Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase☆
Huanru Ding ,Weirui Zhao ,Changjiang Lü,Jun Huang ,Sheng Hu ,Shanjing Yao ,Lehe Mei,*,Jinbo Wang ,Jiaqi Mei
1 School of Biotechnology and Chemical Engineering,Ningbo Institute of Technology,Zhejiang University,Ningbo 315100,China
2 Department of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China
3 School of Biological and Chemical Engineering,Zhejiang University of Science and Technology,Hangzhou 310023,China
4 Department of Chemical Engineering,The University of Utah,Salt Lake City 84102,America
1.Introduction
4-Hydroxyphenylpyruvic acid(4-HPPA),asα-keto acid compound,is an important intermediate in the metabolism of tyrosine[1,2].The presence of high concentration of 4-HPPA in body blood and urine could be regarded as an indicator of tyrosinemia[3,4].Owing to its special structure and properties,4-HPPA has reflected tremendous value in food,pharmaceutical and chemical industry.It could be used to produce hydroxy phenyllactic acid[5,6],which is used as an antimicrobial agent and food preservative compound.4-HPPA is also the intermediate of some drugs(such as benzylisoquinoline alkaloid and atromentin)[7-10],self-sustaining gel[11]and a series of industrial chemicals(such as 4-hydroxybenzaldehyde,4-hydroxymandelate and Watasenia preluciferin)[12-14].Moreover,4-HPPA has proved to be an inhibitor ofacetylcholinesterase and may be usefulin the treatmentofAlzheimer's disease recently[15].Currently,4-HPPA is mainly produced via chemical synthesis[14,16-18],which is usually a multistep process and uses harsh petrochemicals and causes environmentalpollution.Therefore,considerable interest can be generated in the development of eco-friendly technology for the production of 4-HPPA through biocatalysis.

Fig.1.Functional mechanism of different enzymes catalyzing amino acids to α-keto acids.
For economic,environmental and social bene fits,amino acids,derived from biomass,are considered to be promising materials as feedstocks to produce industrial chemicals because they contain chemical functionalities similar to conventional petrochemicals[19,20].This offers the possibility to circumvent process steps,energy and reagents compare with functionalizing the base petrochemicals[19,20].So preparing α-keto acids from amino acids through biocatalysis is relatively ideal.Three classes of amino acid-metabolizing enzymes can generate α-keto acids,namely,amino acid dehydrogenases,transferases and deaminases(Fig.1).Aminotransferases are notcost-effective formass production because another α-keto acid is needed as the essential amino acceptor[21,22].Amino acid dehydrogenases are NADH/NADPH-dependentenzymes,so a cofactor recycling systemis required to supply reducing equivalents[23].Compared with amino acid dehydrogenases and transferases,L-amino acid deaminases(L-AADs,EC 1.4.3.2)can catalyze the stereospecific oxidative deamination of natural L-amino acids to generate the corresponding α-keto acids,ammonia and hydrogen peroxide without coupling systems or cofactors[24].Mostof the microbial L-amino acid deaminases are secreted and the produced hydrogen peroxide in the catalyzed reaction would destroy the nascent α-keto acid[25,26].However,L-AADs from Proteus,Providencia and Morganella species are anchored to the outer side ofthe cytomembrane and employ a noncanonical catalytic mechanism without H2O2production during deamination[26,27].A widely accepted hypothesis is that the membrane-bound L-AADs(mL-AADs)are directly linked to the respiratory chain on the bacterial membrane,and electrons are transferred to cytochrome oxidases to reduce O2to H2O[27].Therefore,mL-AAD is the best choice for production of α-keto acids from L-amino acids.
Most mL-AADs are single-pass transmembrane proteins,which are anchored to cytomembrane surface through their N-terminal transmembrane helixes[27,28].The twin-arginine translocation(Tat)signal peptides(Tat pathway)at mL-AADs'N-terminus are indispensable for facilitating mL-AADs to export to the periplasmic space and anchoring mL-AADs to the outer side of the cytomembrane[27,28].Owing to mL-AADs are anchored to the outer side ofthe cytomembrane,secretion of mL-AADs to periplasm efficiently is key important to prepare whole cell biocatalyst.The conserved secretory pathway(Sec,conducted by pelB signal peptide)and twin-arginine translocation path way(Tat,conducted by Tat signal peptide)are two common pathways used in protein secretion[29,30].When overexpressed of mL-AADs from different species in E.coli,their own Tat peptides were proved to be effective to facilitate the proteins secreting to periplasmic space[31-33].Meanwhile,some researchers also expressed the full length mL-AADs fused with pelB leader signal peptide(Sec secretion pathway)[26,28,34].However,there was no report focus on which expression strategy is more efficient and beneficialto prepare whole cell biocatalyst with high cell-associated mL-AAD activity.
Each mL-AAD has its own particular substrate spectrum.The mL-AAD from Proteus vulgaris could be identified as candidates for 4-HPPAproduction because itshowshigh activity towards hydrophobic amino acids,such as tyrosine and leucine[31].In this work,we aimed to develop an environment friendly process for 4-HPPA production from L-tyrosine using a recombinant E.coli whole-cell biocatalyst expressing mL-AAD from P.vulgaris.To develop the whole-cell biocatalyst with high cell-associated mL-AAD activity,the secretory efficiency of mLAAD conducted by its own twin-arginine signal peptides(Tat pathway)and pelB signal peptide(Sec pathway)was investigated and compared firstly.After that,the suitable conditions for mL-AAD overexpression were determined.Finally,the reaction conditions of the biotrans formation process were optimized and the 4-HPPA was prepared under optimal condition.
2.Experimental
2.1.Materials,strains and vectors
Plasmids,bacterial strains and the primers used in this study were presented in Table 1.Nco I and Bam H I were obtained from Takara Biotechnology Co.,Ltd.(Dalian,China).Fastpfu and T4 ligase were provided by Beijing TransGen Biotech Co.,Ltd.(China).4-HPPAstandard was purchased from Sigma-Aldrich(St.Louis,MO,USA).2,4-dinitrophenylhydrazine(DNP)and trichloroacetic acid(TCA)was acquired from China Medicine Co.Ltd.(China).L-tyrosine,is opropyl-β-D-thiogalactoside(IPTG),kanamycin,a mpicillin and chloram phenicol were provided by Shanghai Sangon Co.,Ltd.(China).Unless specified,all other chemicals were analytical grade or above.

Table 1 Oligonucleotide primers,plasmids and strains used in this study
2.2.L-AAD Plasmids construction and transformation
The mL-AAD gene from Proteus vulgaris(GenBank accession no.AB030003.1)with addition of Bam H I and Xho I restriction sites at the forward and reverse ends respectively was synthesized by GenScript Biotech Co.(Nanjing,China).By virtue of these two sites,the gene was inserted into expression vector pET-20b(+),generating pET20bmlaad.For cloning mlaad into pET28a(+),the mL-AAD gene was amplified with PCR by two primers(mL-AAD28F/R)displayed in Table 1.The puri fied PCR products were treated with Nco I and Bam H I and then were reassembled into expression vector pET-28a(+),generating pET-28a-mlaad.
2.3.Preparation of the whole-cell biocatalyst
For preparation of seed cultures,recombinant E.coli cells were grown in LB medium for 12 h at 200 r·min-1and 310 K.The seed cultures were then inoculated(2%,V/V)into 50 ml LB medium and cultivated at 310 K and 200 r·min-1in a 250 ml Erlenmeyer flask.When the culture optical density at 600 nm was in the range of 0.6-0.8,IPTG was added into the broth at a final concentration of 0.5 mmol·L-1(after optimized,IPTG final concentration was changed to 0.05 mmol·L-1).After 6 h induction at 150 r·min-1and 300 K,the cells were collected via centrifugation(10000 r·min-1,5 min and 277 K)and washed with 200 mmol·L-1sodium phosphate buffer.When required,the following antibiotics were added to the LB medium atthe corresponding concentrations:ampicillin,100μg·ml-1;kanamycin,50 μg·ml-1;and chloramphenicol,30 μg·ml-1.
2.4.Biocatalytic activity assays
To obtain cell-associated mL-AADactivity,reactions were carried out to catalyze 5 mmol·L-1tyrosine for 10 min at310 K and quenched with equal volume of 20%TCA solution.After centrifugation(10000 r·min-1,1 min),the supernatant was recovered for the measurement of 4-HPPA as described below.The cell-associated mL-AAD activity was calculated according to the following Eq.(1):

The total cell-associated mL-AAD activity of cells in 1-L culture was calculated according to the following Eq.(2):

where ν is the cell-associated mL-AAD activity(the amount of 4-HPPA formed by 1 g(dry cell mass)of cells per min,μmol·min-1·g-1);U is the total cell-associated mL-AAD activity of cells in one liter culture,μmol·min-1;n is the amount of 4-HPPA,μmol;mTis the dry cell mass in the reaction mixture,g;the reaction time t was 10 min and m is the dry cell mass in one liter culture,g.
2.5.Optimization of L-tyrosine deamination reaction
To optimize pH,the reaction was conducted at310 K,and pHranged from 5 to 10.For optimization of temperature,the pH was fixed at 9,with temperature varying from 293 K-328 K.Cell concentration modified from 0.12 to 1.23 g·L-1was performed to determine the optimal concentration of biocatalyst,under the conditions of 310 K,pH 9.
2.6.Determination of 4-HPPA concentration
Quantitative determination of 4-HPPA was performed by means of DNPs.Details:100 μl sample solution with appropriate dilution multiple,90 μl TCA(20%)and 40 μl DNP(20 mmol·L-1)were mixed in a 1.5 ml centrifuge tube.Hydrazone was generated from the reaction liquid after 15 min.Then 800 μl NaOH(0.8 mol·L-1)was added into the mixture.The absorbance was immediately detected by UV under 520 nm 15 min later.
3.Results and Discussion
3.1.Expression of mL-AAD via different secretion pathway
In the practice of overexpressing of mL-AADs in E.coli,two expression strategies had been used[26,28,31-34].One was to express mL-AADs in their wild-type using their own Tat peptides(Tat secretory pathway)to facilitate the enzymes secreting to periplasmic space.The other,which was more widely adopted,expressed the full length mL-AADs fused with pelB peptides[26,28,34],which might help mL-AADs locate to the bacterial periplasm by virtue of both Tat and Sec secretory pathways[35](the original Tat signal peptide at the wild-type mL-AADcould not be removed in this case,because Tat signal peptide was essential not only for secreting mL-AADs into the periplasmic space,butalso foranchoring the proteins to the outerside ofthe cytomembrane[27],and deletion of Tat signal peptide would make the enzymes unable to bind to the cytomembrane normally[27]).However,there was no report focused on which strategy was more bene ficial for preparing whole-cell biocatalyst with high cell-associated mL-AAD activity.In order to determine and compare the expression efficiency of the two strategies,two pET systems(expression vector pET-28a(+)and pET-20b(+))were employed to express mL-AAD from P.vulgaris.Differences between these two vectors were attributed to whether they have pelB leader or not.In BL21(DE3)-pET-28a-mlaad,the wild-type mL-AAD was produced,obtained a folded conformation in the cytoplasm and secreted to periplasmic space through Tat pathway.While the fusion mL-AAD with pelB peptide was formed in BL21(DE3)-pET-20b-mlaad,the fusion protein might be secreted to periplasmic space through both Tat and Sec pathways[35].When Sec pathway way was used,the premature fusion proteins were exported to periplasmic space,where they were processed into mature proteins[29,30].The cell-associated mL-AAD activity and cell biomass of these two different recombinants were compared in Table 2.The cellassociated mL-AAD activity of recombinant BL21(DE3)-pET-28a-mlaad was 18.9%higher than that of BL21(DE3)-pET-20b-mlaad.In addition,the cell biomass of BL21(DE3)-pET-28a-mlaad was 33.3%higher than that of BL21(DE3)-pET-20b-mlaad.This indicated expression of mL-AAD in wild-type form using its own Tat signal peptide to secret to periplasmic space was more favorable to prepare whole cell biocatalyst with 4-HPPA transformation activity.Therefore,the recombinant BL21(DE3)-pET-28a-mlaad was used to explore the bioprocess for producing 4-HPPA.

Table 2 Effect of different secretion strategies on the whole-cell biocatalytic activity and biomass
3.2.Determine the suitable conditions for mL-AAD expression
Overexpression of membrane proteins often lead to insuf ficient ATP produced in E.coli cells,which would disturb cell growth and prevent biomass formation[36,37].So we determined whether the expression of mL-AAD of P.vulgaris affected cell growth,we compared the growth characteristics of BL21(DE3)-pET-28a-mlaad and non-transformed cells(Fig.2).Cell growth curves showed that the engineered mL-AAD-expressing strain grew at a much slower rate than the nontr ans formed strain,suggesting that the overexpression of mL-AAD in BL21(DE3)resulted in perturbing cell growth.In order to attenuate the toxicity of exogenous expression to the host,BL21(DE3)plysS is usually employed.So we also attempted to express mL-AAD in BL21(DE3)plysS.As expected,BL21(DE3)plysS-pET-28a-mlaad produced more biomass than BL21(DE3)-pET-28a-mlaad,but functioned with a lower cell-associated mL-AAD activity(Table 3).Balanced between cell biomass and the cell-associated mL-AAD activity,the BL21(DE3)-pET-28a-mlaad was more suitable to prepare whole cellbiocatalyst,because its total cell-associated mL-AAD activity in 1 L was much higher than that of BL21(DE3)plysS-pET-28a-mlaad.

Fig.2.Cell growth profile of BL21(DE3)and BL21(DE3)-pET-28a-mlaad in LB after IPTG induction.(Induction conditions:0.5 mmol·L-1 IPTG,300 K,150 r·min-1 and 6 h).Data represent the mean±SD from three independent determinations.

Table 3 Comparison of the whole-cell biocatalytic activity and biomass of BL21(DE3)-pET-28amlaad and BL21(DE3)plysS-pET-28a-mlaad
Next,the effect of IPTG induction concentration on the cellassociated mL-AAD activity and cell biomass of BL21(DE3)-pET-28amlaad was investigated(Fig.3).The biomass of un-induced engineered BL21(DE3)-pET-28a-mlaad was higher than that of induced engineered cells,which further confirmed that the overexpression of mL-AAD had negative effect on the host strains.The highest cell-associated mL-AAD activity was obtained at 0.05 mmol·L-1.When the IPTG induction concentration was above 0.05 mmol·L-1,the activity had no obvious improvement along with the increase of IPTG induction concentration.In general,the lower inducer concentration(0.05 mmol·L-1)resulted in not only a more superior biocatalytic activity,but also a larger amount of cell biomass.Therefore,the IPTG concentration of 0.05 mmol·L-1was used to prepare whole cell biocatalyst.
3.3.Optimization for mL-AAD catalyzed reaction
In order to apply BL21(DE3)-pET-28a-mlaad to 4-HPPA production,the reaction conditions were optimized.The cell-associated mL-AAD activity under different pH conditions was analyzed in Na2HPO4-NaH2PO4buffers(Fig.4(a)).The highest biocatalyst activity was obtained at pH 9 and the activity retained more than 70%of its maximal activity between 8 and 10.When pH below 8,the biocatalyst activity experienced a sharply decrease.Next,the effect of reaction temperature on cellassociated mL-AAD activity was determined ranging from 293 to 328 K.Apparently,the highest biocatalyst activity appeared at 310 K with a value of 275.89 μmol·min-1·g-1(Fig.4(b)).Besides,when the temperature below 310 K,the activity experienced a slow increase with temperature rising,while the activity declined sharply when temperature above 310 K.The optimal biocatalyst concentration for biotransformation was also investigated(Fig.4(c)).4-HPPA production rate was optimal at 0.74 g·L-1and did not increase at higher cell concentrations,which was mainly owing to substrate and catalyst saturation.Because the poor water solubility of L-tyrosine(>5 mmol·L-1L-tyrosine was difficult to be dissolved in our reaction system),the amount of cell catalysts saturated by dissolved L-tyrosine was at low level in this study,higher cell density in reaction system was unnecessary and wasteful in economic view.Overall,the optimal conditions were as follows:0.74 g·L-1cell biomass,310 K and pH 9.
3.4.Production of 4-HPPA using BL21(DE3)-pET-28a-mlaad
4-HPPA was easily converted to 4-hydroxybenzaldehyde in alkaline condition,especially when pH ranged from 10 to 12[14].So we determined whether the 4-HPPA deteriorated under our optimized experiment condition firstly(pH 9,310 K,200 r·min-1)(Fig.5).Despite 4-HPPA concentration continued decreased consistently over time,there was still 92%4-HPPA left after 12 h(Fig.5).The results indicated that it was feasible to produce 4-HPPA in the optimized condition for a long run time.In addition,we tried to retard the deterioration of 4-HPPA through adding antioxidant(such as ascorbic acid,sodium sul fite)into the biocatalytic mixture.However,the reaction rates were seriously compromised(data was not shown).It might be largely because of the antioxidant reduced available oxygen the bioconversion needed.Ultimately,production of 4-HPPA was conducted without any additional antioxidants.

Fig.3.Effect of different IPTG-induction concentration on biomass and cell-associated mL-AAD activity.Data represent the mean±SD from three independent determinations.
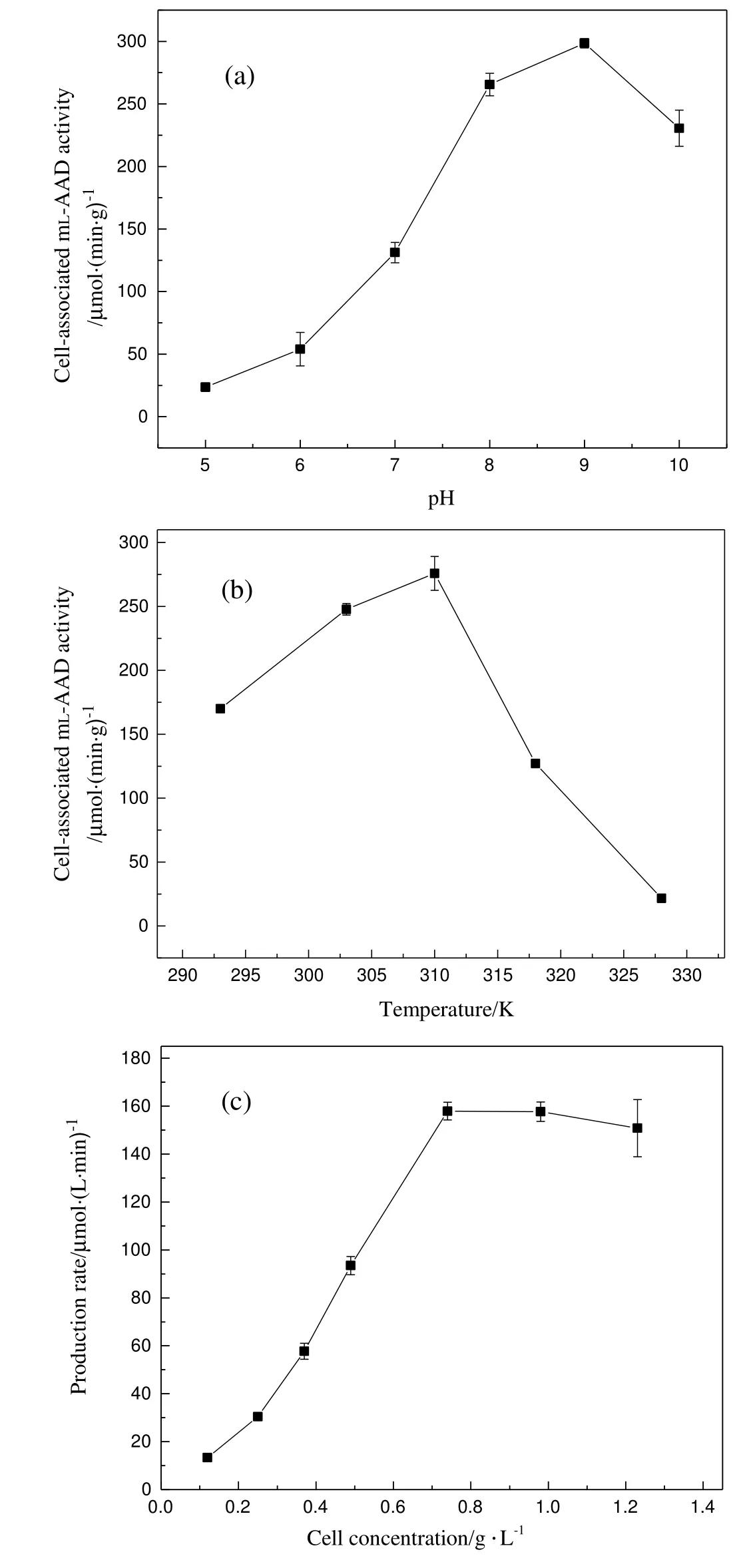
Fig.4.In fluence of pH(a),temperature(b),cell concentration,(c)on whole cell biotransformation.Data representthe mean±SDfrom three independent determinations.

Fig.5.Deterioration of 4-HPPA under optimized experimentcondition.Data represent the mean±SD from three independent determinations.
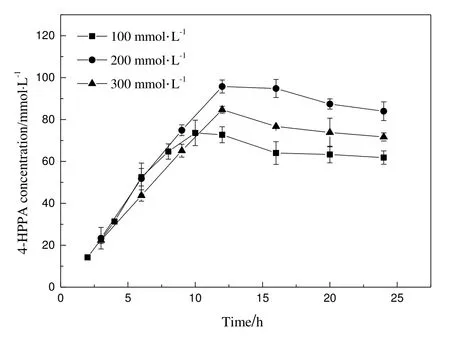
Fig.6.Preparation of 4-HPPA from different amount of tyrosine.Data represent the mean±SD from three independent determinations.
4-HPPA bioconversions were conducted in 50 ml tube under the optimized condition with different L-tyrosine substrate contents(100 to 300 mmol·L-1)within 10 ml reaction mixture(Fig.6).Because L-tyrosine is an aliphatic amino acid with poor water solubility,the reaction was conducted in L-tyrosine solution with solid powder(particles)suspended in the liquid.The L-tyrosine powder dissolved as L-tyrosine was consumed.In the case of100 mmol·L-1L-tyrosine,reaction mixture became clearat10 h,suggesting thatallsubstrate was almostconsumed,and 4-HPPA concentration reached(72.72 ± 3.81)mmol·L-1with a yield of 72.7%.For 200 mmol·L-1and 300 mmol·L-1L-tyrosine,the highest yield of 4-HPPA was obtained at 12 h,reaching(95.75±3.11)mmol·L-1and(84.71 ± 1.63)mmol·L-1respectively,with some amount of tyrosine unconverted.Both the reaction rate and the highest yield at 300 mmol·L-1substrate content were much lower than those of 100 mmol·L-1and 200 mmol·L-1,suggesting higher L-tyrosine content(300 mmol·L-1)reduced the mixing transfer efficiency and oxygen transfer rate.Considering the low price of L-tyrosine and the yields of 4-HPPA,the substrate concentration of 200 mmol·L-1might be suitable for industrialized production,albeit with the conversion rate at this substrate concentration was lower than that of 100 mmol·L-1substrate concentration.Besides,ESI MS was carried out to confirm 4-HPPA,and the proton peak at m/z=179.0347 in the mode of anion was obtained.
With the expanding spectrum of 4-HPPA applications,developing efficient and economic methods for 4-HPPA production is an important challenge.Till now,several methods had been developed to synthesize 4-HPPA,most were chemical methods[14,16-18,38].For example,4-HPPA could be split from(5Z)-5-[(4-hydroxyphenyl)methylidene]imidazolidine-2,4-dione,which was prepared from p-hydroxybenzaldehyde and hydantoin[38,39].Albeit with almost 94%conversion rate could be obtained in this method,it not only consumed mass supported-reagents and took several steps and a long time,but also used harsh solvents and produced kinds of by-products.Although Alton Meister developed a biocatalytic method for 4-HPPA synthesis with much less process steps and environment pollution,which using coupling system of secreted L-AADs(sL-AADs)from venoms and catalases(4-HPPA with a yield of 55%in 12-18 h)[40],this method was not economical for actual application as follow reasons.First,H2O2,which was produced during the deamination reaction catalyzed by sL-AADs,could degrade 4-HPPA and denature the enzyme[26,41].Hence catalases should be used to remove H2O2,which would sharply raise the cost of 4-HPPA preparation.In addition,it was difficult to produce sL-AADs through gene engineering ways.When overexpression of sL-AADs in various expression hosts,the non-active form or low expression level of the enzyme were commonly be observed,which was mainly because H2O2was generated during expression process[24,33].So the high cost for L-AADs preparation could not be decreased.Thus,production of 4-HPPA in a large scale via this method was infeasible.In this study,4-HPPA was produced via recombinant mL-AADs E.coli cells with a high yield(72.7%).Compared with the sL-AADs,mL-AADs could be easily expressed in E.coli.Moreover,non-H2O2was produced during deamination reactions conducted by mL-AADs,which could avoid the degradation of 4-HPPA and denaturation of enzyme in the reaction process.Therefore,the way developed here is ideal and promising for industrial application.
4.Conclusions
In this study,we developed an eco-friendly and high-efficiency process for one-step production of 4-HPPA from inexpensive L-tyrosine using an E.coli whole-cell biocatalyst expressing mL-AAD from P.vulgaris.By using 0.71 g·L-1whole-cell biocatalyst,72.72 mmol·L-14-HPPA could be produced from 100 mmol·L-1L-tyrosine within 10 h.Based on the results,we conclude that the process developed here could replace the multi-step chemical processes and is promising for industrial production of 4-HPPA.To make the transformation more efficiently,the activity and stability of recombinant mL-AAD should be improved by directed evolution or rational design.
[1]G.N.Chen,Y.W.Chi,X.P.Wu,J.P.Duan,N.B.Li,Chemical oxidation of p-hydroxyphenylpyruvic acid in aqueous solution by capillary electrophoresis with an electrochemiluminescence detection system,Anal.Chem.75(23)(2003)6602-6607.
[2]Y.Huang,X.L.Zhang,L.J.Xu,H.Q.Chen,G.N.Chen,Characterization of keto-enol tautomerism of p-hydroxyphenylpyruvic acid using CE with amperometric detection and spectrometric analysis,J.Sep.Sci.32(23-24)(2009)4155-4160.
[3]C.Y.Zhang,Chinese Medical Encyclopedia,Biochemistry,Shanghai Science and Technology Press,Shanghai,1989.
[4]Y.W.Chi,J.P.Duan,X.Z.Qi,G.N.Chen,Electrochemical study on the ketoenol tautomerization of p-hydroxyphenylpyruvic acid in aqueous solution,Bioelectrochemistry 60(1-2)(2003)37-45.
[5]W.M.Mu,S.H.Yu,L.J.Zhu,B.Jiang,T.Zhang,Production of 3-phenyllactic acid and 4-hydroxyphenyllactic acid by Pediococcus acidilactici DSM 20284 fermentation,Eur.Food Res.Technol.235(3)(2012)581-585.
[6]W.M.Mu,Y.Yang,J.H.Jia,T.Zhang,B.Jiang,Production of 4-hydroxyphenyllactic acid by Lactobacillus sp SK007 fermentation,J.Biosci.Bioeng.109(4)(2010)369-371.
[7]E.J.Lee,P.J.Facchini,Tyrosine aminotransferase contributes to benzylisoquinoline alkaloid biosynthesis in opium poppy,Plant Physiol.157(3)(2011)1067-1078.
[8]P.Schneider,S.Bouhired,D.Hoffmeister,Characterization of the atromentin biosynthesis genes and enzymes in the homobasidiomycete Tapinella panuoides,Fungal Genet.Biol.45(11)(2008)1487-1496.
[9]A.S.Kende,J.Lan,J.F.Fan,Total synthesis of a dibromotyrosine alkaloid inhibitor of mycothiol S-conjugate amidase,Tetrahedron Lett.45(1)(2004)133-135.
[10]Y.F.Bai,H.P.Bi,Y.B.Zhuang,C.Liu,T.Cai,X.N.Liu,X.L.Zhang,T.Liu,Y.H.Ma,Production of salidroside in metabolically engineered Escherichia coli,Sci.Rep.4(6640)(2014).
[11]R.Muzzarelli,P.Iari,W.S.Xia,M.Pinotti,M.Tomasetti,Tyrosinase-mediated quinone tanning of chitinous materials,Carbohydr.Polym.24(4)(1994)295-300.
[12]M.Gunsior,J.Ravel,G.L.Challis,C.A.Townsend,Engineering p-hydroxyphenylpyruvate dioxygenase to a p-hydroxymandelate synthase and evidence for the proposed benzene oxide intermediate in homogentisate formation,Biochemistry-U.S.43(3)(2004)663-674.
[13]S.Inoue,K.Okada,H.Tanino,H.Kakoi,A new synthesis of watasenia preluciferin by cyclization of 2-amino-3-benzyl-5-(p-hydroxyphenyl)pyrazine with p-hydroxyphenylpyruvic acid,Chem.Lett.9(3)(1980)299-300.
[14]C.H.Doy,Alkaline conversion of 4-hydroxyphenylpyruvic acid to 4-hydroxybenzaldehyde,Nature 186(4724)(1960)529-531.
[15]D.Szwajgier,Anticholinesterase activity of selected phenolic acids and flavonoidsinteraction testing in model solutions,Ann.Agric.Environ.Med.22(4)(2015)690-694.
[16]S.N.Acerbo,W.J.Schubert,F.F.Nord,Investigations on lignins and lignification.XIX.*the mode of incorporation of p-hydroxyphenylpyruvic acid into lignin,J.Am.Chem.Soc.80(8)(1958)1990-1992.
[17]H.S.Raper,A.Wormall,The tyrosinase-tyrosine reaction.II.The theory of deamination,Biochem.J.19(1)(1925)84-91.
[18]G.C.Du,Y.Song,L.Liu,J.H.Li,J.Chen,Advances in production and application of α-keto acids,J.Food Sci.Biotechnol.32(11)(2013)1121-1127.
[19]Y.L.Teng,E.L.Scott,A.V.Zeeland,J.Sander,The use of L-lysine decarboxylase as a means to separate amino acids by electrodialysis,Green Chem.13(3)(2011)624-630.
[20]A.V.Pukin,C.G.Boeriu,E.L.Scott,J.Sanders,M.Franssen,An efficient enzymatic synthesis of 5-aminovaleric acid,J.Mol.Catal.B-Enzym.65(1-4SI)(2010)58-62.
[21]P.Schadewaldt,F.Adelmeyer,Coupled enzymatic assay for estimation of branchedchain L-amino acid aminotransferase activity with 2-oxo acid substrates,Anal.Biochem.238(1)(1996)65-71.
[22]T.N.Stekhanova,A novel highly thermostable branched-chain amino acid aminotransferase from the crenarchaeon Vulcanisaeta moutnovskia,Enzym.Microb.Technol.96(2017)127-134.
[23]P.Odman,W.B.Wellborn,A.S.Bommarius,An enzymatic process to alphaketoglutarate from L-glutamate:the coupled system L-glutamate dehydrogenase/NADH oxidase,Tetrahedron-Asymmetry 15(18)(2004)2933-2937.
[24]L.Liu,G.S.Hossain,H.D.Shin,J.H.Li,G.C.Du,J.Chen,One-step production of α-ketoglutaric acid from glutamic acid with an engineered L-amino acid deaminase from Proteus mirabilis,J.Biotechnol.164(1)(2013)97-104.
[25]P.Q.Niu,X.X.Dong,Y.C.Wang,L.M.Liu,Enzymatic production of alpha-ketoglutaric acid from L-glutamic acid via L-glutamate oxidase,J.Biotechnol.179(1)(2014)56-62.
[26]G.S.Hossain,J.H.Li,H.D.Shin,G.C.Du,M.Wang,L.Liu,J.Chen,One-step biosynthesis of alpha-keto-gamma-methylthiobutyric acid from L-methionine by an Escherichia coli whole-cell biocatalyst expressing an engineered L-amino acid deaminase from Proteus vulgaris,PloS One 9(e114291)(2014).
[27]Y.C.Ju,S.L.Tong,Y.X.Gao,W.Zhao,Q.Liu,Q.Gu,J.Xu,L.W.Niu,M.K.Teng,H.H.Zhou,Crystal structure of a membrane-bound L-amino acid deaminase from Proteus vulgaris,J.Struct.Biol.195(3)(2016)306-315.
[28]G.S.Hossain,J.H.Li,H.D.Shin,R.R.Chen,G.C.Du,L.Liu,J.Chen,Bioconversion of L-glutamic acid to alpha-ketoglutaric acid by an immobilized whole-cell biocatalyst expressing L-amino acid deaminase from Proteus mirabilis,J.Biotechnol.169(2014)112-120.
[29]A.Q.Zhao,X.Q.Hu,Y.Li,C.Chen,X.Y.Wang,Extracellular expression of glutamate decarboxylase B in Escherichia coli to improve gamma-aminobutyric acid production,AMB Express 6(55)(2016).
[30]Z.X.Dong,J.Zhang,G.C.Du,J.Chen,H.Z.Li,B.H.Lee,Periplasmic export of bile salt hydrolase in Escherichia coli by the twin-arginine signal peptides,Appl.Biochem.Biotechnol.177(2)(2015)458-471.
[31]E.Takahashi,K.Ito,T.Yoshimoto,Cloning of L-amino acid deaminase gene from Proteus vulgaris,Biosci.Biotechnol.Biochem.63(12)(1999)2244-2247.
[32]J.O.Baek,J.W.Seo,O.Kwon,S.I.Seong,I.H.Kim,C.H.Kim,Expression and characterization of a second L-amino acid deaminase isolated from Prstructureoteus mirabilis in Escherichia coli,J.Basic Microbiol.51(2)(2011)129-135.
[33]Y.Song,J.H.Li,H.D.Shin,G.C.Du,L.Liu,J.Chen,One-step biosynthesis of αketoisocaproate from L-leucine by an Escherichia coli whole-cell biocatalyst expressing an L-amino acid deaminase from Proteus vulgaris,Sci.Rep.5(12614)(2015).
[34]Y.Hou,G.S.Hossain,J.H.Li,H.D.Shin,L.Liu,G.C.Du,Production of phenylpyruvic acid from L-phenylalanine using an L-amino acid deaminase from Proteus mirabilis:comparison of enzymatic and whole-cell biotransformation approaches,Appl.Microbiol.Biotechnol.99(20)(2015)8391-8402.
[35]F.J.Tooke,M.Babot,G.Chandra,G.Buchanan,T.Palmer,A unifying mechanism for the biogenesis of membrane proteins co-operatively integrated by the Sec and Tat pathways,eLIFE 6(e26577)(2017).
[36]S.Wagner,L.Baars,A.J.Ytterberg,A.Klussmeier,C.S.Wagner,O.Nord,P.A.Nygren,K.J.V.Wijk,J.W.D.Gier,Consequence of membrane protein overexpression in Escherichia coli,Mol.Cell.Proteomics 6(9)(2007)1527-1550.
[37]S.Wagner,M.M.Klepsch,S.Schlegel,A.Appel,R.Draheim,M.Tarry,M.Hogbom,K.J.V.Wijk,D.J.Slotboom,J.O.Persson,J.W.D.Gier,Tuning Escherichia coli for membrane protein overexpression,Proc.Natl.Acad.Sci.U.S.A.105(38)(2008)14371-14376.
[38]A.J.Cooper,J.Z.Ginos,A.Meister,Synthesis and properties of the alpha-keto acids,Chem.Rev.83(3)(1983)321-358.
[39]G.Billek,Eine neue synthese der 4-hydroxyphenylbrenztraubensaure.2.zur synthese der phenylbrenztraubensauren,Monatsh.Chem.92(2)(1961)335-342.
[40]A.Meister,Enzymatic preparation of alpha-keto acids,J.Biol.Chem.197(1)(1952)309-317.
[41]C.A.Bunton,Oxidation of alpha-diketones and alpha-keto-acids by hydrogen peroxide,Nature(London)163(1949)444.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Transport hindrances with electrodialytic recovery of citric acid from solution of strong electrolytes
- Experimental investigation on CO2-light crude oil interfacial and swelling behavior
- Process development for producing a food-grade glucose solution from rice straws
- Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
- Biodegradation of natural and synthetic estrogens in moving bed bioreactor
- Molten waste plastic pyrolysis in a vertical falling film reactor and the in fluence of temperature on the pyrolysis products☆
