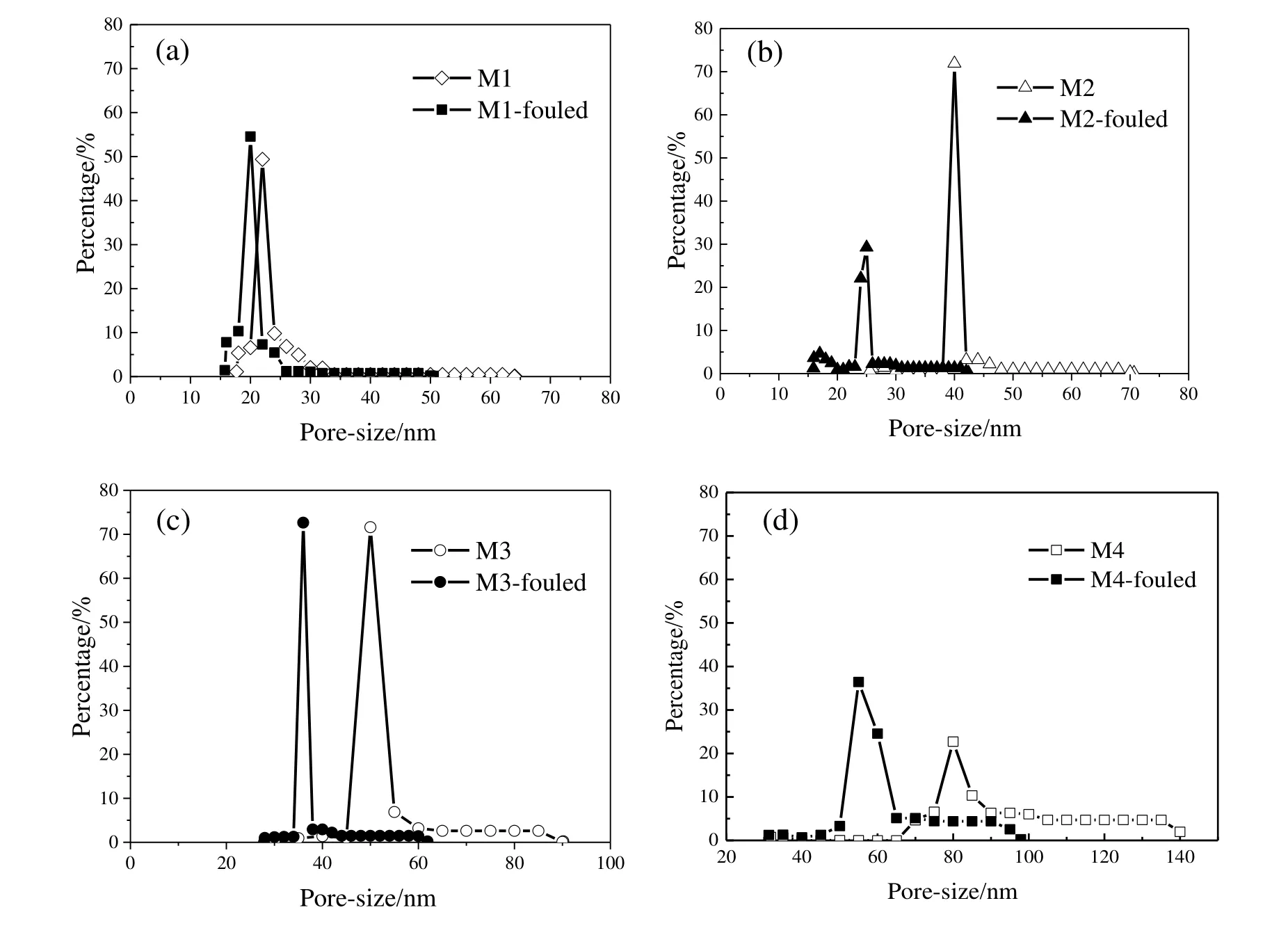
Fig.7.Effect of fouling process on the membrane pore size distribution caused by dextran mixture(40000:70000:100000:150000:500000=1:1:1:1:1):(a)M1,(b)M2,(c)M3,(d)M4.
A constant pressure operation allows determination of a steady flux and flux decline.If initially the flux is too high(athigh TMP),membrane will be over-fouled and cause irreversible fouling within a short time.The rapid flux decline at the initial stage of the four membranes can be seen in Fig.6(a).Therefore,TMP stepping UF experiments were employed to determine the critical and limiting fluxes in the process of gradual fouling as shown in Fig.12.Flux increased with the TMP,steady flux within 10 min can be achieved when filtration experiments were conducted atlow TMP.Fouling is indicated by flux decline through TMP stepping operations.The critical flux is defined as the flux below which a decline of flux with time does not occur and above it fouling occurs[15].The permeate flux declined rapidly athigher TMP and eventually no improvement in the flux by increasing TMP to a larger values.The maximum flux is the flux limitation for which fouling reached an extent of greatest degree.Because the TMP did not increase continuously but with intervals of 0.005-0.010 MPa,the critical flux only can be determined in a flux range.With increasing membrane pore size from 24 nm to 94(M1 to M4),the critical flux ranges were 105-114,63-73,38-44 and 34-43 L·m-2·h-1,respectively.The TMP corresponding to the critical flux was also decreased with the increase of pore size.It indicated that ultra filtration exhibited higher critical flux and limiting flux compared to micro filtration if the membrane was fouled gradually.It is undesirable to use membranes with larger pores(M4)in the actual water treatment system because of the flux decreases even at a low TMP.

Fig.8.Cross-section SEM images of the pristine and fouled membranes and surface SEM images of the fouled membranes.(a)cross-section ofthe pristine membranes,(b)cross-section of the fouled membrane,and(c)surface of the fouled membrane.
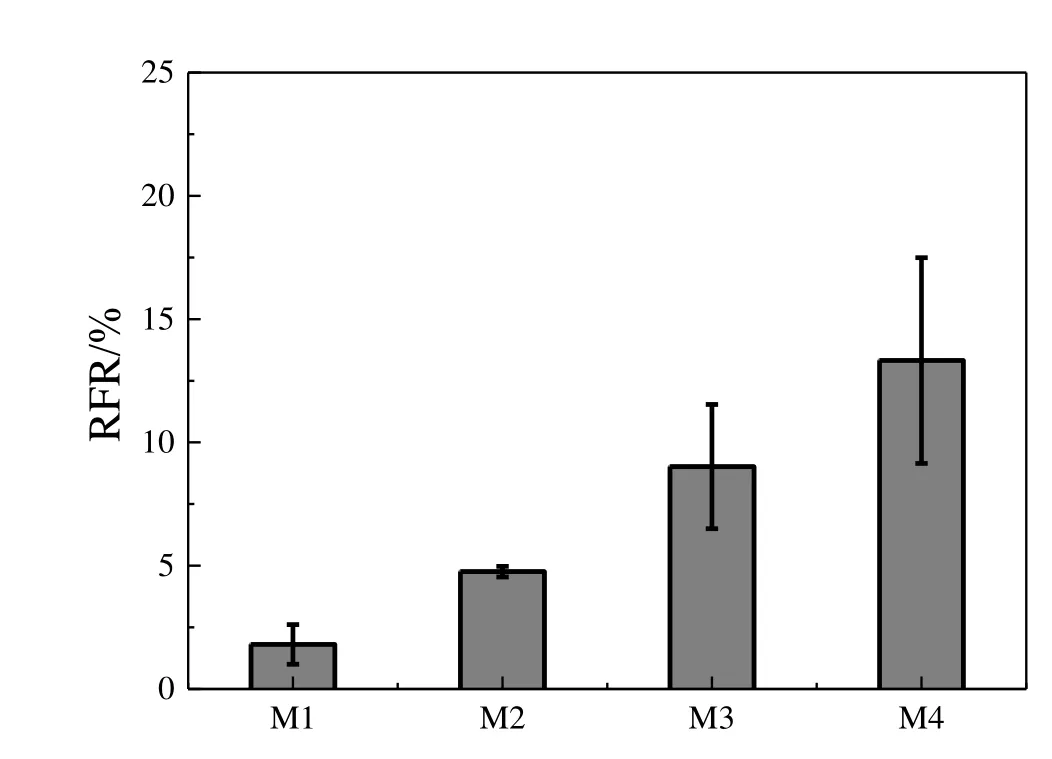
Fig.9.RFR after adsorption(24 h)of various solute mixtures(40000:70000:100000:150000:500000=1:1:1:1:1)at a total concentration of 1.0 g·L-1.
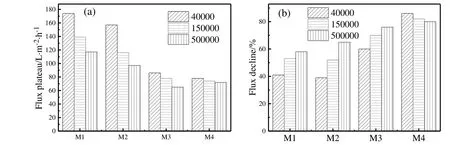
Fig.10.Steady flux(a)and flux decline(b)of the four membranes in three solute systems(the total solute concentration was 1.0 g·L-1).
Before reaching limiting flux,the relative lower resistance of M4 compared with M2 and M3 can be attributed to its larger pore.However,this membrane experienced the shortest time to reach the limiting flux.The time scale is very important for the process of gradual fouling behavior[23].When reaching limiting flux,a further increase in TMP leads to more serious fouling and the increased driving force cannot compensate the deposited gel-layer.Throughout the entire process of TMP stepping experiment,loose membrane(M4)presented lower flux at the late period of filtration.The permeate fluxes at the time of 130 min for four membranes were 163,99,57 and 44 L·m-2·h-1,corresponding to M1,M2,M3 and M4.This result was related to the formation of a compacted gel-layer,which will be discussed in the later section in detail.
3.5.Membrane resistance distribution and fouling mechanism discussions
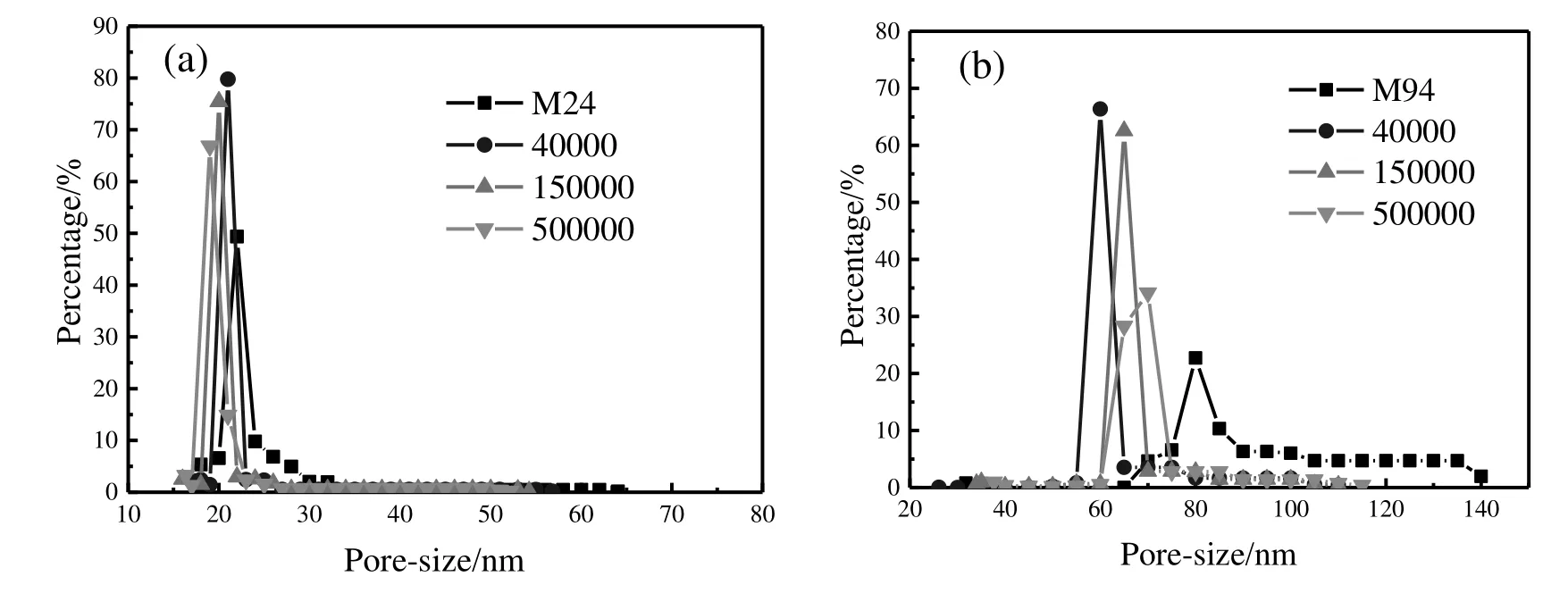
Fig.11.The pore size reduction after fouled by dextran with different molecular weights for M1(a)and M4(b).
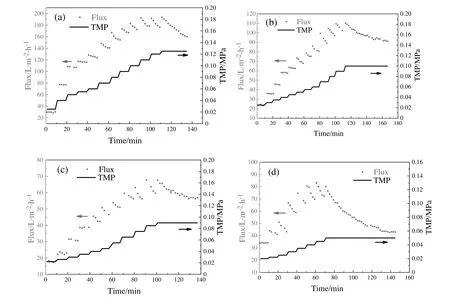
Fig.12.Flux profiles in TMP stepping experiments for four membranes ata cross flow velocity of 0.8 m·s-1 with dextran mixtures concentration of 1.0 g·L-1.(a)M1;(b)M2;(c)M3;(d)M4.
Membrane fouling resistance was calculated according to Darcy's law and the resistance-in-series model.Membrane intrinsic resistance(Rm),four types of fouling resistance and total fouling resistance(Rf)are listed in Table 3.It can be seen that M2 had the greatest Rm(1.35×1012)because its lowest PWF and the larger pore size membrane(M4)had the lowest value of Rm(9.38×1011).The total filtration resistance increased with the membrane pore size from 24 nm to 94 nm.This is the reason why membrane with larger pore size had lower steady flux values(Section 3.2).Greater extent of dextranadsorption occurred on the membrane with larger pore size(M4)illustrated by the static adsorption experiments.Larger membrane pores were easier to be blocked,which contributed to the low flux plateau and higher fouling extent(Section 3.2).This result is consistent with the increase pore blocking resistance from M1 to M3.What's more,larger membrane pores were vulnerable to blockage and constriction,which had greater impacts on the flux decline.Therefore,the gel-layer on the membrane with larger pore size was more compacted because of stronger permeate drag[30],and higher gel-layer and concentration polarization resistance occurred on membrane with larger pore size.The pore blocking resistance decreased and gel-layer resistance increased dramatically when pores increased from 52 nm to 94 nm,which was attributed to the rapid formation of gel-layer on the membrane with larger pore size.The fouling ratios of the four membranes to the total filtration are presented in Fig.13.It can be concluded that the fouling caused by organic matters was attributed to concentration polarization,gel layer formation as well as membrane pore blocking according to the resistance distribution of normalized fouling.There were some discrepancies of membrane fouling behavior between M1 to M3 and M4.The fouling behavior of M1,M2 and M3 can be described as follows:the extent of pore reduction and pore blocking resistance increased with the increase of membrane pore size and molecular weight of dextran,as well as concentration of dextran.Membranes with larger pores were subject to be more severely fouled,resulting in serious flux decline and lower critical flux.The pore size of M4(94 nm)is close to the level of micro filtration whose fouling behavior is mainly controlled by membrane pore size and molecular weight of dextran.It's worth to note that dextran with lower molecular weight caused higher flux decline and serious pore blocking because larger pores did not reject dextran with lower molecular weight.The effect of pore blocking was reached rapidly and then further formed compacted gel-layer.Eventually,the greatest total fouling resistance occurred on the membrane with the largest pore size(M4).

Table 3 Filtration resistance of different fouling stages for the four membranes

Fig.13.Normalized fouling of four types of fouling for the four membranes.
3.6.Long-term pilot study applied in surface water treatment
To investigate the performance of the optimized PVDF membrane in real application,a pilot experiment was carried out employing two PVDF membranes with mean pore sizes of 25 nm and 60 nm,respectively.Nature organic matters(NOM),colloid and suspended particles are main foul ants for UF membrane during surface water treatment.Many studies described that the NOM fouling is primarily governed by adsorption and gel formation,colloid and particle fouling are governed by cake deposition[31].Therefore,physical cleaning and chemically enhanced backwash were necessary to mitigate fouling in the long-term operation.TMP(Fig.14)was monitored to describe fouling propensity during the long-term operation in this study.
As shown in Fig.14,constant permeate flux filtration was conducted with two membranes M25 and M60,indicating pore sizes of 25 nm and 60 nm.The initial water flux was set to 40.5 L·m-2·h-1for both of the two membrane modules.From Fig.14,it can be seen that the initial TMP ofM60 was lower than 0.03 MPa and M25 was 0.03 MPa approximately.No serious fouling occurred and the major filtration resistance was membrane intrinsic resistance at the initial filtration stage,which can account for the fact that M60 showed lower initial TMP.Subsequently,the TMP of M60 increased fast and reached to 0.048 MPa at the operate time of 120 h.At the same time,the TMP of M25 maintained at 0.03 MPa.M60,with the larger pore size membrane,was the system that experienced serious fouling phenomenon as discussed above.Serious irreversible membrane fouling occurred on the M60 because fouling can't be mitigated by physical and chemical cleaning.On the other hand,the TMP of M25 basically maintained at 0.03 MPa for an extended period of time,indicating that no serious fouling occurred for this membrane at the permeate flux of 40.5 L·m-2·h-1or the fouling can be removed by cleaning procedure.The fluctuation of TMP for M25 performed at constant permeate flux(40.5 L·m-2·h-1)attributed to the change of operate conditions,such as the feed turbidity and feed temperature.During the operation period of 240-290 h,without chemical detergent(HCl)added to backwash fluid in the operate steps of backwashing,TMP increased to a relatively higher plateau about 0.035 MPa.This phenomenon revealed that the filtration resistance increase resulted from the deposition of organic matters and particulates.Then,the water flux increased to 48 L·m-2·h-1when operation time was 410 h.TMP increased quickly and eventually stabilized at 0.04 MPa.This means M25 runs stably at the target products of 48 L·m-2·h-1according to the steady TMP and exhibits better antifouling performance compared to M60.At the operation time about 570 h,the permeate flux was increased to 60 L·m-2·h-1in order to obtain higher water productions.The increase of TMP can be attributed to more contaminants deposited on the inner pores and membrane surface when higher permeate flux through the membrane.According to the observations above,although the smaller pore size membrane was able to reject lots of materials resulting in fouling layer,this kind of fouling was more reversible,which can be removed by combined physical and chemical cleaning.However,more serious pore blocking and gellayer or cake-layer occurred on the membrane with larger pore size(M60)as discussed in Section 3.5 and fouling was more irreversible.
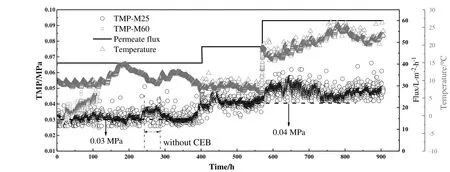
Fig.14.The profiles of TMP vs time for two membranes with pore size of 25 nm and 60 nm.
4.Conclusions
This paper investigated the fouling behavior of membranes with different pore sizes.The results provided clear information to understand fouling behavior caused by membrane micro-structure.Flux plateau,flux decline and pore reduction have been used to characterize the fouling extent.Critical flux and filtration resistance of four types of fouling have been used to illustrate fouling mechanism.Conclusions are summarized as below.
(1)Membrane with the smallest pore size of 24 nm was the system that experienced the lowest fouling extent and slowest fouling rate.The higher the molecular weight and concentration of dextran,the higher the fouling extent of membrane when its pore size is increased from 24 nm to 52 nm.It is concluded that fouling behavior is mainly controlled by membrane intrinsic pores and molecular weight of solutes if membrane pores increase to the level of micro filtration(M4).
(2)Membrane with the smallest pore size of 24 nm exhibited higher critical flux and limiting flux compared with the membranes with larger pore size when the membrane was fouled gradually.Membrane fouling potential was in good accordance with the sequence of membrane pore size:M1<M2<M3<M4.
(3)Membrane fouling caused by organic matter was mainly attributed to concentration polarization,gel layer formation as well as membrane pore blocking.The effect of pore blocking was reached rapidly and then further formed compacted gel-layer,as well as high concentration polarization for the membrane with the largest pore-size of 94 nm.
(4)Two membrane modules were applied in a pilot study of surface water treatment.Serious irreversible membrane fouling occurred on the membrane pore size of 60 nm at the permeate flux of 40.5 L·m-2·h-1and fouling can't be mitigated by physical and chemical cleaning.In contrast,membrane module,with the pore size of25 nm,exhibited better anti-fouling performance when permeate fluxes were 40.5,48 and 60 L·m-2·h-1because of the noticeable fouling phenomenon and the fouling was reversible.
Acknowledgments
The authors wish to acknowledge to Nanjing Jiuying Membrane Technology Co.,Ltd.for its kindness to supply PVDF hollow fiber membranes.
[1]S.M.Riley,J.M.S.Oliveira,J.Regnery,T.Y.Cath,Hybrid membrane bio-sy stems for sustainable treatment of oil and gas produced water and fracturing flow back water,Sep.Purif.Technol.171(2016)297-311.
[2]D.Metcalfe,P.Jarvis,C.Rockey,S.Judd,Pre-treatment of surface waters for ceramic micro filtration,Sep.Purif.Technol.163(2016)173-180.
[3]A.Zouboulis,D.Zamboulis,K.Szymanska,Hybrid membrane processes for the treatment of surface water and mitigation of membrane fouling,Sep.Purif.Technol.137(2014)43-52.
[4]F.Qu,H.Liang,J.Zhou,J.Nan,S.Shao,J.Zhang,G.Li,Ultra filtration membrane fouling caused by extracellular organic matter(EOM)from Microcystis aeruginosa:effects of membrane pore size and surface hydrophobicity,J.Membr.Sci.449(2014)58-66.
[5]G.D.Kang,Y.M.Cao,Application and modification of poly(vinylidene fluoride)(PVDF)membranes—A review,J.Membr.Sci.463(2014)145-165.
[6]J.Ji,F.Liu,N.A.Hashim,M.R.M.Abed,K.Li,Poly(vinylidene fluoride)(PVDF)membranes for fluid separation,React.Funct.Polym.86(2015)134-153.
[7]F.Liu,N.A.Hashim,Y.Liu,M.R.M.Abed,K.Li,Progress in the production and modiif cation of PVDF membranes,J.Membr.Sci.375(2011)1-27.
[8]Z.He,D.J.Miller,S.Kasemset,L.Wang,D.R.Paul,B.D.Freeman,Fouling propensity of a poly(vinylidene fluoride)micro filtration membrane to several model oil/water emulsions,J.Membr.Sci.514(2016)659-670.
[9]D.J.Miller,X.Huang,H.Li,S.Kasemset,A.Lee,D.Agnihotri,T.Hayes,D.R.Paul,B.D.Freeman,Fouling-resistant membranes for the treatment of flowback water from hydraulic shale fracturing:A pilot study,J.Membr.Sci.437(2013)265-275.
[10]K.Parameshwaran,A.G.Fane,B.D.Cho,K.J.Kim,Analysis of micro filtration performance with constant flux processing of secondary ef fluent,Water Res.35(2001)4349-4358.
[11]Z.Cui,E.Drioli,Y.M.Lee,Recent progress in fluoropolymers for membranes,Prog.Polym.Sci.39(2014)164-198.
[12]Z.Cui,N.T.Hassankiadeh,S.Y.Lee,J.M.Lee,K.T.Woo,A.Sanguineti,V.Arcella,Y.M.Lee,E.Drioli,Poly(vinylidene fluoride)membrane preparation with an environmental diluent via thermally induced phase separation,J.Membr.Sci.444(2013)223-236.
[13]G.Amy,Fundamental understanding of organic matter fouling of membranes,Desalination 231(2008)44-51.
[14]H.Susanto,M.Ulbricht,Influence of ultra filtration membrane characteristics on adsorptive fouling with dextrans,J.Membr.Sci.266(2005)132-142.
[15]R.W.Field,D.Wu,J.A.Howell,B.B.Gupta,Critical flux concept for micro filtration fouling,J.Membr.Sci.100(1995)259-272.
[16]M.Turker,J.Hubble,Membrane fouling in a constant- flux ultra filtration cell,J.Membr.Sci.34(1987)267-281.
[17]P.E.Barker,R.M.Alsop,G.J.Vlachogiannis,Fractionation,puri fication and concentration of dextran solutions by ultra filtration,J.Membr.Sci.20(1984)79-91.
[18]T.Miyoshi,K.Yuasa,T.Ishigami,S.Rajabzadeh,E.Kamio,Y.Ohmukai,D.Saeki,J.Ni,H.Matsuyama,Effect of membrane polymeric materials on relationship between surface pore size and membrane fouling in membrane bioreactors,Appl.Surf.Sci.330(2015)351-357.
[19]T.Lin,B.Shen,W.Chen,X.Zhang,Interaction mechanisms associated with organic colloid fouling of ultra filtration membrane in a drinking water treatment system,Desalination 332(2014)100-108.
[20]L.Jin,S.L.Ong,H.Y.Ng,Comparison of fouling characteristics in different pore-sized submerged ceramic membrane bioreactors,Water Res.44(2010)5907-5918.
[21]C.-F.Lin,A.Yu-Chen Lin,P.Sri Chandana,C.-Y.Tsai,Effects of mass retention of dissolved organic matter and membrane pore size on membrane fouling and flux decline,Water Res.43(2009)389-394.
[22]W.Zhang,L.Ding,Z.Zhang,J.Wei,M.Y.Jaffrin,G.Huang,Threshold flux and limiting flux for micellar enhanced ultra filtration as affected by feed water:experimental and modeling studies,J.Clean.Prod.112(Part 1)(2016)1241-1251.
[23]P.Bacchin,P.Aimar,R.W.Field,Critical and sustainable fluxes:theory,experiments and applications,J.Membr.Sci.281(2006)42-69.
[24]R.W.Field,G.K.Pearce,Critical,sustainable and threshold fluxes for membrane filtration with water industry applications,Adv.Colloid Interf.Sci.164(2011)38-44.
[25]E.Yuliwati,A.F.Ismail,Effect of additives concentration on the surface properties and performance of PVDF ultra filtration membranes for re finery produced wastewater treatment,Desalination 273(2011)226-234.
[26]M.Kohmoto,Y.Oono,Cantor spectrum for an almost periodic Schrödinger equation and a dynamical map,Phys.Lett.A 102(1984)145-148.
[27]J.Hellemans,P.Forrez,R.Dewilde,Experiment illustrating Bernoulli equation and Hagen-Poiseuille law,Am.J.Phys.48(1980)254-256.
[28]M.M.Dal-Cin,F.McLellan,C.N.Striez,C.M.Tam,T.A.Tweddle,A.Kumar,Membrane performance with a pulp mill ef fluent:relative contributions offouling mechanisms,J.Membr.Sci.120(1996)273-285.
[29]H.-Y.Gan,Z.-H.Shang,J.-D.Wang,X.-L.Liu,Effect of the pore sizes of nylon af finity membranes on γ-globulin adsorption,Chromatographia 53(2001)450-453.
[30]A.D.Marshall,P.A.Munro,G.Trägårdh,In fluence of permeate flux on fouling during the micro filtration of β-lactoglobulin solutions under cross- flow conditions,J.Membr.Sci.130(1997)23-30.
[31]V.Lahoussine-Turcaud,M.R.Wiesner,J.-Y.Bottero,Fouling in tangential- flow ultrafiltration:the effect of colloid size and coagulation pretreatment,J.Membr.Sci.52(1990)173-190.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Transport hindrances with electrodialytic recovery of citric acid from solution of strong electrolytes
- Experimental investigation on CO2-light crude oil interfacial and swelling behavior
- Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase☆
- Process development for producing a food-grade glucose solution from rice straws
- Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
- Biodegradation of natural and synthetic estrogens in moving bed bioreactor
