有丝分裂原活化蛋白激酶信号通路介导大鼠星形胶质细胞氧糖剥夺后水通道蛋白4的表达①
千超,刘锋,肖学谦,李峰,高喜松,党连锋,张毓
有丝分裂原活化蛋白激酶信号通路介导大鼠星形胶质细胞氧糖剥夺后水通道蛋白4的表达①
千超,刘锋,肖学谦,李峰,高喜松,党连锋,张毓
目的探讨有丝分裂原活化蛋白激酶(MAPKs)是否介导缺血后大鼠星形胶质细胞水通道蛋白4(AQP4)的表达。方法分离培养新生Sprague-Dawley大鼠脑星形胶质细胞。第2代细胞分成对照组、氧糖剥夺(OGD)组和阻断组。后两组复制OGD 5 h后复氧模型,12 h后阻断组分别换入含U0126(1 μmol/L和10 μmol/L,U1组和U10组)、SP600125(1 μmol/L和10 μmol/L,SP1组和SP10组)和SB203580(1 μmol/L和10 μmol/L,SB1组和SB10组)的培养液。复氧后0.5 h、1 h、1.5 h、2 h、3 h、4 h、8 h和12 h检测OGD组细胞体积,复氧后0.5 h、1 h、1.5 h、8 h和12 h Western blotting法检测OGD组AQP4表达;复氧后24 h,检测各组乳酸脱氢酶(LDH)活性,Western blotting法检测各组AQP4,磷酸化细胞外调节蛋白激酶(p-ERK)、c-Jun氨基末端激酶(p-JNK)和p38 MAPK(p-p38 MAPK)表达。结果复氧后1.5 h、2 h、3 h和4 h时,OGD组细胞体积较对照组显著增大(P<0.001),OGD组AQP4水平较对照组显著升高(P<0.001),并在复氧后1.5 h达到峰值。OGD组p-ERK、p-JNK和p-p38 MAPK、AQP4水平较对照组显著增加(P<0.001),阻断组p-ERK、p-JNK和p-p38 MAPK较OGD组显著下降(P<0.001),SB10组AQP4较OGD组显著下降(P<0.001)。除SP1组、SB1组外,各干预组LDH活性较OGD组显著下降(P<0.01),SB10组最低(P<0.001)。结论MAPK信号通路,特别是p38 MAPK可介导大鼠星形胶质细胞AQP4蛋白表达,加重细胞坏死。
氧糖剥夺;星形胶质细胞;有丝分裂原活化蛋白激酶;信号通路;水通道蛋白4;水肿;大鼠
水通道蛋白4(aquaporins 4,AQP4)在中枢神经系统的水运输中起重要作用[1-2],并在脑星形胶质细胞中高表达[3]。近年研究表明,调节星形胶质细胞AQP4的表达可能是治疗脑水肿的新策略[4]。丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPKs)是调节细胞渗透压的重要信号通路,动物研究表明,缺血性脑组织中MAPKs的表达及其磷酸化水平发生变化[5-6];高渗应激可通过p38 MAPK途径增加大鼠星形胶质细胞AQP4的表达[7]。但也有学者得出相反结果[8]。本研究观察MAPK信号通路与脑水肿后星形胶质细胞AQP4调节的关系。
1 材料与方法
1.1 材料
新生Sprague-Dawley大鼠5只(SPF级):西安交通大学医学实验动物中心。无酚红细胞培养液、胎牛血清、U0126和SP600125:SIGMA公司。PBS缓冲液和胰蛋白酶:GIBCO公司。SB203580:BIOMOL公司。胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP)、AQP4、磷酸化 p38 MAPK(phosphorylation of p38 MAPK,p-p38 MAPK)、磷酸化细胞外调节蛋白激酶(phosphorylation of extracellular regulated protein kinases,p-ERK)、磷酸化c-Jun氨基末端激酶(phosphorylation of c-Jun N-terminal kinase,p-JNK)和β-actin抗体:CELL SIGNAL TECHNOLOGY公司。乳酸脱氢酶(lactic dehydrogenase,LDH)活性检测试剂盒:广州碧云天公司。蛋白定量试剂盒:PIERCE公司。激光共聚焦显微镜:徕卡公司。酶标仪和电泳仪:BIO-RAD公司。生物安全柜和细胞培养箱:THERMO公司。
1.2 星形胶质细胞的培养与鉴定
无菌条件下分离大鼠大脑皮质,0.25%胰蛋白酶37℃水浴消化15 min,离心,收集细胞,接种于含10%胎牛血清的DMEM-F12培养基,5%CO2、37℃培养箱培养[7,9]。
取第2代细胞接种于细胞培养板,甲醛固定,PBS缓冲液漂洗3次,加入GFAP抗体100 μl,4℃孵育过夜。PBS漂洗3次,加FITC荧光标记二抗150 μl,37℃孵育1 h;PBS洗涤3次,封片,荧光显微镜下观察。
1.3 造模方法
取第2代星形胶质细胞,以每孔5×105浓度接种于6孔细胞培养板,并做细胞爬片。将细胞分成对照组、OGD组和阻断组。后两组参考文献复制氧糖剥夺(oxygen-glucose deprivation,OGD)和复氧模型[10]。第2代星形胶质细胞培养24 h后,弃去培养液,PBS缓冲液漂洗2次,加入不含葡萄糖的无血清DMEM培养液,95%N2、5%CO2、37℃培养5 h。然后换入正常细胞培养液,5%CO2、37℃培养。12 h后,阻断组分别换入含 U0126(1 μmol/L 和 10 μmol/L,U1 组和U10组)、SP600125(1 μmol/L和10 μmol/L,SP1组和SP10组)和SB203580(1 μmol/L和10 μmol/L,SB1组和SB10组)的培养液。其他两组正常换液。
1.4 细胞体积检测
复氧后0.5 h、1 h、1.5 h、2 h、3 h、4 h、8 h和12 h,细胞培养液加1 mmol/L甲基葡萄糖(3-O-methylglucose,3-OMG)和0.5 μCi/ml的3H-3-OMG,继续培养12 h后收集上清,进行放射性检测[7]。细胞用4℃预冷缓冲液(pH 7.4)漂洗后,用1 N NaOH 0.5 ml裂解,按照试剂盒说明书进行检测蛋白含量。
1.5 LDH活性检测
复氧后24 h,取各组细胞培养液,4℃预冷PBS缓冲液漂洗3遍,加入LDH工作液60µl混匀,室温(约25℃)避光孵育30 min,450 nm处测定吸光度。
1.6 免疫荧光染色
加入阻断剂后,各组继续培养2 h,弃培养液,4℃预冷PBS洗3次,每孔加入4%多聚甲醛固定20 min,PBS洗3次。0.3%Triton X-100透化。加AQP4一抗(1∶400)4℃孵育过夜,PBS洗3次,加Alexa标记二抗和TRITC标记鬼笔环肽,37℃孵育1 h,PBS缓冲液清洗3次,封片,激光共聚焦显微镜下观察。
1.7 Western blotting
各组弃去培养液,PBS漂洗3次,加入RIPA细胞裂解液800µl裂解30 min。12,000 r/min离心15 min,收集上清,95℃变性5 min。各组取蛋白质样品10 μg,15%SDS-PAGE电泳分离,转移至PVDF膜,2%脱脂奶粉封闭2 h,加入AQP4、p-p38 MAPK、p-ERK、p-JNK和β-actin一抗,4℃过夜。PBST摇床漂洗3次,每次10 min,加入二抗,37℃孵育2 h。增强化学发光法检测目的蛋白,用Image-Pro plus6.0软件测定灰度值。
复氧后0.5 h、1 h、1.5 h、8 h和12 h检测OGD组AQP4表达;复氧后24 h检测各组AQP4、p-p38 MAPK、p-ERK、p-JNK表达。
1.8 统计学分析
采用SPSS 20.0进行数据处理。检测结果以(xˉ±s)表示,多组均数比较采用单因素方差分析(one-way ANOVA),组间比较选用LSDt检验法。显著性水平α1=0.05,非常显著性水平α2=0.01。
2 结果
2.1 星形胶质细胞鉴定
细胞GFAP阳性,胞体较大,具有短而粗大的突起,形态不规则,轮廓较清晰,为星形胶质细胞。OGD后,细胞体积增大。见图1。
2.2 OGD复氧后星形胶质细胞改变
复氧1.5 h、2 h、3 h和4 h时,细胞体积和对照组相比显著增大(P<0.001),尤以1.5 h时细胞体积最大。见表1。
复氧后,星形胶质细胞AQP4表达较对照组显著升高(P<0.001),复氧后1.5 h达到峰值。见图2、表2。
2.3 LDH活性
OGD组LDH活性显著高于对照组(P<0.001);各阻断组除SB1组外,LDH活性均较OGD组下降(P<0.05),SB10组最低(P<0.001)。见表3。
2.4 Western blotting
复氧后,OGD组p-ERK、p-JNK、p-p38 MAPK和AQP4水平均较对照组显著升高,阻断组p-ERK、p-JNK和p-p38 MAPK均较OGD组显著下降(P<0.001),SB10 组 AQP4 水平最低(P<0.001)。见图 3、图4、表4、表5。
2.5 免疫荧光染色
有较多AQP4蛋白荧光信号,SB10组较OGD组明显减少(图5)。
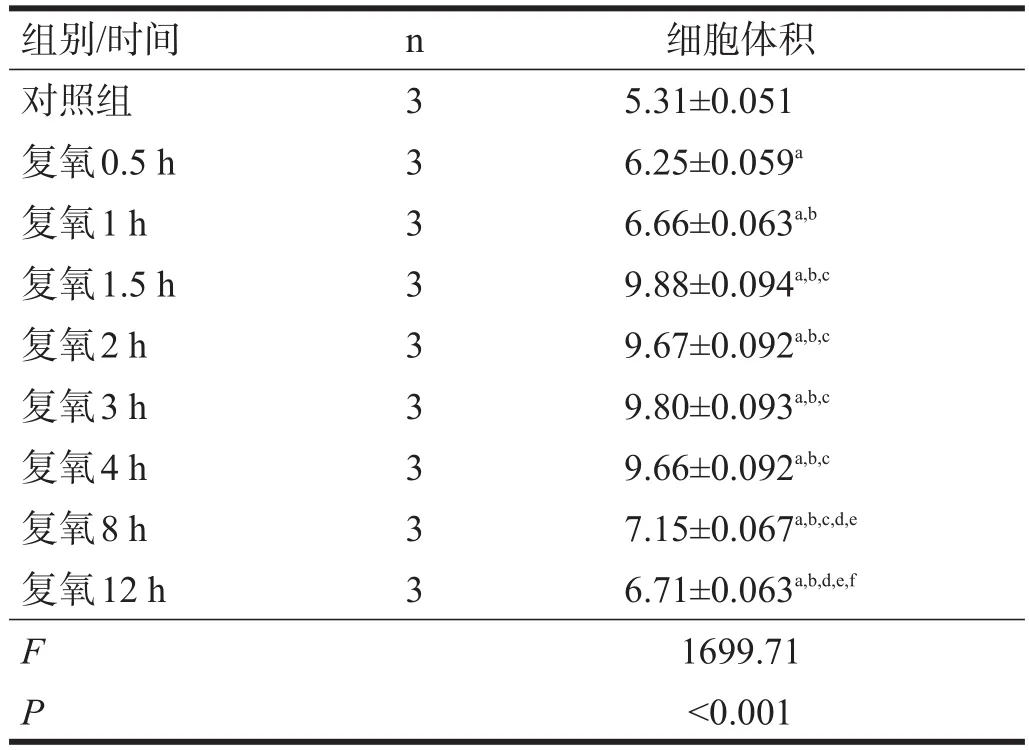
表1 复氧后各时间点细胞体积(μl/mg)

图1 星形胶质细胞培养
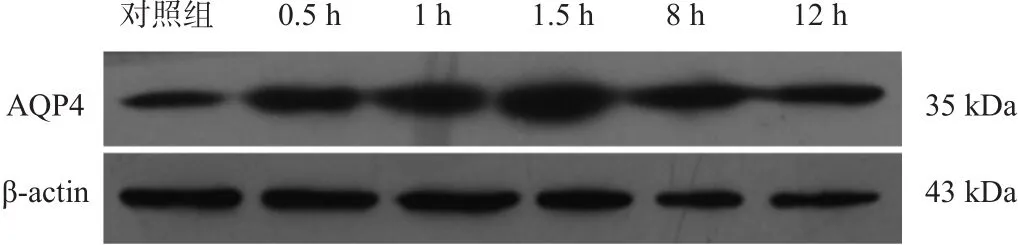
图2 复氧后各时间点AQP4蛋白表达(Western blotting)
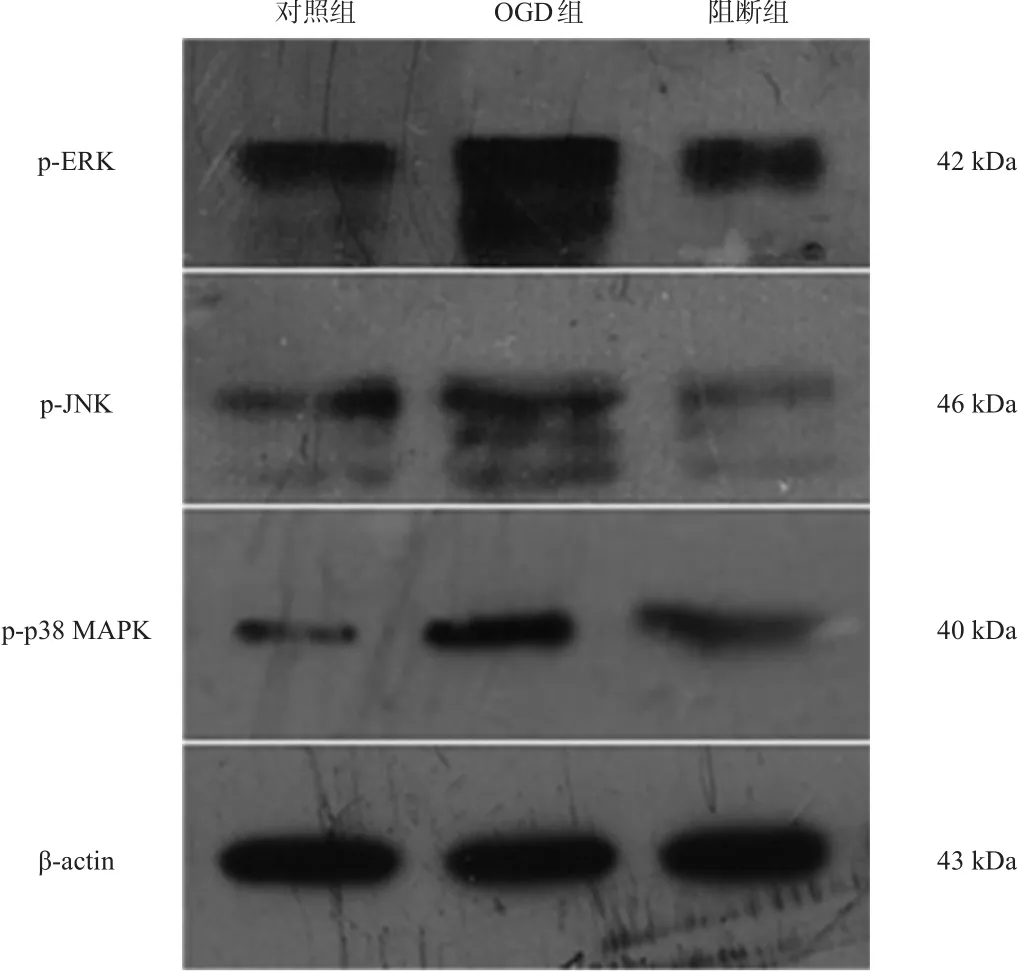
图3 各组p-ERK、p-JNK、p-p38 MAPK蛋白表达(Western blotting)

图4 各组AQP4蛋白表达(Western blotting)

表2 复氧后各时间点AQP4蛋白表达(灰度)
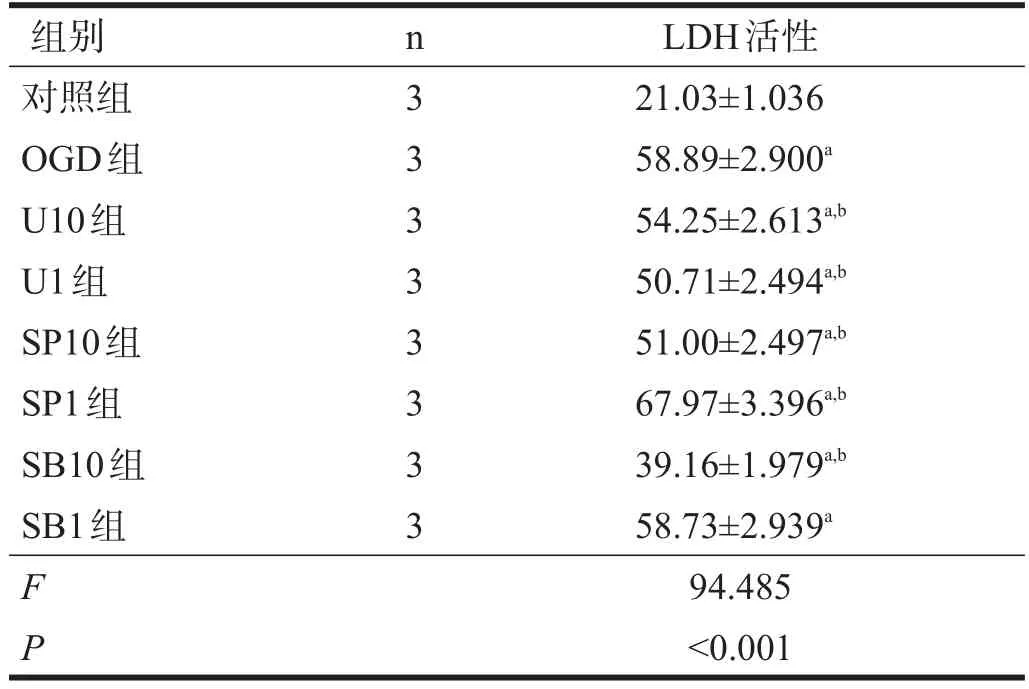
表3 复氧后各组LDH活性(%)
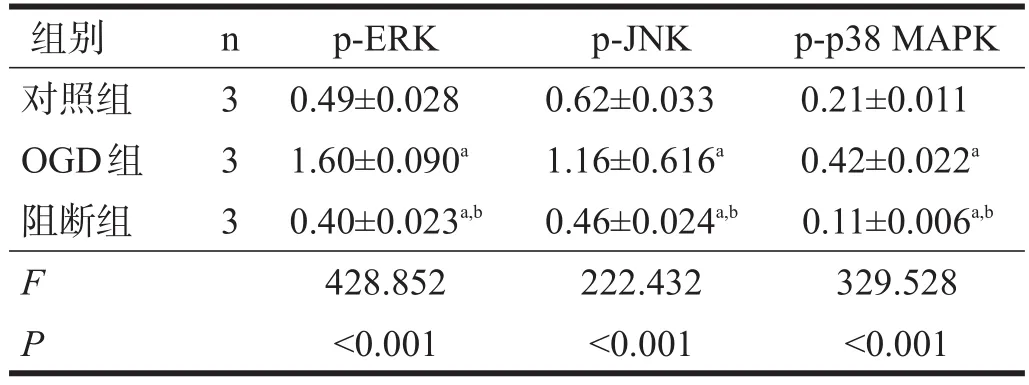
表4 各组p-ERK、p-JNK、p-p38 MAPK蛋白表达(灰度)

表5 各组AQP4蛋白表达(灰度)
3 讨论
本研究显示,星形胶质细胞OGD水肿后,AQP4表达增加;阻断MAPK通道各个因子,均可抑制AQP4异常高表达,尤以p38 MAPK特异性抑制剂SB203580效果最佳。本研究还显示,SB203580可以减少水肿后星形胶质细胞死亡,与最新报道一致[11];可以减少星形胶质细胞水肿,与Nito等[12]的研究结果一致。提示星形胶质细胞OGD后AQP4蛋白的表达主要与p38 MAPK信号通路有关,Qi等[13]也得到相似的结论。
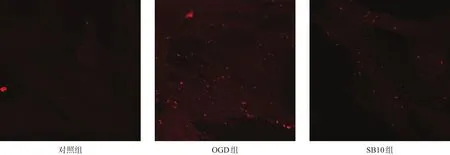
图5 星形胶质细胞AQP4蛋白表达(免疫荧光染色,100×)
JNK特异性抑制剂SP600125和ERK特异性抑制剂U0126都可以减少AQP4蛋白的表达,但对细胞凋亡的作用较小。我们分析可能是SP600125只能阻断JNK1表达,而对JNK2/3无效。有学者发现SP600125可以抑制脑缺血后神经元的凋亡[8],但可能对本研究涉及的星形胶质细胞作用不大。
大量资料表明,脑卒中、脑外伤、脑膜炎和脑肿瘤患者,脑组织中星形胶质细胞AQP4均高表达[14-19]。Frydenlund等[20]发现,脑动脉闭塞再灌注24 h时,AQP4在梗死周围皮质高表达。体内实验也表明,p38 MAPK信号通路在脑卒中后被激活,且短暂性脑缺血后,半暗带区域星形胶质细胞诱导p38 MAPK的延迟激增。Piao等[21]发现,短暂性脑缺血后,经SB203580干预,可以减少梗死面积和神经元死亡,有效保护脑组织。
Tait等[22]却认为,AQP4缺失可能通过减少脑部过量水分的消除,增加蛛网膜下腔出血后脑水肿。Manley等[23]也证明,AQP4敲除小鼠脑缺血后水肿减少。这些差异可能是由于使用的模型不同所致。需要对AQP4敲除小鼠脑损伤后的水转运进行更详细的研究和分析。
脑损伤后AQP4的异常表达不仅与p38 MAPK信号通路有关,也与蛋白激酶C和核因子κB信号通路相关。Arima等[24]研究表明,p38 MAPK信号通路是诱发AQP4异常表达的必然通路,但不是唯一通路。本研究发现,大鼠脑细胞损伤后AQP4的异常表达与p38 MAPK、ERK和JNK都有关,但与p38 MAPK关系最密切。p38 MAPK信号通路阻断剂SB203580能够抑制星形胶质细胞水肿后细胞死亡。这为临床治疗神经损伤水肿提供了基础。有待进一步研究。
[1]Hubbard JA,Szu JI,Binder DK.The role of aquaporin-4 in synaptic plasticity,memory and disease[J].Brain Res Bull,2017,6(17):30115-30116.
[2]Aghayev K,Bal E,Rahimli T,et al.Aquaporin-4 expression is not elevated in mild hydrocephalus[J].Acta Neurochir,2012,154(4):753-759.
[3]Ximenes SA.Metal ion toxins and brain aquaporin-4 expression:an overview[J].Front Neurosci,2016,1(10):233-238.
[4]Filippidis AS,Carozza RB,Rekate HL.Aquaporins in brain edema and neuropathological conditions[J].Int J Mol Sci,2016,18(1):E55.
[5]Yang J,Zhang R,Shi C,et al.AQP4 association with amyloid deposition and astrocyte pathology in the Tg-ArcSwe mouse model of Alzheimer's disease[J].Alzheimers Dis,2017,57(1):157-169.
[6]Raghupathi R.Cell death mechanisms following traumatic brain injury[J].Brain Pathol,2004,14(2):215-222.
[7]Nito C,Kamada H,Endo H,et al.Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes[J].J Neurotrauma,2012,29(14):2404-2412.
[8]Xiong LL,Tan Y,Ma HY,et al.Administration of SB239063,a potent p38 MAPK inhibitor,alleviates acute lung injury induced by intestinal ischemia reperfusion in rats associated with AQP4 down regulation[J].Int Immunopharmacol,2016,38(3):54-60.
[9]Jayakumar AR,Rao KV,Panickar KS,et al.Trauma-induced cell swelling in cultured astrocytes[J].Neuropathol Exp Neurol,2008,67(5):417-427.
[10]Yu J,Jiang Z,Ning L,et al.Protective HSP70 induction by Z-ligustilide against oxygen-glucose deprivation injury via activation of the MAPK pathway but not of HSF1[J].Biol Pharm Bull,2015,38(10):1564-1572.
[11]Chen YQ,Gao F,Jiang R,et al.Down-regulation of AQP4 expression via p38 MAPK signaling in temozolomide-induced glioma cells growth inhibition and invasion impairment[J].Cell Biochem,2017,118(12):4905-4913.
[12]Nito C,Kamada H,Endo H,et al.Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A(2)signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion[J].Cereb Blood Flow Metab,2008,28(10):1686-1696.
[13]Qi LL,Fang SH,Shi WZ,et al.CysLT2 receptor-mediated AQP4 up-regulation is involved in ischemic-like injury through activation of ERK and p38 MAPK in rat astrocytes[J].Life Sci,2011,88(3):50-60.
[14]De Bellis M,Pisani F,Mola MG,et al.Translational read through generates new astrocyte AQP4 isoforms that modulate supramolecular clustering,glial endfeet localization,and water transport[J].Glia,2017,65(5):790-803.
[15]Yang X,Ransom BR,Ma JF,et al.The role of AQP4 in neuromyelitis optica:more answers,more questions[J].J Neuroimmunol,2016,298(15):63-70.
[16]Ikeshima KH.Neuroimmunological implications of AQP4 in astrocytes[J].Int J Mol Sci,2016,17(8):1306-1322.
[17]Hinson SR,Lennon VA,Pittock SJ.Autoimmune AQP4 channelopathies and neuromyelitis optica spectrum disorders[J].Handb Clin Neurol,2016,133:377-403
[18]Li S,Hu X,Zhang M,et al.Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes[J].Behav Brain Res,2015,289(1):1-8.
[19]Assentoft M,Larsen BR,MacAulay N.Regulation and function of AQP4 in the central nervous system[J].Neurochem Res,2015,40(12):2615-2627.
[20]Frydenlund DS,Bhardwaj A,Otsuka T,et al.Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice[J].Proc Natl Acad Sci USA,2006,103(36):13532-13536.
[21]Piao CS,Kim JB,Han PL,et al.Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult[J].Neurosci Res,2003,73(4):537-544.
[22]Tait MJ,Saadoun S,Bell BA,et al.Increased brain edema in AQP4-null mice in an experimental model of subarachnoid hemorrhage[J].Neuroscience,2010,167(1):60-67.
[23]Manley GT,Fujimura M,Ma T,et al.Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke[J].Nat Med,2000,6(2):159-163.
[24]Arima H,Yamamoto N,Sobue K,et al.Hyperosmolar mannitol stimulates expression of aquaporins 4 and 9 through a p38 mitogen-activated protein kinase-dependent pathway in rat astrocytes[J].Biol Chem,2003,278(45):44525-44534.
Role of Mitogen-activated Protein Kinase Pathways in Expression of Aquaporin-4 in Astrocytes after Oxygen-glucose Deprivation in Rats
QIAN Chao,LIU Feng,XIAO Xue-qian,LI Feng,GAO Xi-song,DANG Lian-feng,ZHANG Yu
Deparment of Neurosurgery,No.215 Hospital of Shaanxi Nuclear Industry,Xianyang,Shaanxi 712000,China
Correspondence toLIU Feng.E-mail:liufeng2112010@163.com
ObjectiveTo investigate whether mitogen-activated protein kinases(MAPKs),which were involved in changes in osmolality,might mediate aquaporin-4(AQP4)expression in astrocytes after oxygen-glucose deprivation(OGD)in rats.MethodsAstrocytes were obtained from new born Sprague-Dawley rats.The P2cells were divided into control group,OGD group and inhibitors of U0126,SB203580 and SP600125 groups.The latter groups underwent OGD for five hours and reoxygenated,the inhibitors of U0126,SB203580 and SP600125(1 μmol/L and 10 μmol/L,respectively)groups,named U1,U10,SB1,SB10,SP1 and SP10 groups,respectively,were cultured with the inhibitors for twelve hours.The volume of cells in OGD group was measured half,one,one and half,two,three,four,eight and twelve hours after reoxygenation,and the expression of AQP4 was detected half,one,one and half,eight and twelve hours after reoxygenation with Western blotting.The expression ofAQP4,and phosphorylation of extracellular regulated protein kinases(p-ERK),c-Jun N-terminal kinase(p-JNK)and p38 MAPK(p-p38 MAPK)was detected 24 hours after reoxygenation in all the groups,while the activity of lactate dehydrogenase(LDH)was measured.ResultsThe volume of cells increased in OGD group one and half,two,three and four hours after reoxygenation compared with those in the control group(P<0.001),and the expression of AQP4 also increased in OGD group after reoxygenation(P<0.001),especially 1.5 hours of reoxygenation.The expression of AQP4,p-ERK,p-JNK and p-p38 MAPK increased in OGD group after five hours of OGD compared with those in the control group(P<0.001),and the expression of p-ERK,p-JNK and p-p38 MAPK decreased in the inhibitors groups compared with those in OGD group(P<0.001),and the expression of AQP4 decreased in SB10 group(P<0.001).The activity of LDH was less in all the inhibitors groups except SP1 and SB1 groups than in OGD group(P<0.01),and was the least in SB10 group(P<0.001).ConclusionMAPKs signal pathway,especially p38 MAPK,may promote AQP4 expression in astrocytes after OGD in rat,and play a role in cells death.
陕西省核工业215医院神经外科,陕西咸阳市712000。作者简介:千超(1974-),男,汉族,陕西咸阳市人,硕士,副主任医师,主要研究方向:颅底肿瘤的分子机制。通讯作者:刘锋。E-mail:liufeng2112010@163.com。
10.3969/j.issn.1006-9771.2017.12.006
oxygen-glucose deprivation;astrocyte;mitogen-activated protein kinases;signal pathway;aquaporin-4;edema;rats
R742
A
1006-9771(2017)12-1397-06
[本文著录格式]千超,刘锋,肖学谦,等.有丝分裂原活化蛋白激酶信号通路介导大鼠星形胶质细胞氧糖剥夺后水通道蛋白4的表达[J].中国康复理论与实践,2017,23(12):1397-1402.
CITED AS:Qian C,Liu F,Xiao XQ,et al.Role of mitogen-activated protein kinase pathways in expression of aquaporin-4 in astrocytes after oxygen-glucose deprivation in rats[J].Zhongguo Kangfu Lilun Yu Shijian,2017,23(12):1397-1402.
2017-04-02
2017-06-08)

