Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
Jierong Yang ,Wangliang Li,Qingfen Liu *,Huizhou Liu *
1 Key Laboratory of Green Process and Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
1.Introduction
Antibiotics mycelium,an abundant byproduct of fermentation in pharmaceutical industry,is a useful and renewable feedstock for biodegradable materials and fine chemical products,but has the risk of inducing drug-resistant bacteria[1,2].Penicillin mycelium,as the most largely produced antibiotic,has protein,carbohydrate and lipid contents in its dry mycelium of about 37.8%,48.3%and 10.4%[3].Therefore,improper disposal would lead to a waste of resource and cause environmental pollution[4].
To recover poly saccharides or protein efficiently,dissolution of antibiotics mycelium is the necessary step in which strong internal interactions such as hydrogen bonds should be broken.The solubility of mycelium in traditional solvents,such as hot water,alcohol,acid,alkali and traditional organic solvents is very low[5,6].Even enhanced by ultrasound,microwave and enzymatic catalysis,only part of antibiotics mycelium can be extracted[7-9].Furthermore,these solvents can lead to denaturation and degradation of protein.Thus,in the past few decades,the utilization of mycelium was limited in the field of producing fertilizers and animal fodder,which was forbidden because they bring serious environmental pollution and induce drug-resistant bacteria and superbugs in the natural environment[10,11].
Ionic liquids(ILs)are environmentally friendly solvents and show good performance in the dissolution of biomass,such as cellulose[12-14],lignin[15,16],chitin[17],and keratin[18].As a kind of biomass,antibiotics mycelium also contains protein and chitin,which can be dissolved in ILs.Design of ILs provides opportunities for highefficiency dissolution of mycelium under mild condition,which is important for the low-energy utilization of antibiotics mycelium.Furthermore,by adding anti-solvents to biopolymers/ILs solution,different types of materials can be prepared from biological macromolecules,which provides feasibility for its high-valued utilization[12,13].
The study on the mechanism of biomass dissolution in ILs indicated that hydrogen bond formed between anions of ILs and biomass plays an important role,while the interaction between ILs cations and biomass was negligible[12].Zhang et al.found that Ac-anion favored the formation of hydrogen bonds with hydrogen atoms of hydroxyls,and the aromatic protons in[Emim]+,especially the most acidic H2,preferred to associate with the oxygen atoms of hydroxyls[19].Zhuo et al.found that the interaction of glucose with the anions was stronger than that with cation[20].Molecular dynamics simulations and density functional theory were also used to study the interaction and confirmed that hydrogen bond formed between anion of ILs and biomass[21,22].Until now,the active sites of forming hydrogen bonds between ILs and biomass have not been thoroughly studied.Although the effects of cations and anions on the dissolution of biomass were investigated,quantitative study on the relationship of the solubility and accepting ability of anions or donating ability of cations has never been reported.
The performance and mechanism of penicillin G mycelium dissolution were investigated using ILs as solvent.The driving force of mycelium dissolution and active sites of forming hydrogen bonds were studied using NMR and FT-IR spectroscopy.In addition,the relationship of the solubility and accepting ability of anions or donating ability of cations was quantitatively investigated with the Kamlet-Taft model.
2.Materials and Methods
2.1.Materials
The penicillin G mycelium was kindly supplied by North China Pharmaceutical Co.,Ltd.The mycelium was washed by deionized water, filtered under reduced pressure and dried at 40.0°C for 48.0 h.Dried mycelium was ground into powder and kept at 40.0°C for use.
1-Alkyl-3-methyl imidazolium based ILs([Bmim]Cl,[Bmim]BF4,[Bmim]MeSO4,[Bmim][MeO]HPO2and[Bmim]PF6)and diethylammonium acetate([N22]Ac)were kindly provided by Henan Linzhou KeNeng Co.,Ltd.1-Butyl-3-methyl imidazolium acetate([Bmim]Ac),tetrabutylammonium chloride([N4444]Cl),choline acetate([Choline]Ac)and 1-benzyl-3-methylimidazolium acetate([Benzylmim]Ac)were provided by Shanghai Cheng Jie Chemical Co.,Ltd.All ILs used were dried in a vacuum oven at 50.0°C for 24.0 h prior to use.Dimethyl sulfoxide(DMSO),pyridine and methanol were purchased from XiLong Chemical Industry Co.,Ltd.N,N-diethyl-4-nitroaniline was purchased from Fluorochem Co.,Ltd.The purity of all reagents above is higher than 98.0%.Reichardt's dye 30(90.0%)was purchased from Sigma-Aldrich.
2.2.Dissolution of antibiotics mycelium
Dried penicillin Gmycelium was added into ILs or IL/co-solvent mixture with the ratio of dried penicillin Gmycelium to ILs as 50.0 g·mol-1.The mixture was then heated at a given temperature(25.0-120.0°C)on a hot plate with magnetic stirring at the speed of 300 r·min-1.Then,the mixture was diluted by pyridine and centrifuged for 10.0 min at 8000 r·min-1to remove undissolved solid fraction.The solubility data were calculated according to mass of penicillin mycelium dissolved in per mole of IL.
2.3.Kamlet–Taft parameters for the ILs
The measurement of the Kamlet-Taft parameters of a series of ionic liquids was carried out by the procedure reported by Doherty[23].Three different types of dyes:N,N-diethyl-4-nitroaniline,4-nitroaniline and Reichardt's dye were used.The absorbance spectra of each IL/dye sample were measured between 350 and 700 nm using a UH5300 UV/VIS spectrophotometer at 25.0 °C or 90.0 °C and the maximum wavelength(λmax)was recorded.The Kamlet-Taft parameters(α and β)were calculated using the following equations[24]:


2.4.Analysis methods
In order to investigate the effect of mycelium concentration on the proton and carbon chemical shifts of[Bmim]Ac,solutions of mycelium in[Bmim]Ac were prepared by adding 0.0 mg,4.6 mg and 10.0 mg mycelium into the solution of 0.5 ml DMSO-d6 and 150 mg[Bmim]Ac.Undissolved fractions were removed by centrifugal separation for 10.0 min at 8000 r·min-1.Upon dissolution,these samples were transferred into 5 mm NMR tubes.All experiments were carried out on an AVANCE III spectrometer operated at room temperature with 1-4 scans for1H NMR and 128-768 scans for13C NMR[19,25].
FT-IR spectra over the frequency range between 1100 and 3600 cm-1were collected at room temperature using a T27-Hyperion-Vector22 spectrometer with KBr pellets.All the spectra were recorded with a resolution of 2 cm-1using 32 scans.A baseline correction was performed on each spectrum.
3.Results and Discussion
3.1.Dissolution of penicillin mycelium
Fig.1 shows the schematic diagram of the dissolution process of penicillin mycelium in ILs.Firstly,penicillin mycelium was mixed with ILs and the dissolution process was conducted at the temperature of 25.0 °C-120.0 °C for 5.0 h.Then,the dissolved fractions precipitated out from ILs by adding water and were separated by filtration.The spent ILs can be reused for penicillin mycelium dissolution after regeneration.Dissolution percentage of mycelium of different ILs at 90.0°C was shown in Table S1(see Supplementary Material),and the result indicated that[Bmim]Ac was the most efficient.

Fig.1.Schematic diagram of the dissolution of penicillin mycelium.

Fig.2.The effectofdissolution time(a),temperature(b),and ratio of[Bmim]Ac/mycelium(m/m)(c)on dissolution performance in[Bmim]Ac.
The time-dependent experiments at25 °C and 90 °C were performed and shown in Fig.2a.The equilibrium of dissolution reached at 60 min at the both tested temperatures.To ensure full dissolution at different temperatures,the dissolution process was conducted for 5.0 h.The effect of temperature on dissolution performance in[Bmim]Ac was shown in Fig.2b.The dissolution percentage of penicillin mycelium in[Bmim]Ac increased with the increase of temperature.Higher temperature provided more energy to break the hydrogen bonds within mycelium and improved the dissolution process.When the temperature increased from 25.0 °C to 120.0 °C,dissolution of penicillin mycelium increased from 69.74%to 91.45%.It's worth mentioning that penicillin mycelium was dissolved in ILs at room temperature.Compared to dissolution of biomass at high temperatures,such as zein at 95 °C[26]and keratin at 130 °C[27],dissolution of penicillin myceliumcan be achieved at a much lowertemperature,which provided an energy-saving and sustainable process to dispose penicillin mycelium.
The effect of[Bmim]Ac/mycelium(m/m)ratio on dissolution performance was studied at 90.0°C and the result was shown in Fig.2c.At the[Bmim]Ac/mycelium(m/m)ratio of(0.95:1)-(3.90:1),dissolution percentage increased fast,and with a further increase in the amount of[Bmim]Ac,dissolution percentage increased slowly.Almost completely dissolution of mycelium was achieved at the[Bmim]Ac/mycelium(m/m)ratio of 3.90:1,at which dissolution percentage is 89.91%and the solubility was 45.17 g·mol-1.
Aprotic polar solvent produced more solvated cations and “free”anions,which disrupted inter-and intra-molecular hydrogen bonds in biomass and improved biomass dissolution by forming of new hydrogen bonds between ILs and biomass[28-30].Therefore,the typical aprotic polar solvent DMSO was used to enhance the dissolution of mycelium.Conductivity of[Bmim]Ac/DMSO solvent was used to characterize the correlation between IL dissociation and dissolving ability[31].As shown in Fig.3a,conductivity of[Bmim]Ac/DMSO solvent increased with the increasing of DMSO/[Bmim]Ac(v/m)ratio in the range of 0 to 1.0,and then decreased due to the dilution of ions when the ratio was higher than 1.0.The dissolution percentage of penicillin mycelium showed a similar trend with conductivity of[Bmim]Ac/DMSO cosolvent,proving that DMSO produced more free ions and increased the dissolution of mycelium.The optimal ratio of DMSO to[Bmim]Ac(v/m)was 1.0.Although DMSO is widely used as organic solution,solubility of mycelium in DMSO at 25.0°C was very low,only 0.014 g·ml-1.As shown in Fig.3b,with the addition of DMSO,dissolution percentage of penicillin mycelium in[Bmim]Ac increased from 69.74%to 94.50%at room temperature.The results indicated that synergistic effect of ionic liquids and co-solvent DMSO was found with the DMSO/[Bmim]Ac(v/m)ratio in the range of 0.0-1.0.
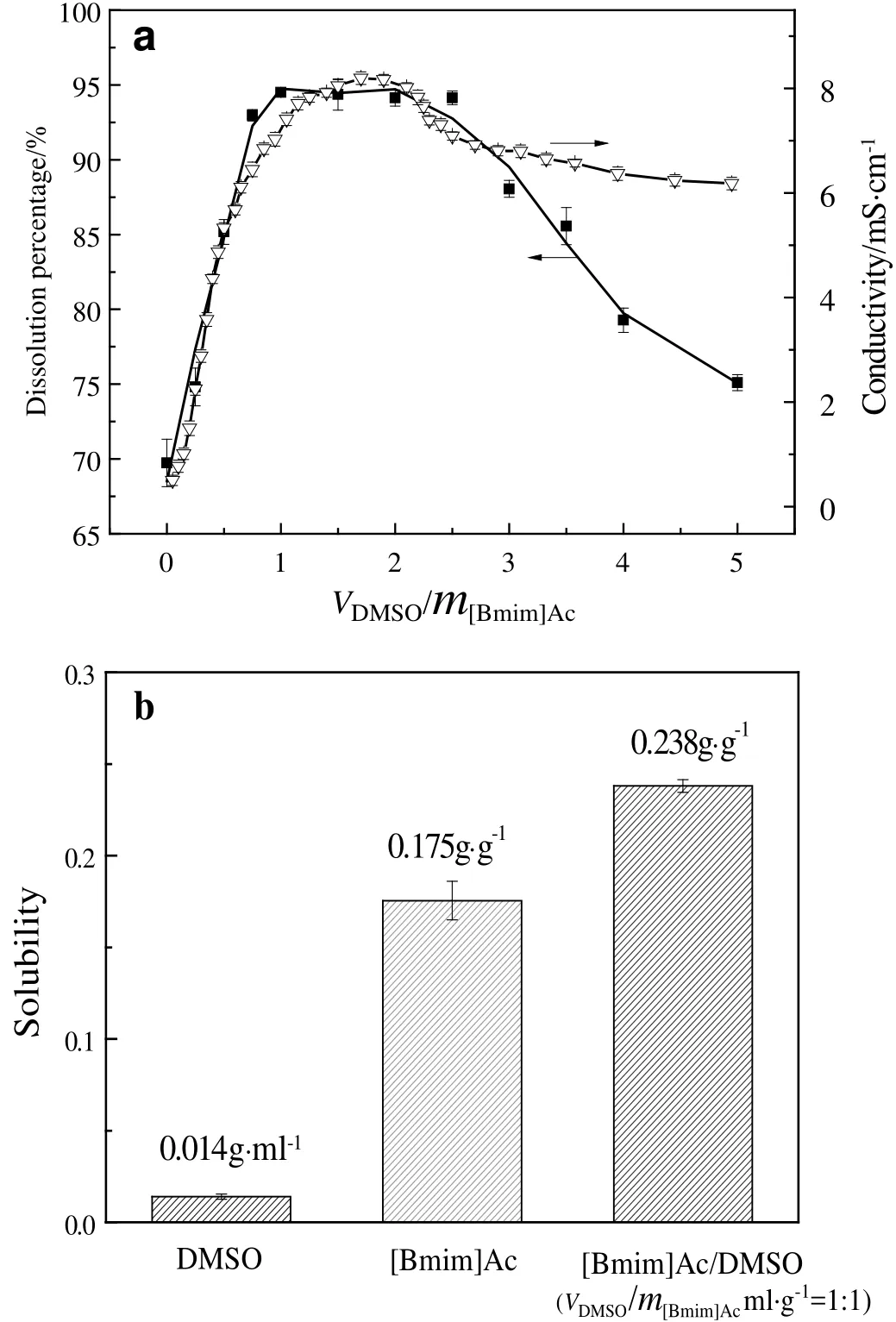
Fig.3.(a)The correlation between conductivity of[Bmim]Ac/DMSO solvent and dissolving ability and(b)solubility of penicillin mycelium in DMSO,[Bmim]Ac and[Bmim]Ac/DMSO solvent.
3.2.Driving force of penicillin mycelium dissolution in[Bmim]Ac
To further study the driving force of mycelium dissolution,the interaction between penicillin mycelium and[Bmim]Ac was characterized with1H and13C NMR spectra.Proton1H chemical shift of[Bmim]Ac with different penicillin mycelium concentrations was investigated and shown in Fig.4a.Compared to pure[Bmim]Ac,the protons H2,H4 and H5 in imidazolium cation show an up field shift with the increasing concentration of penicillin mycelium,while there is a negligible change in other protons of both anion and cation.Among the protons in imidazolium cation,H2 is the most acidic and shows the most significantup field change in chemicalshift.While,H4 and H5 protons present a less obvious up field shift,which can be explained by the form of weaker hydrogen bond.The formation of stronger hydrogen bond leads to a down field shift,and vice versa[32].Because H atoms of hydroxyl and amino groups are stronger hydrogen bond donors than the protons of imidazolium cation,Ac-prefers to form stronger hydrogen bond with H atoms of hydroxyl and amino groups in mycelium[33].Therefore,hydrogen bonds between Ac-and[Bmim]+were broken,and Ac-ions were replaced by hydroxyls or amino to form hydrogen bond with protons in[Bmim]+,especially with H2.The weaker hydrogen bonding acceptor ability of hydroxyl or amino in mycelium causes an up field shift for aromatic protons[34,35].
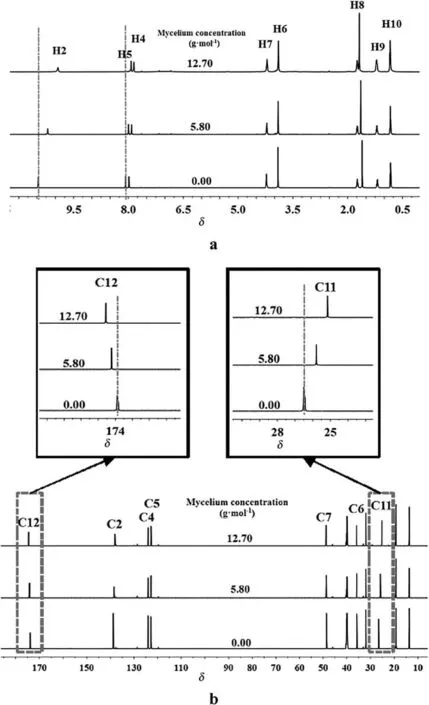
Fig.4.(a)1H NMR spectra and(b)13C NMR spectra of[Bmim]Ac/mycelium solution at various mycelium concentrations.From bottom to top:0.00,5.80 and 12.70 g·mol-1.
The effect of penicillin mycelium concentration on13C chemical shifts of[Bmim]Ac was investigated and shown in Fig.4b.The signal of car boxyl carbon C12 in[Bmim]Ac moves down field,while the methyl carbon C11 in[Bmim]Ac presents an up field shift.The significant downfield shift of C12 atom indicates that acetate anions create stronger hydrogen bond with proton H of hydroxyl or amino groups in penicillin mycelium,which leads to a decrease of electron cloud density around C12 atoms.The up field shift of C11 is attributed to the increase of electron density around C11 atoms,because the stronger hydrogen bond between carboxyl and hydroxylor amino groups causes electronic distribution of acetate anion.In addition,the up field shifts of imidazolium cation C2,C4 and C5 atom signal were also found,which was attributed to the hydrogen bond between protons in[Bmim]+and hydroxyl oxygen or amino nitrogen in penicillin mycelium and led to an increase of electron cloud density around[Bmim]+.The1H and13C NMR spectra suggested that hydrogen bonds were the dominant driving force of mycelium dissolution.
To further verify the active site of[Bmim]Ac,FT-IR spectra of[Bmim]Ac and[Bmim]Ac/mycelium solution were studied.As seen in Fig.5,the peaks at 1384,1568,and 1176 cm-1are ascribed to O--C--O asymmetric and symmetric stretching and C--Ostretching,respectively.The peaks at 3056 cm-1and 3140 cm-1are ascribed to imidazole ring C2--H,C4--H and C5--H stretching[36].With the dissolution of penicillin mycelium,the peak at 1384 cm-1,1568 cm-1,3056 cm-1and 3140 cm-1shifted to a higher frequency and the peak at 1176 cm-1shifted to a lower frequency.The red shift of C--O stretching is ascribed to the formation of stronger hydrogen bond between C--O and mycelium.The blue shift of C2--H,C4--H and C5--H stretching is ascribed to the formation of weaker hydrogen bond,which is consistent with the results of NMR spectra.The results of FT-IR indicate that O atom in C--O and H atom in imidazole ring are the active site of[Bmim]Ac.The blue shift of O--C--O stretching is consistent with the change caused by hydrogen bond between acetate anion and penicillin mycelium[37].
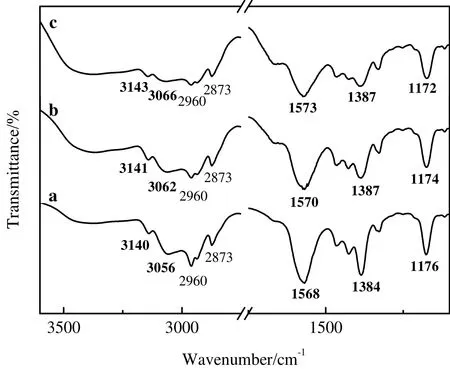
Fig.5.The FT-IR spectra of(a)[Bmim]Ac and(b)[Bmim]Ac/mycelium solution at mycelium concentrations of 6.40 g·mol-1 and(c)14.50 g·mol-1.
The possible mechanism of penicillin mycelium dissolution in ILs was shown in Fig.6.Firstly,O of both C=O and C--O in Ac-forms hydrogen bonds with proton H of--OH group of polysaccharides or--NH group of protein.Subsequently,aromatic protons H2,H4 and H5 of cation form hydrogen bonds with hydroxyl oxygen of--OH group of polysaccharides or amino nitrogen of--NH group of protein.
3.3.Effect of structure of ILs on dissolution capacity
Dissolution percentage of penicillin mycelium in imidazole-based ILs was shown in Table S1.Itcan be seen that the dissolution percentage of penicillin mycelium was significantly influenced by the anions.Among the ILs,[Bmim]Ac is the most efficient ionic liquid for the dissolution of penicillin mycelium with the dissolution percentage 89.91%at 90.0°C,while,the dissolution percentage of mycelium in[Bmim]MeSO4was 61.62%.The dissolution percentage of penicillin mycelium in[Bmim]BF4and in[Bmim]PF6was low.The dissolution percentage of penicillin mycelium in ILs decreased in the sequence:[Bmim]Ac>[Bmim]Cl>[Bmim][MeO]HPO2>[Bmim]MeSO4>[Bmim]BF4>[Bmim]PF6.Itindicated that anions of ILs played an important role in dissolving penicillin mycelium.In terms of cellulose and keratin,the dissolving capacity depends on the ILs'ability of breaking hydrogen bonds and sulfide bonds[12,18].The dissolving capacity also depends on the ILs'a bility of breaking intermolecular forces between mycelium molecular.It can be seen that anions with higher hydrogen bond-accepting ability such as OAc-,Cl-,[MeO]HPO2-and MeSO4-,showed higher ability,while anions with lower hydrogen bond-accepting ability such as BF4-and PF6-had lowerability to dissolve penicillin mycelium.This suggested that hydrogen bond-accepting ability affected the ILs'ability of breaking intermolecular forces in mycelium and led to the dissolution of penicillin mycelium.
The donating ability of cations or hydrogen bond accepting ability of the anions in the ILs can be characterized by α or β parameter of Kamlet-Taft,which is closely linked to the solubility of biomass[38-40].A higher β value indicates a stronger hydrogen bond accepting ability.Therefore,parameter β was used to examine the influence of anionic structure on dissolving capacity.To study the correlation of β and dissolving capacity,solubility of mycelium was used to evaluate the dissolving capacity.As shown in Table 1,it can be seen that β value of ILs with different anions varied significantly.Fig.7 shows a linear relationship between solubility of penicillin mycelium and β value of ILs.Solubility of penicillin mycelium increased with increasing hydrogen bond accepting ability of anions.This conclusion was similar to the result about microcrystalline cellulose that stronger hydrogen bond accepting ability of anions results in higher solubility of microcrystalline cellulose,which is due to the formation of hydrogen bonds[39].The result confirmed that hydrogen bond-accepting ability of anions was crucial to the dissolution of penicillin mycelium in ILs.
Table S2 shows the influence of cationic structures on dissolving performance of penicillin mycelium(see Supplementary Material).It can be seen that cations of ILs also have influence on the penicillin mycelium dissolution.In the case of CH3COO-,the solubility decreased in the following sequence:[Bmim]Ac>[Choline]Ac>[Benzylmim]Ac>N22Ac.For Cl-,dissolving ability of ILs decreased in the sequence:[Bmim]Cl>[N4444]Cl.The solubility data in Table S2 indicated that the ILs with N-heterocyclic aromatic cations,such as[Bmim]Ac,[Benzylmim]Ac,[Bmim]Cl showed better dissolving performance.While solubility in the ILs without N-heterocyclic aromatic cations,such as[N4444]Cl and[N22]Ac,was relatively low,due to the proton donating property of N-heterocyclic aromatic ring.

Fig.6.Possible mechanism of penicillin mycelium dissolution in[Bmim]Ac.(a)Interaction between[Bmim]Ac and hydroxyl group of polysaccharides and.(b)Interaction between[Bmim]Ac and amide group of protein.
Kamlet-Taft parameter α was used to study the effect of cations on dissolution of penicillin mycelium.As shown in Table 2,the values of α parameter varied greatly.For CH3COO-,the solubility of mycelium increased with the increasing of α parameter value.Fig.8 shows an approximate linear relationship between solubility of penicillin mycelium and α value of ILs.As reported in the literature[38],a higher α value indicates a stronger hydrogen bond donating ability,indicating that penicillin mycelium solubility increased with increasing hydrogen bond donating ability of cations.This indicated that a stronger hydrogen bond donating ability of cations improved penicillin mycelium solubility.The effect of anion and cation on penicillin mycelium solubility further emphasized that the formation of hydrogen bond was the driving force of penicillin mycelium dissolution.
4.Conclusions
The dissolution of penicillin mycelium was studied using ILs as solvents.Among the mentioned ILs,[Bmim]Ac had the highest dissolving capacity of 91.45%at 120.0°C with the[Bmim]Ac/mycelium(m/m)ratio of 3.90:1.At room temperature,the dissolution percentage of mycelium increased from 69.74%to 94.50%by adding DMSO at the optimalratio of DMSOto[Bmim]Ac of1.0(v/m).NMR and FT-IRspectra showed that hydrogen bond was the driving force of dissolution.The atoms H2,H4 and H5 in imidazolium cation and O in C=O and C--O in Ac-were the active sites to form hydrogen bonds with the proton H of--OH group of polysaccharides or--NH group of protein.Quantitative study of the Kamlet-Taft model showed that dissolution of penicillin mycelium was influenced by the hydrogen bond accepting ability of anions and donating ability of cations.There is a linearly positive correlation between the solubility of penicillin mycelium and β parameter of the ILs.Further study of designing functional ILs to improve the dissolution and degradation of antibiotics is still required.
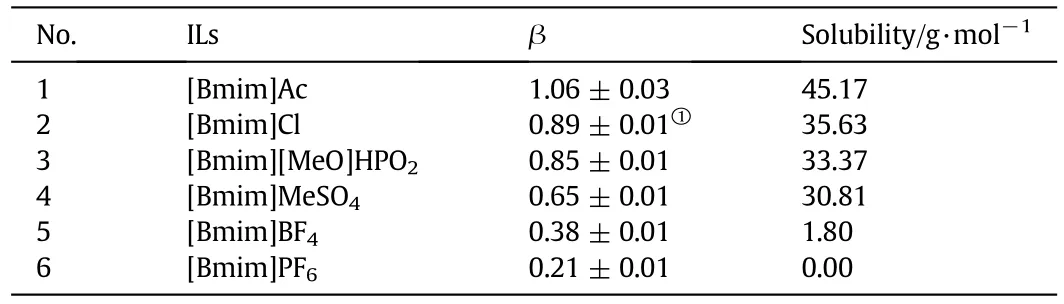
Table 1 The β parameter values of ILs with different anions
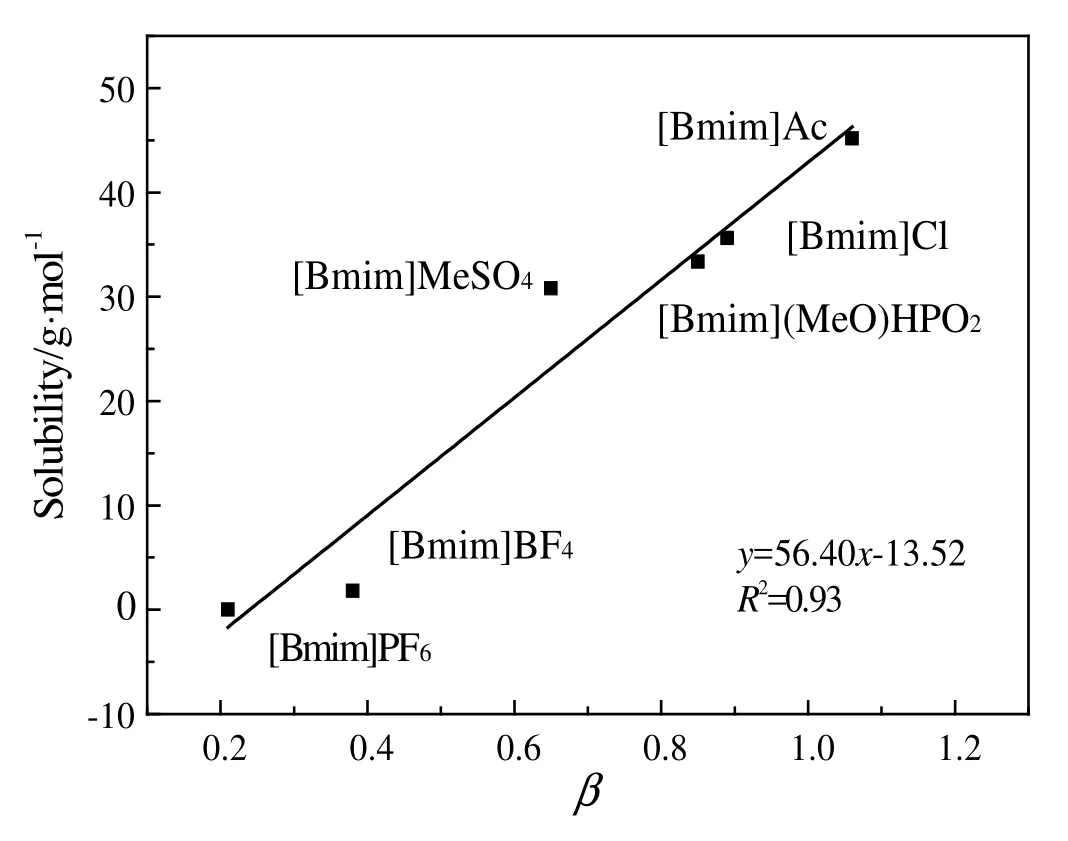
Fig.7.The linear correlation between solubility of penicillin mycelium and β parameter of the ILs.

Table 2 The α parameter values of ILs with different cations
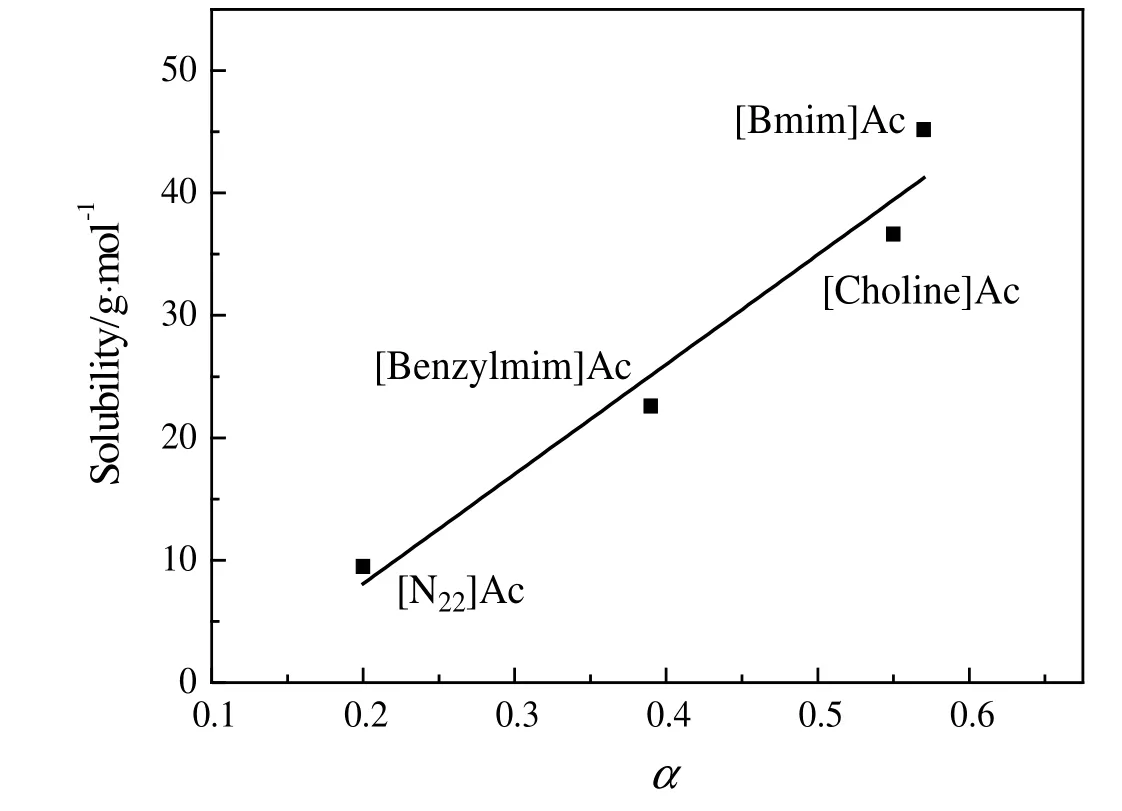
Fig.8.The linear correlation between solubility of penicillin mycelium andα parameter of the ILs.
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2017.04.003.
[1]A.B.Caracciolo,P.Grenni,F.Falconi,M.C.Caputo,V.Ancona,V.F.Uricchio,Pharmaceutical waste disposal:Assessment of its effects on bacterial communities in soil and groundwater,Chem.Ecol.27(1)(2011)43-51.
[2]A.D.Anderson,J.M.Nelson,S.Rossiter,F.J.Angulo,Public health consequences of use of antimicrobial agents in food animals in the United States,Microb.Drug Resist.9(4)(2003)373-379.
[3]J.L.Shimp,J.E.Kinsella,Composition of the mycelium of Penicillium roqueforti,J.Food Sci.42(3)(1977)681-684.
[4]V.V.Belakhov,A.V.Garabadzhiu,Directions of practical application of mycelial wastes of microbiological production of antibiotics in various areas of industry and agriculture,Russ.J.Gen.Chem.84(13)(2015)2664-2676.
[5]J.H.Sietsma,J.G.H.Wessels,Solubility of(1-3)-beta-D/(1-6)-beta-D-glucose in fungal walls:Importance of presumed linkage between glucan and chitin,J.Gen.Microbiol.125(1)(1981)209-212.
[6]T.Q.Wang,H.X.Li,M.Y.Wang,T.W.Tan,Integrative extraction of ergosterol,(1→3)-alpha-D-glucan and chitosan from Penicillium chrysogenum mycelia,Chin.J.Chem.Eng.15(5)(2007)725-729.
[7]N.New,W.F.Stevens,Production of fungal chitosan by solid substrate fermentation followed by enzymatic extraction,Biotechnol.Lett.24(2)(2002)131-134.
[8]J.W.Li,S.D.Ding,X.L.Ding,Optimization of the ultrasonically assisted extraction of polysaccharides from Zizyphus jujuba cv.jinsixiaozao,J.Food Eng.80(1)(2007)176-183.
[9]H.O.Kim,J.W.Yun,A comparative study on the production of expolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures,J.Appl.Microbiol.99(4)(2005)728-738.
[10]O.A.Arikan,W.Mulbry,C.Rice,Management of antibiotic residues from agricultural sources:Use of composting to reduce chlortetracycline residues in beef manure from treated animals,J.Hazard.Mater.164(2-3)(2009)483-489.
[11]J.I.R.Castanon,History of the use of antibiotic as growth promoters in European poultry feeds,Poult.Sci.86(11)(2007)2466-2471.
[12]H.Wang,G.Gurau,R.D.Rogers,Ionic liquid processing of cellulose,Chem.Soc.Rev.41(4)(2012)1519-1537.
[13]B.Ma,A.Qin,X.Li,C.J.He,Preparation of cellulose hollow fiber membrane from bamboo pulp/1-butyl-3-methylimidazolium chloride/dimethylsulfoxide system,Ind.Eng.Chem.Res.52(27)(2013)9417-9421.
[14]S.M.Raeisi,M.Tabatabaei,B.Ayati,A.Ghafari,S.H.Mood,A novel combined pretreatment method for rice straw using optimized EMIM[Ac]and mild NaOH,Waste Biomass Valoriz.7(2016)97-107.
[15]T.Rashid,C.F.Kait,I.Regupathi,T.Murugesan,Dissolution of kraft lignin using Protic Ionic Liquids and characterization,Ind.Crop.Prod.84(2016)284-293.
[16]M.M.Hossain,L.Aldous,Ionic liquids for lignin processing:dissolution,isolation,and conversion,Aust.J.Chem.65(11)(2012)1465-1477.
[17]Y.Wu,T.Sasaki,S.Irie,K.Sakurai,A novel biomass-ionic liquid platform for the utilization of native chitin,Polymer 49(9)(2008)2321-2327.
[18]A.Idris,R.Vijayaraghavan,A.F.Patti,D.R.MacFarlane,Distillable protic ionic liquids for keratin dissolution and recovery,ACS Sustain.Chem.Eng.2(7)(2014)1888-1894.
[19]J.M.Zhang,H.Zhang,J.Wu,J.Zhang,J.S.He,J.F.Xiang,NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids,Phys.Chem.Chem.Phys.12(8)(2010)1941-1947.
[20]K.L.Zhuo,Y.J.Chen,J.Che,G.Y.Bai,J.J.Wang,Interactions of 1-butyl-3-methylimidazolium carboxylate ionic liquids with glucose in water:a study of volumetric properties,viscosity,conductivity and NMR,Phys.Chem.Chem.Phys.13(32)(2011)14542-14549.
[21]H.B.Liu,K.L.Sale,B.M.Holmes,B.A.Simmons,S.Singh,Understanding the interactions of cellulose with ionic liquids:A molecular dynamics study,J.Phys.Chem.B 114(12)(2010)4293-4301.
[22]Y.Y.Yao,Y.Li,X.M.Liu,X.C.Zhang,J.J.Wang,X.Q.Yao,S.J.Zhang,Mechanistic study on the cellulose dissolution in ionic liquids by density functional theory,Chin.J.Chem.Eng.23(2015)1894-1906.
[23]T.V.Doherty,M.Mora-Pale,S.E.Foley,R.J.Linhardt,J.S.Dordick,Ionic liquid solvent properties as predictors of lignocellulose pretreatment efficacy,Green Chem.12(11)(2010)1967-1975.
[24]Y.Fukaya,K.Hayashi,M.Wada,H.Ohno,Cellulose dissolution with polar ionic liquids under mild conditions:Required factors for anions,Green Chem.10(2008)44-46.
[25]J.M.M.Araújo,R.Ferreira,I.M.Marrucho,L.P.N.Rebelo,Solvation of nucleobases in 1,3-dialkylimidazolium acetate ionic liquids:NMR spectroscopy insights into the dissolution mechanism,J.Phys.Chem.B 115(36)(2011)10739-10749.
[26]H.M.Choi,I.Kwon,Dissolution of zein using protic ionic liquids:N-(2-hydroxyethyl)ammonium formate and N-(2-hydroxyethyl)ammonium acetate,Ind.Eng.Chem.Res.50(50)(2011)2452-2454.
[27]A.Idris,R.Vijayaraghavan,U.A.Rana,D.Fredericks,A.F.Patti,D.R.MacFarlane,Dissolution of feather keratin in ionic liquids,Green Chem.15(2)(2013)525-534.
[28]Y.L.Zhao,X.M.Liu,J.J.Wang,S.J.Zhang,Insight into the cosolvent effect of cellulose dissolution in imidazolium-based ionic liquid systems,J.Phys.Chem.B 117(30)(2013)9042-9049.
[29]W.Fawcett,R.P.Brooksby,D.Verbovy,I.Bakó,G.Pálinkás,Studies of anion solvation in polar aprotic solvents,J.Mol.Liq.118(1-3)(2005)171-178.
[30]A.R.Xu,X.Guo,R.Xu,Understanding the dissolution of cellulose in 1-butyl-3-methyl imidazolium acetate+DMAc solvent,Int.J.Biol.Macromol.81(2015)1000-1004.
[31]J.M.Andanson,E.Bordes,J.Devémy,F.Leroux,A.A.H.Pádua,M.F.C.Gomes,Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids,Green Chem.16(5)(2014)2528-2538.
[32]Y.Chen,Y.Y.Cao,Y.W.Zhang,T.C.Mu,Hydrogen bonding between acetate-based ionic liquids and water:three types of IR absorption peaks and NMR chemical shifts change upon dilution,J.Mol.Struct.1058(1)(2014)244-251.
[33]S.T.Handy,M.Okello,The 2-position of imidazolium ionic liquids:Substitution and exchange,J.Org.Chem.70(5)(2005)1915-1918.
[34]H.Shimura,M.Yoshio,K.Hoshino,T.Mukai,H.Ohno,T.Kato,Noncovalent approach to one-dimensional ion conductors:Enhancement of ionic conductivities in nanostructured columnar liquid crystals,J.Am.Chem.Soc.130(2008)1759-1765.
[35]M.R.Chierotti,R.Gobetto,Solid-state NMR studies of weak interactions in supramolecular systems,Chem.Commun.39(31)(2008)1621-1634.
[36]A.Mehrdad,Z.Niknam,Investigation of interaction between polyethylene oxide and ionic liquid 1-octyl-3-methyl-imidazolium bromide in aqueous solutions by spectroscopic and viscometric methods,J.Mol.Liq.223(2016)100-106.
[37]X.Y.Tan,X.X.Li,L.Chen,F.W.Xie,Solubility of starch and microcrystalline cellulose in 1-ethyl-3-methylimidazolium acetate ionic liquid and solution rheological properties,Phys.Chem.Chem.Phys.18(39)(2016)27584-27593.
[38]B.Lu,A.Xu,J.Wang,Cation does matter:how cationic structure affects the dissolution of cellulose in ionic liquids,Green Chem.16(3)(2014)1326-1335.
[39]A.R.Xu,J.J.Wang,H.Y.Wang,Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems,Green Chem.12(2)(2010)268-275.
[40]C.Chiappe,D.Pieraccini,Ionic liquids:solvent properties and organic reactivity,J.Phys.Org.Chem.18(4)(2005)275-297.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Transport hindrances with electrodialytic recovery of citric acid from solution of strong electrolytes
- Experimental investigation on CO2-light crude oil interfacial and swelling behavior
- Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase☆
- Process development for producing a food-grade glucose solution from rice straws
- Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
- Biodegradation of natural and synthetic estrogens in moving bed bioreactor
