Biodegradation of natural and synthetic estrogens in moving bed bioreactor
Mohammad Mehdi Amin ,Bijan Bina ,Karim Ebrahim ,Zeynab Yavari*,Farzaneh Mohammadi
1 Environment Research Center,Research Institute for Primordial Prevention of Non-communicable Disease,Isfahan University of Medical Sciences,Isfahan,Iran
2 Department of Environmental Health Engineering,School of Health,Isfahan University of Medical Sciences,Isfahan,Iran
3 Student Research Committee,Isfahan University of Medical Sciences,Isfahan,Iran
1.Introduction
Many regulatory agencies,such as WHO,USEPA and EU have listed EDC as priority pollutants[1-3].Estrogenic hormones are a group of EDCs that can interfere with endocrine system in humans and animals[4,5].Estrone(E1),17β-estradiol(E2)Natural estrogens,and 17α-ethinyl estradiol(EE2)synthetic estrogen,have considered as substances,which have high potential for endocrine-disrupting effects.Many of wastewater treatment plants(WWTPs)do not have enough potential for elimination of micropollutants.Therefore,numerous studies have found the ef fluent of WWTPs as main sources for introduction of these compounds in aquatic environment[5-8].Consequently,the occurrence of EDCs,menace health of humans and wildlife and inducement detrimental effects on aquatic life especially in receiving water[7,9,10].Biodegradation,adsorption,air stripping and photolysis are four important mechanisms for EDC removal within wastewater treatment process[11-13].Nevertheless,the biodegradation and the sorption are propound to be the most substantial contribution in decomposition of these compounds.Other studies which have been conducted in WWTPs,reported roughly 50%-95%of estrogenic compounds(E1,E2,and EE2)can be removed in conventional activated sludge plants[14-16].Researchers announced the fate of EDCs in microbial degradation,affected by some operation parameters such as hydraulic retention time(HRT)and solid retention time(SRT),physicochemical characteristics of hormones and biomass particle[17,18].SRT and HRT are two mostcrucialoperationalparameters in biologicalwastewater treatment processes,particularly in a nitrification/denitrification process.At long SRT,slow growing bacteria such as nitrifying cultures,would have enough time to proliferation[19].De Mes et al.reported that at high HRT and SRT,removal efficiency of estrogenic compounds can be enhanced[20].In the work by Joss et al.the removal of E1,E2,and EE2 in WWTP was more than 90%,specifically in nitrification and denitrification processes which SRT was 12-15 days[21].According to applied results of Koh et al.,which measured the mass balance in a nitrifying/denitrifying activated sludge plantatSRT>13 days,the biodegradation rate of estrogens was 70%[22].The biodegradability of natural and synthetic estrogens has been reported by Hashimoto and Murakami in an oxidation-ditch process.They represented that the degradation of E2 was achieved very fast at SRT>15 days;while EE2 had lag phase of 2 h and the degradation was accomplished after 24 h[23].
Contrary to conventional and advanced wastewater treatment processes,the attached growth processes are effective and have many advantages over the suspended growth.The Moving Bed Bioreactor(MBBR),as an attached growth process is executable technology and has been recently gaining amicability.The inherent preponderance in the following aspect including,high biomass activity and redox conditions in biofilm improve the elimination of micropollutants,organic substances,nitrification and denitrification[24,25].Ehsan Ahmadi reported that,more than 92%of phthalates removal was achieved in MBBR.Yunlong Luo et al.investigated the removal of micropollutants in a sponge-based MBBR.They determined that removal rate has been between 25.9%and 96.8%[25].To our knowledge,previous experiments were notconducted for the identification ofthe effectofHRT and SRT for elimination rates ofE1,E2,and EE2 in MBBR.This paperseeks to address the effectof these operational parameters(SRT and HRT)for removal of estrogens.The analytes were extracted by a pre-concentration technique,Dispersive Liquid-Liquid Microextraction(DLLME),and detected by gas chromatography followed with mass spectrometry(GC-MS).
2.Materials and Methods
2.1.Reagents
All chemicals were of analytical reagent grade.The target steroidal compounds included Estrone(E1),17β-estradiol(E2),17α-ethinyl estradiol(EE2),pyridine and derivatization reagent N-O bis(trimethyl)tri fluoroacetamide(BSTFA)containing 1%of trimethylchlorosilane(TMCS)were purchased from Sigma.MeOH of GC grade obtained from Merck.All glassworks are washed with nitric acid(6 mol·L-1)and then consecutive with deionized water before use.Separate stock solutions for E1 and E2 in 200 mg·L-1and 100 mg·L-1for EE2 supplied by dissolving an appropriate amountofeach substance in methanoland were stored at 4°C prior to use.The working solutions of specific standards were prepared through the serial dilution of stock solutions and pure deionized water.
2.2.MBBR system
The experiments conducted using the lab scale MBBR which schematic diagram presented in Fig.1.
A cylindrical shaped reactor was used with an internal diameter of 150 mm and 500 mm in height,operating volume of 5 L and filled with 40%(determined from the batch experiments)of acclimatized carrier of floating media.Table 1 summarizes characteristics of the media.
Air diffusers and in fluentpipe were installed atthe bottom of the reactor.The Coarse bubble diffusers had flowed for 4 min-1and provided oxygen concentration between 4 and 5 mg·L-1for bacterial activity and circulation of carriers.The sampling ports for sample collection were set in reactor.The reactor operated in an up- flow mode.Syntheticwastewater fed continuously from storage tank into bioreactor through pump(Etatron-Italy).Synthetic wastewater composition including glucose(600 mg·L-1)as carbon source and(NH4)2SO4(90 mg·L-1),KH2PO4and K2HPO4(9 mg·L-1)as nutrient was employed in all experiments.Also,the wastewater was enriched with the following components,as mg·L-1,including:4.4 CaCl2·2H2O,12.2 MgSO4·7H2O,0.05 MnCl2·4H2O,0.132 ZnSO4·7H2O,18.2 FeCl3,0.01 CuSO4·5H2O,0.04 CoCl2·6H2O,0.15 Na2MoO4·2H2O,0.054 KI and 0.045 H3BO3[26].To set up the system,reactor was acclimatized for microbial growth by synthetic wastewaterwithouthormonesand activated sludge within 120 days.Afterwards,synthetic wastewater was spiked with hormones at different organic loading rates and HRTs were continuously introduced to the bioreactor(Table 2).The elimination rate of natural and synthetic hormones was carried out over a period of 120 days.NaHCO3and NaOH were used to adjust the pH and alkalinity.

Table 1 Characteristics of the floating media

Table 2 Technical data and operation results for the moving bed biofilm reactor
2.3.Analytical methods

Fig.1.Schematic diagram of the lab scale MBBR system.
Analysis of soluble and chemical oxygen demand(sCOD)and rapid biodegradability chemical oxygen demand(rbCOD)in ef fluent,biosolids(as MLSS)and biomass as(MLVSS),NH4-N,NO2-N,NO3-N,and PO4-P was performed periodically according to standard methods[27].The attached growth bio-solid concentration was determined by difference in mass of10 new carriers withoutbiomass and with biomass which were dried at 105°C for 24 h[28].The biofilm structure was detached in HCl 5%during one week and analyzed by scanning electron microscopy(SEM).Ef fluent samples(100 ml)for analysis of Estrone,17β-estradiol and 17α-ethinyl estradiol were taken out every other day from the reactor, filtered through glass fiber filters(GF/F,0.45 μm,Whatman™),and stored at 4 °C before the analysis.The content of E1,E2 and EE2 in sludge phase was measured according to Qingling Zeng and Kaushalya C.Wijekoon[29,30].
2.4.Dispersive liquid–liquid microextraction
DLLME is a pre-concentration technique which has been used for the extraction of micropollutants[31].This method consists of a formation of cloudy solution in the conical test tube by injecting an appropriate mixture of extractive which is denserthan waterand dispersive solvent thatcan be solved in both phases to aqueous sample containing analytes[32,33].Subsequently,this cloudy solution was centrifuged and formed two distinct phases at the bottom of test tube[34].A volume of 5.0 ml sample of ef fluent from the reactor was poured in a 10 ml capped tube with a conical bottom and spiked with 10 μl of n-octyl phenol as internal standard.Under optimal conditions,100 μl of chloroform(extractive solvent)and 500 μl of methanol(dispersive solvent)were injected rapidly into tube.A cloudy solution formed and was centrifuged for 5 min at 5000 r·min-1.The lower phase extracted by a micro syringe and transferred into a 2 mL vial.The extracted evaporated to dryness under a gentle flow of nitrogen.The dry residue was reconstituted for derivatization.
2.5.Derivatization procedure
As steroids encompass both of carbonyl and hydroxyl groups,derivatization is necessary to enhance chromatographic separation to decrease the polarity and increase the volatility thermal stability of analytes[35].Brie fly,10 μl of BSTFA containing 1%of TMCS and 20 μl pyridine were added to dry residue and heated at 70°C for 30 min in a water bath[36].Pyridine prevents the inter-conversion between EE2 and E1 during derivatization[37].The derivatives were cooled to room temperature and 3 μl was injected to GC-MS for analysis.All experiments were performed in triplicates.
2.6.GC–MS analysis
GC-MS analysis was performed using a gas chromatograph(7890A Agilent Technologies,USA)interfaced with a mass spectrometry(5975C series).Helium carrier gas was maintained at a constant flow rate of 1.5 ml·min-1.The GC column temperature was programmed from 100(initial equilibrium time 1 min)to 200°C via a ramp of 10 °C·min-1,200-260 °C via a ramp of 15 °C·min-1,and 260-300 °C via a ramp of 3 °C·min-1and held at 300 °C for 2 min.The MS was operated in SIM scan mode from m/z,50-600 for qualitative and quantitative analyses[38].The molecular ions[M]+with m/z 342,416 and 425 correspond to E1,E2 and EE2,respectively[26].Sample injection(1 μl)was in splitless mode.Limit of detection(LOD),limit of quantification(LOQ)and extraction recovery of target analytes were presented in Table 3.In addition,Fig.2 depicts the chromatogram of Estrone,17β-estradiol and 17α-ethinyl estradiol.
3.Results and Discussion
3.1.Removal of conventional parameters
Fig.3 depicted the removal efficiency of operating parameters including rbCOD and NH4-N and ef fluent concentration of E1,E2 and EE2 in MBBR system during operation at different SRTs.The results showed the stable removal of rbCOD achieved in the whole experiment(95%-97%),and sCOD concentration in ef fluent was<20 mg·L-1.The removal efficiency of NH4-N enhanced over time from 57.5%to 82%with increasing the SRT from 11 to 46 days.The current data agree well with the results of Yu Luo,who expressed that the NH4-N removal was 73.6%-87%[39].The overall removal of total phosphorous(TP)was 20.2%±0.8%during the study.PO4-P removal measured by Yu Luo was less than those reported by Qingling Zeng who used anaerobic,anoxic,and aerobic reactors for elimination of estrogen and nutrients[11,39].
3.2.Effect of SRT and HRT in removal of steroid hormones
During MBBR process,two crucial operational parameters(i.e.SRT and HRT)can impress the reduction of estrogen.A lab scale MBBR was evaluated for biological removal of E1,E2 and EE2 at various HRTs(4,8,12,and 16 h)and corresponding SRTs(11,23,35,and 46 days).Minimal reduction rates of 81%,98.6%,and 71%were obtained for E1,E2,and EE2,at SRT 11 days,respectively.By a gradual increase of HRT and SRT,the elimination rate of E1,E2,and EE2 was enhanced and achieved 98%,99.9%,and 95%,respectively at high SRT(46 days).Generally,the overall removal rate of natural and synthetic estrogens in MBBR was more than 90%in SRTs 46 and 35 days.Fig.4 presents the relationship between the SRT and HRT for removal of E1,E2,and EE2.These results demonstrated link between SRT and biodegradability of estrogenic compounds.SRT amends microbial community for the degradation of hormones and establishes biodiversity of microbial community for enrichment of the slowly growing bacteria such as nitrifying bacteria which are responsible for the degradation of recalcitrant pollutants such as EE2[40].Ethinyl estradiol(EE2)has an ethinyl group functional which makes much more resistance for microbial degradation in low SRT[26,40].Likewise,long HRT increments the contact time to biomass and augments the estrogens removal.
The elimination rate ofE1 and EE2 drastically raised about19%when HRT was increased from 4 to 16 h.While for E2,variations were not noticeable.In other words,the elimination rate of E1 and EE2 was affected by different SRTs and HRTs while was not for E2.These findings suggest that the higher removal efficiency of estrogens is associated with a SRT of 46 days and HRT of 16 h.This finding is supported by Clara et al.who introduced SRT of 10 days as“critical SRT”for elimination of micropollutants.Also,they pinpointed that SRT more than 10 days,can enhance the biodegradability of some compounds such as natural estrogens,ibuprofen and bisphenol A[41].Lee et al.and Suarez et al.found that in biological processes,EE2 has more resistance compared to E1 and E2[42,43].Also Suárez et al.[43]suggested that by applying high SRT,removal efficiency for fluoxetine,citalopram and ethinylestradioldrastically increased more than 10%.Edson B etal.identified in MBR that,high efficiency of estrogen removal was obtained at SRT and HRT of 60 days and 12 h,respectively[26].

Table 3 LOD,LOQ,and recoveries of E1,E2,and EE2

Fig.2.GC-MS(SIM)chromatograms of E1,E2,and EE2.
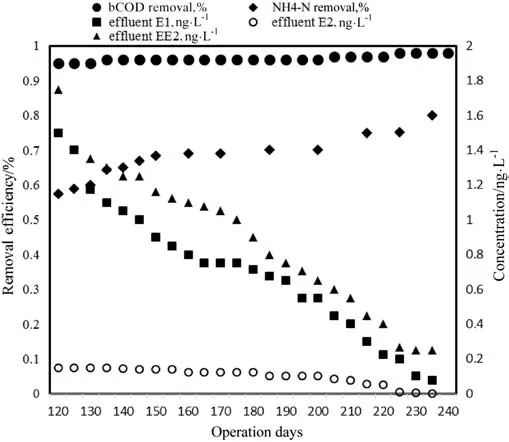
Fig.3.Removal of conventional parameters in MBBR system during operation of reactor.
3.3.Nitrification and estrogen removal rate

Fig.4.Removal efficiency of E1,E2,and EE2 at different SRTs and HRTs.
Estrogen removal efficiency in MBBR improved at high SRT,which notion is owing to development of nitrification.Table 3 summarizes the data for hormones that were acquired during operation of reactor.The experimental data illustrated a link between removal of E1,E2,and EE2 and nitrification rate in various SRTs.It is apparent from Fig.5 that in low SRT(11 days)which nitrification rates were slow too,nitrification rate was 54%and estrogen removal rates were 1.16,1.41,and 1.09 μg·(g VSS)-1h-1for E1,E2,and EE2,respectively.High elimination rates of estrogens were observed at 46 days SRT,which nitrification rate augmented to 84%.In this SRT,specific removal rate of E1,E2 and EE2 was 0.23,0.25,and 0.21 μg·(g VSS)-1,respectively.

Fig.5.Relationship between N and estrogen removal rate.
Conclusion drawn from these data is that nitrify enrichment condition has af firmative effect on estrogen elimination.According to data,the removal rates of E1(81%-98%)and EE2(76%-95%)were slightly lower than E2(98.58%-99.9%).E2 was very efficiently eliminated in this system.At first stage of degradation,E2 converted to E1.On the other hand,E2 and E1 were considered biodegraded metabolically by heterotrophic organisms in wastewater.However,the ethinyl group in EE2 makes it more recalcitrant and steric ally hindered for metabolism by heterotrophs.These findings were confirmed by other researchers[18,40,44].Nitrification cause to EE2 oxidized initially by nitrifier enrichment cultures to intermediates compounds thereinafter avail as a substrate for heterotrophic cultures[18].In addition,by increasing the biofilm thickness,transfer of oxygen to deeper parts of biofilm was limited by diffusion,which leads to denitrification.As a rule of thumb,E1 and E2 can be degraded in nitrifying and denitrifying conditions,while for the degradation of EE2 nitrifying cultures is necessary[30,45].These results are supported by Yi et al.(2007),who demonstrated in activated sludge,EE2 degraded enriched with nitrifying bacteria strains[17].Numerous studies reported that the degradation of E2 and EE2 could be performed by heterotrophic bacteria,ammonia oxidizing bacteria(AOB)and nitrifying activated sludge(NAS)through cometabolism[17,18].Also,they confirmed that the biotransformation of EE2 cometabolically,is accomplished in enrich nitrifying cultures[17,18].As a whole,these findings express that the cometabolism can be considered as a crucial process for the removal of estrogens from WWTPs.
3.4.Steroids in MBBR biofilm
Microbial population and physical properties of biofilm determine the affinity of estrogenic compounds for sorption or biodegradation.The fractions of E1,E2 and EE2,which were biodegraded and adsorbed,and the residual contents in biofilm at different SRTs(11-46 days)are shown in Fig.6(a,b and c).Of the overall E1 removal efficiencies(81%-98%),the contribution of biodegradation,sorption,and release to ef fluent was 79%-96.5%,1.4%-1.8%and 2%-19%,respectively.Approximately>98%ofE2 was biodegraded,and just<1.5%was adsorbed to biomass.The concentration of E2 in ef fluent was negligible.As above mentioned,SRT has no substantial effect on E2.In other words,E2 completely transformed within operation of reactor.These results are in agreement with Pholchan et al.[46]who indicated that the biodegradation rate of E2 in SBR(sequencing batch reactors)(>0.98%)was independent of SRT.

Fig.6.Contribution of biodegradation,adsorption,and release of E1,E2,and EE2 at different SRT s.

Table 4 Experimental results at different sludge retention times
For EE2,biodegradation rate was more important than biosorption at high SRT.The contribution of biodegradation and adsorption to biofilm was 76%-95%and 1.2%-5.7%,respectively and 5%-24%of EE2 released to ef fluent.Whereas nitrification has a positive effect on EE2 removal,the microbialdegradation of EE2 may induce in enriched nitrifying cultures that promote in high SRT.Our research has underlined the importance of biodegradation and sorption to bio floc as principle mechanisms.In MBBRprocess elimination rate wasascribed to sorption onto biomass and the conformance biotransformation.At the beginning operation of the reactor sorption onto biomass particles was assumed as a predominant process.This spontaneous adsorption is due to physisorption and slightly chemisorption.At long HRT,the microbial communities attached to the media have enough time for the degradation of hormones.This was presumably due to the presence of acclimatized film degrading microorganisms(heterotroph and autotroph)on media.Then,during biotransformation,the estrogenic compounds by losing the phenolic hydroxyl group get a negative charge.The charge repulsion between negatively charged compounds and biomass consequently prohibits the removal of them.These conclusions were supported by Luo Yu,who investigated the removal of micropollutants by MBBR.They found that the biodegradation was considered as main removal pathway for most compounds and biosorption to sludge for resistant EDCs[39].Also,Andersen et al.indicated that the small fraction of steroid hormones sorbed to sludge compared with biodegradation[47].Adsorption of estrogens onto sludge was determined by solid-water distribution coefficient(Kd)which is the ratio of steroid concentration in solid and water phase[1].As can be seen from the data(Table 4),lg Kdwas 2.54-2.76 for E1,2.67-2.82 for E2 and 2.85-2.94 for EE2[3].In the study by Clara et al.lg Kdfor E2 and EE2 was measured 2.64-2.97 and 2.71-3.00,respectively[48].Yunlong Luo found that for compounds with lg Kdbelow 2.48,the sorption onto solids can be considered to be insignificant[1].For persistence compounds,accumulation to biofloc is considered as principle pathway.Ethinyl estradiol has high hydrophobicity compared to E1 and E2.It appears that,the fraction of biosorption to biomass at low SRT was more important for elimination of EE2 from wastewater.It seems that these conclusions have a good agreement with Johnson and Sumpter which noted that the recalcitrant and hydrophobic nature of EE2 allows agglomeration in sludge[49].Altogether,it can be concluded that the elimination of estrogens by adsorption to biomass is not important for the last destination of these compounds compared to biodegradation.
4.Conclusions
The removal efficiency of natural and synthetic steroid estrogens in WWTPs can be in fluenced by hydraulic and sludge retention times.The results showed that at prolonged SRT and HRT,90%-95%removal in estrogenic loading related to biodegradation and 3%-5%of these compounds were absorbed to biomass.High potential for the estrogen removal by biodegradation in MBBR was observed,allowing less estrogen concentration in liquid phase for the adsorption of these compounds onto bioflocs.The high presence of acclimatized film degrading microorganisms(heterotroph and autotroph)on media,causes the estrogens to readily bind with this organic matter.Estrogen specific removal rate was between 0.22-1.45 μg·(g·VSS)-1·d-1for natural and synthetic hormones.Results from the whole experiments,illustrated the biodegradability of hormones in order E2,E1 and EE2.Accordingly,E2 and EE2 are easily and recalcitrant estrogenic compounds to microbial degradation,respectively.Therefore,removal efficiency of estrogenic compounds was increased by MBBR compared to other biological wastewater treatment processes.
Acknowledgments
This article is the result of Ph Dthes is approved in the Isfahan University of Medical Sciences(IUMS).The authors wish to acknowledge to Vice Chancellery of Research of IUMS for the financial support,Research Project,#394774.
[1]Y.Luo,W.Guo,H.H.Ngo,L.D.Nghiem,F.I.Hai,J.Zhang,S.Liang,X.C.Wang,A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment,Sci.Total Environ.473-474(2014)619-641.
[2]H.Andersen,H.Siegrist,B.Halling-Sorensen,T.A.Ternes,Fate of estrogens in a municipal sewage treatment plant,Environ.Sci.Technol.37(2003)4021-4026.
[3]H.Hamid,C.Eskicioglu,Fate of estrogenic hormones in wastewater and sludge treatment:A review of properties and analytical detection techniques in sludge matrix,Water Res.46(2012)5813-5833.
[4]Y.Yu,L.Wu,Analysis of endocrine disrupting compounds,pharmaceuticals and personal care products in sewage sludge by gas chromatography-mass spectrometry,Talanta 89(2012)258-263.
[5]M.Auriol,Y.Filali-Meknassi,R.D.Tyagi,C.D.Adams,R.Y.Surampalli,Endocrine disrupting compounds removal from wastewater,a new challenge,Process Biochem.41(2006)525-539.
[6]E.Lundström,B.Björlenius,M.Brinkmann,H.Hollert,J.O.Persson,M.Breitholtz,Comparison of six sewage ef fluents treated with different treatment technologies—Population level responses in the harpacticoid copepod Nitocra spinipes,Aquat.Toxicol.96(2010)298-307.
[7]T.A.Ternes,M.Stumpf,J.Muller,K.Haberer,R.-D.Wilken,M.Servos,Behaviour and occurrence of estrogens in municipal sewage treatment plants:I,Investig.Ger.Canada Brazil,Sci.Total Environ.225(1999)81-90.
[8]B.Bina,F.Mohammadi,M.M.Amin,H.R.Pourzamani,Z.Yavari,Determination of 4-nonylphenol and 4-tert-octylphenol compounds in various types of wastewater and their removal rates in different treatment processes in nine wastewater treatment plants of Iran,Chin.J.Chem.Eng.(2017),http://dx.doi.org/10.1016/j.cjche.2017.04.009.
[9]J.Bratby,Endocrine Disruptors in Water and Wastewater Treatment,2012.http://www.doc88.com/p-3522961448945.html.
[10]H.Wu,C.Lai,G.Zeng,J.Liang,J.Chen,J.Xu,J.Dai,X.Li,J.Liu,M.Chen,L.Lu,L.Hu,J.Wan,The interactions of composting and biochar and their implications for soil amendment and pollution remediation:A review,Crit.Rev.Biotechnol.(2016)1-11.
[11]Q.Zeng,Y.Li,S.Yang,Sludge retention time as a suitable operational parameter to remove both estrogen and nutrients in an anaerobic-anoxic-aerobic activated sludge system,Environ.Eng.Sci.30(2013)161-169.
[12]W.J.Sim,J.W.Lee,S.K.Shin,K.B.Song,J.E.Oh,Assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants,Chemosphere 82(2011)1448-1453.
[13]H.Wu,G.Zeng,J.Liang,J.Chen,J.Xu,J.Dai,X.Li,M.Chen,P.Xu,Y.Zhou,F.Li,L.Hu,J.Wan,Responses of bacterial community and functional marker genes of nitrogen cycling to biochar,compost and combined amendments in soil,Appl.Microbiol.Biotechnol.100(2016)8583-8591.
[14]M.Auriol,Y.Filali-Meknassi,C.D.Adams,R.D.Tyagi,T.N.Noguerol,B.Piña,Removal of estrogenic activity of natural and synthetic hormones from a municipal wastewater:Efficiency of horseradish peroxidase and laccase from trametes versicolor,Chemosphere 70(2008)445-452.
[15]B.Pauwels,H.Noppe,H.De Brabander,W.Verstraete,Comparison of steroid hormone concentrations in domestic and hospital wastewater treatment plants,J.Environ.Eng.134(2008)933-936.
[16]L.Gunnarsson,M.Adolfsson-Erici,B.Björlenius,C.Rutgersson,L.Förlin,D.G.J.Larsson,Comparison of six different sewage treatment processes—Reduction of estrogenic substances and effects on gene expression in exposed male fish,Sci.Total Environ.407(2009)5235-5242.
[17]T.Yi,W.F.Harper,The link between nitrification and biotransformation of 17α-ethinylestradiol,Environ.Sci.Technol.41(2007)4311-4316.
[18]B.Pauwels,K.Wille,H.Noppe,H.De Brabander,T.Van De Wiele,W.Verstraete,N.Boon,17α-Ethinylestradiol cometabolism by bacteria degrading Estrone,17β-estradiol and Estriol,Biodegradation 19(2008)683-693.
[19]P.Falås,H.R.Andersen,A.Ledin,J.la Cour Jansen,Impact of solid retention time and nitrification capacity on the ability of activated sludge to remove pharmaceuticals,Environ.Technol.33(2012)865-872.
[20]T.de Mes,G.Zeeman,G.Lettinga,Occurrence and fate of estrone,17β-estradiol and 17α-ethynylestradiol in STPs for domestic wastewater,Rev.Environ.Sci.Biotechnol.(2005)275-311.
[21]A.Joss,H.Andersen,T.Ternes,P.R.Richle,H.Siegrist,Removal of estrogens in municipal wastewater treatment under aerobic and anaerobic conditions:Consequences for plant optimization,Environ.Sci.Technol.38(2004)3047-3055.
[22]Y.K.K.Koh,T.Y.Chiu,A.R.Boobis,M.D.Scrimshaw,J.P.Bagnall,A.Soares,S.Pollard,E.Cartmell,J.N.Lester,In fluence of operating parameters on the biodegradation of steroid estrogens and nonylphenolic compounds during biological wastewater treatment processes,Environ.Sci.Technol.43(2009)6646-6654.
[23]T.Hashimoto,T.Murakami,Removal and degradation characteristics of natural and synthetic estrogens by activated sludge in batch experiments,Water Res.43(2009)573-582.
[24]M.Žubrowska-Sudol,Moving bed technology as an alternative solution for reducing bioreactor volume,Environ.Prot.Eng.38(2012)15-22.
[25]Y.Luo,A sponge-based moving bed bioreactor for micropollutant removal from municipal wastewater,Thesis(2014)http://hdl.handle.net/10453/29223.
[26]E.B.Estrada-Arriaga,P.N.Mijaylova,In fluence of operational parameters(sludge retention time and hydraulic residence time)on the removal of estrogens by membrane bioreactor,Environ.Sci.Pollut.Res.18(2011)1121-1128.
[27]APHA/AWWA/WEF,Standard Methods forthe Examination ofWater and Wastewater,American Water Works Assn,USA,2012.
[28]J.L.Shore,W.S.M'Coy,C.K.Gunsch,M.A.Deshusses,Application of a moving bed biofilm reactor for tertiary ammonia treatment in high temperature industrial wastewater,Bioresour.Technol.112(2012)51-60.
[29]K.C.Wijekoon,F.I.Hai,J.Kang,W.E.Price,W.Guo,H.H.Ngo,L.D.Nghiem,The fate of pharmaceuticals,steroid hormones,phytoestrogens,UV- filters and pesticides during MBR treatment,Bioresour.Technol.144(2013)247-254.
[30]Q.Zeng,Y.Li,G.Gu,Nitrate-dependent degradation of 17α-ethinylestradiolby acclimated activated sludge under anaerobic conditions,J.Chem.Technol.Biotechnol.84(2009)1841-1847.
[31]C.Almeida,J.O.Fernandes,S.C.Cunha,Anoveldispersive liquid-liquid microextraction(DLLME)gas chromatography-mass spectrometry(GC-MS)method for the determination of eighteen biogenic amines in beer,Food Control 25(2012)380-388.
[32]L.Miri,F.Jalali,Dispersive liquid-liquid micro-extraction as a sample preparation method for clonazepam analysis in water samples and pharmaceutical preparations,Journal of Reports in Pharmaceutical Science 2(2013)103-110.
[33]J.Aufartová,C.Mahugo-Santana,Z.Sosa-Ferrera,J.J.Santana-Rodríguez,L.Nováková,P.Solich,Determination of steroid hormones in biological and environmental samples using green microextraction techniques:An overview,Anal.Chim.Acta 704(2011)33-46.
[34]A.Zgoła-Grześkowiak,T.Grześkowiak,Dispersive liquid-liquid microextraction,TrAC Trends Anal.Chem.30(2011)1382-1399.
[35]M.D.Hernando,M.Mezcua,M.J.Gómez,O.Malato,A.Agüera,A.R.Fernández-Alba,Comparative study of analytical methods involving gas chromatography-mass spectrometry after derivatization and gas chromatography-tandem mass spectrometry for the determination of selected endocrine disrupting compounds in wastewaters,J.Chromatogr.A 1047(2004)129-135.
[36]R.Liu,J.L.Zhou,A.Wilding,Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction-gas chromatography-mass spectrometry,J.Chromatogr.A 1022(2004)179-189.
[37]A.Shareef,M.J.Angove,J.D.Wells,Optimization of silylation using N-methyl-N-(trimethylsilyl)-tri fluoroacetamide,N,O-bis-(trimethylsilyl)-tri fluoroacetamide and N-(tert-butyldimethylsilyl)-N-methyltri fluoroacetamide for the determination of the estrogens estrone and 17α-ethinylestradiol,J.Chromatogr.A 1108(2006)121-128.
[38]R.Liu,J.L.Zhou,A.Wilding,Microwave-assisted extraction followed by gas chromatography-mass spectrometry for the determination of endocrine disrupting chemicals in river sediments,J.Chromatogr.A 1038(2004)19-26.
[39]Y.Luo,W.Guo,H.H.Ngo,L.D.Nghiem,F.I.Hai,J.Kang,S.Xia,Z.Zhang,W.E.Price,Removaland fate ofmicropollutants in a sponge-based moving bed bioreactor,Bioresour.Technol.159(2014)311-319.
[40]T.Cajthaml,Z.Křesinová,K.Svobodová,K.Sigler,T.Řezanka,Microbialtransformation of synthetic estrogen 17α-ethinylestradiol,Environ.Pollut.157(2009)3325-3335.
[41]M.Clara,N.Kreuzinger,B.Strenn,O.Gans,H.Kroiss,The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micro pollutants,Water Res.39(2005)97-106.
[42]B.C.Lee,J.Y.Jung,H.K.Kim,Evaluation of estrogenic activity and measurement of EDCs in wastewater treatment plantsvol,Journal of Ocean University of China 5(2006)351-356.
[43]S.Suarez,J.M.Lema,F.Omil,Removal of Pharmaceutical and Personal Care Products(PPCPs)under nitrifying and denitrifying conditions,Water Res.44(2010)3214-3224.
[44]C.Moschet,J.Hollender,Microbial Degradation of Steroid Hormones in the Environment and Technical Systems,Swiss Federal Institute of Technology,Zurich,2009.
[45]B.De Gusseme,B.Pycke,T.Hennebel,A.Marcoen,S.E.Vlaeminck,H.Noppe,N.Boon,W.Verstraete,Biological removal of 17α-ethinylestradiol by a nitrifier enrichment culture in a membrane bioreactor,Water Res.43(2009)2493-2503.
[46]P.Pholchan,M.Jones,T.Donnelly,P.J.Sallis,Fate of estrogens during the biological treatment of synthetic waste waterin a nitrite-accumulating sequencing batch reactor,Environ.Sci.Technol.42(2008)6141-6147.
[47]H.R.Andersen,M.Hansen,J.Kjølholt,F.Stuer-Lauridsen,T.Ternes,B.Halling-Sørensen,Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment,Chemosphere 61(2005)139-146.
[48]M.Clara,B.Strenn,O.Gans,E.Martinez,N.Kreuzinger,H.Kroiss,Removal of selected pharmaceuticals,fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants,Water Res.39(2005)4797-4807.
[49]A.C.Johnson,J.P.Sumpter,Removal of endocrine-disrupting chemicals in activated sludge treatment works,Environ.Sci.Technol.35(2001)4679-4703.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
