Experimental investigation on CO2-light crude oil interfacial and swelling behavior
Mostafa Lashkarbolooki*,Shahab Ayatollahi
1 School of Chemical Engineering,Babol Noshirvani University of Technology,Babol,Iran
2 School of Chemical and Petroleum Engineering,Sharif University of Technology,Tehran,Iran
1.Introduction
The concentration of carbon dioxide(CO2)as one of the important greenhouse gases has tremendously increased as the industrial revolution begun in the mid of 19th century enhances the Earth's climate temperature[1].As a way out to eliminate this gas from atmosphere,CO2sequestration in geologic reservoirs is one of the applicable methods extensively being considered during the past decades[2-5].In details,carbon capture and sequestration drastically reduce emissions from power plants and industrial sources by capturing CO2emissions and injecting them into deep geologic formations including oil and gas fields,saline aquifers,and deep coal seams[2,3,6].Among the CO2sequestration methods in geologic reservoirs,miscible displacement by CO2injection into the oil reservoirs under favorable conditions not only can considerably reduce greenhouse gas emissions but also can effectively enhance oil recovery[7-9].
The capillary forces in the pores and throats would entrap the oil ganglia and retain them as an unre covered phase in the reservoir[10].From the enhanced oil recovery(EOR)perspective,one-third of the original oilin place to be unrecoverable probably due to IFT between crude oil and injected gas or water[11].To achieve a low residual oilsaturation,an ultra-low IFT between the crude oil and injected fluids,which lead to the miscibility of fluids,are required.In the light of this fact,it seems that characterization of the main phenomena during interfacial interactions of crude oil and CO2such as interfacial tension(IFT),swelling and Bond number may have strong effects on ultimate oil recovery and long-term CO2sequestration in the depleted oil reservoirs[9,12-14].
On the other hand,although,CO2is usually not miscible with a crude oil under the reservoir conditions at the first contact,it can develop through two-way inter facial mass transfer between the crude oil and CO2so-called dynamic and/or multi-contact miscibility[12,15,16].
The minimum pressure at which CO2can achieve the multi-contact miscibility with the crude oil is so called minimum miscibility pressure(MMP)[15,16].Determining the MMP between a crude oil and CO2is the principal technical concern in optimization or design ofa CO2flooding project[17].
In the shadow of this fact,several methods have been proposed to experimentally measure the MMP which the most popular one is the slimtube method.In contrast to slim tube which is an old and conventional method,vanishing inter facial tension(VIT)is the newest method recently proposed as the fastest and cheapest technique[12].VIT method is based on this concept that the fluid/ fluid IFT must approach zero when the two fluids become miscible[9].Therefore,experimental measurements of IFT between crude oiland CO2versus pressure have a critical impact on determining MMP of CO2and crude oil,although various contradicting results are reported about the trend existed about the IFT of oleic phase/CO2versus pressure[9,12,13,18-26].In addition,it has been shown thatthe measured equilibrium IFTs between pure hydrocarbons such as heptane and hexadecane reduce linearly with the pressure[25].However,for multicomponent fluids linear reduction with two slopes in the two distinct pressures intervals[21-25]and three slopes in the three distinct pressure intervals[9,12,13,19,20]have been reported.For instance,the experimental results reported by Zolghar et al.[25]showed that IFTs of diesel fuel(multi-component without as phaltene)+CO2versus pressure changed in two slopes in two individual pressure ranges.Moreover,Wang et al.[12]found that the equilibrium IFT between crude oil and CO2reduces almost linearly with the pressure in three distinct pressure ranges for the two light crude oils and in two different pressure ranges for the medium crude oil.For the two light crude oils-CO2systems,the respective linear regression equations of the equilibrium IFT versus pressure data for two initial ranges converge to almost the same equilibrium pressure at IFT=0[12].Although,three distinct pressures with different slopes were reported in literature for different crude oils,no similar equilibrium pressure at IFT=0 obtained in the respective linear regression equations of two initial ranges[9,13].Based on the obtained results they claimed that asphaltene precipitation has the dominant impact on the trend of equilibrium IFT as a function of pressure[9,12,19,20,26].Due to these shortcomings,in the current investigation,a crude oil from one of the southern Iranian oil fields with API°of 35 and low asphaltene content(about 0.1 wt%)is selected to study the mutual interaction between crude oil and CO2under various temperature and pressure conditions.In particular,the Bond number,swelling and IFT between crude oil and CO2is determined.Also,the MMP of the used crude oil/CO2are obtained based on the VIT method as functions of pressure and temperature.
2.Experimental
2.1.Materials
In this investigation,the sample crude oil was supplied from of the southern Iranian oil fields.The general properties of the used crude oil are listed in Table 1.In addition,the used CO2was supplied from Pars Balloon Co,with a mole fraction purity of higher than 0.99.

Table 1 Properties of the used light crude oil
2.2.Apparatus
Among the several proposed IFT measurement methods,the asymmetric pendant drop shape analysis method(the ADSA technique)is probably the most suitable one for measuring the IFT at high pressures and temperatures[18,27].In this regard,IFT-700 apparatus based onthe ADSA technique(Vinci-Technologies Co.,France)(see Fig.1)was used to not only measure the equilibrium IFT between crude oil and CO2but also to calculate the swelling and Bond number.The apparatus consists of several sections including a bulk(CW)and drop(crude oil)tanks,high pressure and temperature view cell,manual pump,pressure and the temperature display unit,light source,CCD camera,personal computer,and image processing software.The view cell is equipped with an entry port at the top and bottom for the drop injection based on the density difference existed between bulk and drop.The visual cell is equipped with three heating elements which are controlled by a PID controlling pattern(Thermostate F32-ME,Julabo,Germany)with the assist of temperature sensor(PT100)with accuracy of 0.1 K.
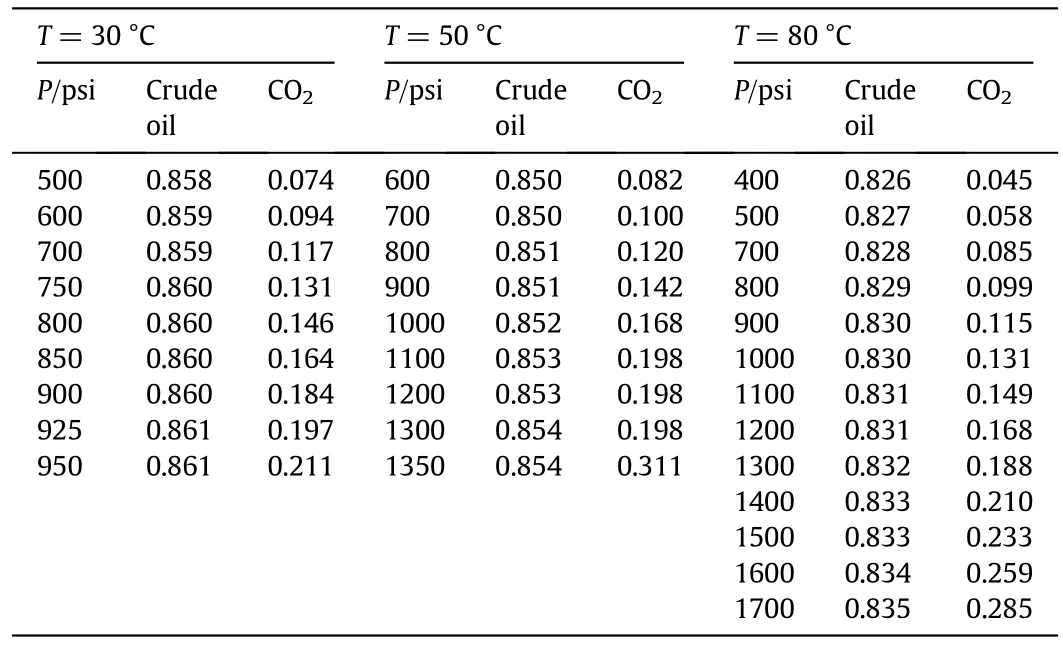
Table 2 Densities(g·cm-3)of the used crude oil and CO2 for all studied conditions(1 psi=6894.76 Pa)
The pressure is monitored by means ofa pressure transducer(Keller,modelPA-33X,Winterthur,Switzerland)up to 70 MPa with relative uncertainty of 0.1%.For monitoring the drops,a CCD camera(1.4 Mpixel,macro zoom lens,and panel light)and a light source were used for viewing the drops with high resolution.
After adjusting the temperature of tanks and view cell at desired temperature,the view cell was filled with CO2as a bulk fluid.After that,when the temperature was stabilized,a drop of crude oil was injected from the drop tank into the cell containing CO2at the desired pressure.Each experiment was repeated for at least five times for each desired temperature and pressure conditions,and each drop was monitored during the time to reach the equilibrium.The complete shape of the drop is analyzed with an advanced Drop Shape Analysis Software using a calibrated and accurate video lens system,and consequently the drop volume,IFT and bond number(see Eq.(1))were measured.

Fig.1.Schematic of the used apparatus.
It should be noted that Bond Number(the ratio of gravity forces to capillary forces)is

where g is the gravity constant;Δρis the density difference between the two fluids;γ is the IFT;R is the curve radius at the apex[13].Densities of the used crude oiland CO2forallstudied conditionsare tabulated in Table 2.
3.Results and Discussion
3.1.Dynamic behavior of crude oil drop,IFT and bond number
IFT,droplet volume and Bond number values of crude oil and CO2system are recorded versus time for all studied operational conditions.Typically,dynamic IFT,volume and bond number of used crude oil/CO2at 80 °C and pressures of 500,800,1000 and 1300 psi(1 psi=6894.76 Pa)are shown in Fig.2a-c,respectively.As it can be seen in Fig.2a-c,the IFT and Bond number almost remained unchanged versus time while the drop volume experienced complicated and unsystematic trend.

Fig.2.Dynamic IFT(a),volume(b)and bond number(c)of BCO/CO2 at T=80°C and different pressures(1 psi=6894.76 Pa).
Ithas been wellunderstood thatas CO2gradually dissolves into the oil phase the volume ofthe dynamic pendantoildrop increases due to swelling phenomenon.But,on the otherhand a reverse trend ofvolume reduction called shrinkage process can occur due to the movement of light components of the pendant oil drop into the CO2phase.Wang et al.[12]reported that the oil-swelling due to CO2dissolution into the oil phase is more pronounced at the start up period(t<30 s),whereas the oil shrinking effect due to light-components extraction becomes dominant at a large time(e.g.,t>30 s)[18,22].In contrast to the previously published literature,these aforementioned phenomena were not observed during the measurements performed in this investigation.As it is obvious in Fig.2,in contrast to IFT and BN,the volume of the pendant crude oil drop continues to change.In addition,based on the increased or decreased volume of the crude oil drop it can be concluded that the oil swelling and light component extraction phenomena occurred.In details,it is seen that the swelling behavior occurs during all-time while light component extraction occurred at different pressures regardless of time.The interesting point is that the change of volume of crude oil has no effect on the IFT and BN.Therefore,it can be concluded that the variation of volume of crude oil has a minimum effect on the inter facial properties of crude oil and CO2,and,consequently,leads to no effect on IFT and the ratio of gravity forces to capillary forces(i.e.BN)as a function of time.In the next stage of this study,the effect of temperature(30,50 and 80°C)on the volume of crude oil drop under pressure of 500 psiwas investigated(see Fig.3).In addition,the shapes of the formed drops at different temperatures while the pressure was kept constant at 500 psi(1 psi=6894.76 Pa)are tabulated in Table 3.
A closer examination into Fig.3 and Table 3,revealed that the influence of temperature on the crude oil drops.The oil swellings not only are higher at low temperatures but also began to increase at the startup of the test which may be due to higher dissolution of CO2in the crude oil at lower temperatures.At higher temperatures(i.e.80°C),the volume of the crude oil drop remained unchanged during the initial 600 s,while after this period of time crude oil volume increased may be due to dissolution of CO2in the oil phase.Therefore,it can be concluded that the composition of crude oil as well as operational conditions have dominant effects on the crude oil volume during CO2flooding.
3.2.Equilibrium behavior of IFT and bond number
In the next step of this investigation,the equilibrium IFT and Bond number values are investigated at different operational conditions.The measured equilibrium IFT values are plotted at 30,50 and 80°C vs pressure to investigate the inter facial behavior of the system(see Fig.4).Equilibrium IFT of the used crude oil/CO2at all studied conditions with their uncertainties are also listed in Table 4.It can be seen from Fig.4 that both pressure and temperature has dominant effects on the equilibrium IFT of CO2and light crude oil.Also,the equilibrium IFT decreases as pressure increases,while it increases as a function of temperature since the solubility of CO2in crude oil is higher at higher pressures and lower at higher temperatures.Moreover,as it is shown in Fig.4,equilibrium IFT values were linearly extrapolated to zero,which was previously defined as the MMP of the crude oil-CO2system[17,18].It is found that the measured equilibrium IFT is reduced almost linearly with the pressure in two distinct pressure intervals.The linear equations in these intervals withtheir correlation coefficient(R2)are reported in Table 5.The obtained results are shown a close match between the linear equation and equilibrium IFT data.

Table 3 Initial and final(before releasing)shape of crude oil drop at constant pressure of 500 psi(1 psi=6894.76 Pa)and three different temperatures
The most significant threshold pressure that can be observed from the measured equilibrium IFT against the pressure curve is the pressure at which this curve showed a sharp slope change.This pressure is located at the intersection of the above two linear correlations tabulated in Table 5.The first and second slopes are the indication of lighter and heavier components(after extraction of the light components by CO2)presented at the interface,respectively[21,25].
Equilibrium IFTs of the used crude oil and CO2versus temperature for three constant pressures and pressure of intersection points(threshold pressure)are shown in Fig.5.These depicted results revealed that there is a linear relation between equilibrium IFT and temperature for threshold pressure while the linear relation is not obtained for constant pressures.

Fig.3.Volume of crude oil drop at constant pressure of 500 psi(1 psi=6894.76 Pa)and three different temperatures as a function of time.

Fig.4.EIFT of BCO/CO2 at three constant temperatures as a function of pressure(1 psi=6894.76 Pa).

Table 4 EIFT of the used crude oil/CO2 at all studied conditions with their uncertainty(1 psi=6894.76 Pa)
In Fig.5,the equilibrium IFT between diesel fuel and CO2[25]at threshold pressure is also plotted as a function of temperature which revealed a linear phenomenon at threshold pressure.In more details,at threshold pressure for the examined light crude oil and diesel fuel,quilibrium IFT experienced an enhancement of about 0.0821 and 0.0252 mN·m-1,respectively.At threshold pressure,crude oil with API°of 35 which is heavier than diesel fuel with API°of 38.5 experienced more IFT enhancement.In other words,the effect of temperature on the equilibrium IFT at threshold pressure is more considerable for heavy components.

Table 5 Linear equation between EIFT(mN·m-1)and P(psi,1 psi=6894.76 Pa)for specified lines depicted in Fig.4
For more examination,in Fig.6,the obtained MMP and threshold pressure of the used crude oil and diesel fuel are depicted versus temperature.For two studied systems,both of MMP and threshold pressure increased with temperature,although the increasing rate as a function of temperature is higher for MMP compared to threshold pressure.
Comparing the obtained results for the light crude oil with API°of 35 with the diesel fuel with API°38.5 demonstrated that the heavier components(i.e.light crude oil)lead to more MMP enhancement with temperature.Consequently,for light crude oil,the higher differences between MMP and threshold pressure could be observed at higher temperatures.
The results ofthe obtained Bond number of all studied conditions are shown in Fig.7 and the irun certainties are listed in Table 6.These results demonstrated that Bond number linearly increases with pressure at constant temperature.Thus,as the BN is increased,a blob that would have been trapped in the capillary dominated case can continue to flow if the gravitational forces are more important[28].Also,it can be observed that BN decreases with temperature at constant pressure.The slope and intercept of the linear equations are also shown in Fig.8.It can be observed that the slope of the linear line of BN versus pressure decreases as a function of temperature but the intercept linearly increases with temperature.
Based on the obtained results,one can conclude that two conformable outcomes could be observed when two reservoirs with the similar crude oil and different temperatures are compared.First,crude oil in the reservoir with lower temperature could have lower MMP.Thus,lower pressure will be required to employ the influence of miscibility to obtain higher oil recovery.Second,the reservoir with lower temperatures due to higher BN can experience more oil recovery because it can overcome to gravitational forces compared to capillary forces.
4.Conclusions
In this investigation,the mutual inter facial interaction is examined for a CO2-light crude oil system.Dynamic and equilibrium IFT,Bond number and swelling/extraction are measured at different pressures and temperatures.The most important results of this investigation can be summarized as follows:
➢The IFT and Bond number almost remained unchanged vs time while a complicated and unsystematic trend was observed for the drop volume.
➢Swelling behavior is mostly dominant during the long time and extraction of light component are occurred at different pressures regardless of the contact time.

Fig.5.Comparison of EIFT at constant pressures with intersection point as a function of temperature(1 psi=6894.76 Pa).

Fig.6.Functionality of intersection point and MMP with temperature and pressure(1 psi=6894.76 Pa)for the used crude oil and diesel fuel.Obtained from Ref.[25].

Fig.7.BN of BCO/CO2 at three constant temperatures as a function of pressure(1 psi=6894.76 Pa).

Fig.8.Slope and intercept of BN versus P (Fig.7)at different temperatures.

Table 6 BN of BCO/CO2 at all studied conditions with their uncertainty(1 psi=6894.76 Pa)
➢The equilibrium IFT increases as temperature increases which possibly can be related to lower CO2solubility at higher temperatures.Therefore,the reservoir with lower temperatures due to lower IFT and higher BN experiences more oil recovery as a result of gravitational forces conquered to capillary forces.
➢The measured equilibrium IFT is reduced almost linearly as a function of pressure in two distinct pressure intervals.
➢MMP and threshold pressure(pressure at the intersection of two linear lines of equilibrium IFT versus pressure)are increased as temperature increased,while the rate of increasing as a function of temperature was higher for MMP compared with threshold pressure.
Acknowledgments
The authors express their sincere gratitude to Mr.Ali Zeinolabedini Hezave for his masterful guidance during the experimentation and organizing this manuscript.
[1]P.J.Crutzen,W.Steffen,How long have we been in the Anthropocene era?Clim.Chang.61(2003)251-257.
[2]H.J.Herzog,Peer reviewed:What future for carbon capture and sequestration?Environ.Sci.Technol.35(2001)148-153.
[3]H.S.Viswanathan,R.J.Pawar,P.H.Stauffer,J.P.Kaszuba,J.W.Carey,S.C.Olsen,G.D.Guthrie,Development of a hybrid process and system model for the assessment of well bore leakage at a geologic CO2sequestration site,Environ.Sci.Technol.42(2008)7280-7286.
[4]Y.A.N.G.Zihao,J.I.N.Min,L.I.Mingyuan,D.O.N.G.Zhaoxia,Y.A.N.Peng,Implication of geochemical simulation for CO2storage using data of york reservoir,Chin.J.Chem.Eng.19(2011)1052-1059.
[5]M.A.Ahmadi,B.Pouladi,T.Barghi,Numerical modeling ofCO2injection scenarios in petroleum reservoirs:Application to CO2sequestration and EOR,J.Nat.Gas Sci.Eng.30(2016)38-49.
[6]S.M.Klara,R.D.Srivastava,H.G.McIlvried,Integrated collaborative technology development program for CO2sequestration in geologic formations--United States Department of Energy R&D,Energy Convers.Manag.44(2003)2699-2712.
[7]Y.Yang,X.Li,P.Guo,Y.Zhuo,Y.Sha,Improving oil recovery in the CO2flooding process by utilizing nonpolar chemical modifiers,Chin.J.Chem.Eng.24(2016)646-650.
[8]D.Yang,P.Tontiwa chwuthikul,Y.Gu,Interfacial interactions between reservoir brine and CO2at high pressures and elevated temperatures,Energy Fuel 19(2005)216-223.
[9]M.Escrochi,N.Mehranbod,S.Ayatollahi,The gas-oil interfacial behavior during gas injection into an asphaltenic oil reservoir,J.Chem.Eng.Data 58(2013)2513-2526.
[10]M.Lashkarbolooki,S.Ayatollahi,M.Riazi,The impacts of aqueous ions on interfacial tension and wettability of an asphaltenic-acidic crude oil reservoir during smart water injection,J.Chem.Eng.Data 59(2014)3624-3634.
[11]J.R.Christensen,E.H.Stenby,A.Skauge,A review of WAG field experience,SPE Reserv.Eval.Eng.4(2001)97-106.
[12]X.Wang,S.Zhang,Y.Gu,Four important onset pressures for mutual interactions between each of three crude oils and CO2,J.Chem.Eng.Data 55(2010)4390-4398.
[13]Y.Kazemzadeh,R.Parsaei,M.Riazi,Experimental study of asphaltene precipitation prediction during gas injection to oil reservoirs by interfacial tension measurement,Colloids Surf.A Physicochem.Eng.Asp.466(2015)138-146.
[14]M.Lashkarbolooki,A.Vaezian,A.Z.Hezave,S.Ayatollahi,M.Riazi,Experimental investigation of the influence of supercritical carbondioxide and supercritical nitrogen injection on tertiary live-oil recovery,J.Supercrit.Fluids 117(2016)260-269.
[15]L.W.Hol,V.A.Josendal,Mechanisms of oil displacement by carbon dioxide,J.Pet.Technol.26(1974)1427-1438.
[16]D.W.Green,G.P.Willhite,Enhanced Oil RecoVery,SPE Textbook Series,vol.6,SPE,Richardson,TX,1998.
[17]M.Dong,S.S.Huang,S.B.Dyer,F.M.Mourits,A comparison of CO2minimum miscibility pressure determinations for weyburn crude oil,J.Pet.Sci.Eng.31(2001)13-22.
[18]D.Yang,Y.Gu,Interfacial interactions between crude oil and CO2under reservoir conditions,Pet.Sci.Technol.23(2005)1099-1112.
[19]A.Hemmati-Sarapardeh,S.Ayatollahi,M.H.Ghazanfari,M.Masihi,Experimental determination of interfacial tension and miscibility of the CO2-crude oil system;temperature,pressure,and composition effects,J.Chem.Eng.Data 59(2014)61-69.
[20]X.Wang,Y.Gu,Oilrecovery and permeability reduction ofa tightsandstone reservoirin immiscible and miscible CO2flooding processes,Ind.Eng.Chem.Res.50(2011)2388-2399.
[21]M.Nobakht,S.Moghadam,Y.Gu,Mutual interactions between crude oil and CO2under different pressures,Fluid Phase Equilib.265(2008)94-103.
[22]M.Nobakht,S.Moghadam,Y.Gu,Determination of CO2minimum miscibility pressure from the measured and predicted equilibrium interfacial tensions,Ind.Eng.Chem.Res.47(2008)8918-8925.
[23]H.Li,D.Yang,P.Tontiwachwuthikul,Experimental and theoretical determination of equilibrium interfacial tension for the Solvent(s)-CO2-heavy oil systems,Energy Fuel 26(2012)1776-1786.
[24]Y.Gu,P.Hou,W.Luo,Effects of four important factors on the measured minimum miscibility pressure and first-contact miscibility pressure,J.Chem.Eng.Data 58(2013)1361-1370.
[25]A.Zolghadr,M.Escrochi,S.Ayatollahi,Temperature and Composition Effecton CO2Miscibility by Interfacial Tension Measurement,J.Chem.Eng.Data 58(2013)1168-1175.
[26]M.Bayat,M.Lashkarbolooki,A.Z.Hezave,S.Ayatollahi,Investigation of gas injection flooding performance as enhanced oil recovery method,J.Nat.Gas Sci.Eng.29(2016)37-45.
[27]M.Lashkarbolooki,S.Ayatollahi,M.Riazi,Mechanistic study on the dynamic interfacialtension ofcrude oil+water systems:Experimentaland modeling approaches,J.Ind.Eng.Chem.35(2016)408-416.
[28]D.S.Schechter,Z.Denqen,F.M.Orr,Capillary imbibition and gravity segregation in low IFT systems.In SPE annual technical conference and exhibition,Society of Petroleum Engineers,SPE-22594-MS January,1991.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
