半番鸭与番鸭精巢组织差异表达转录组测序分析
李丽, 缪中纬,辛清武,朱志明, 章琳俐,庄晓东,郑嫩珠,3
(1福建省农业科学院畜牧兽医研究所,福州 350013;2福建昌龙集团,福建漳州 363000;3福建农林大学食品科学学院,福州351100)
畜牧·兽医·资源昆虫
半番鸭与番鸭精巢组织差异表达转录组测序分析
李丽1, 缪中纬1,辛清武1,朱志明1, 章琳俐1,庄晓东2,郑嫩珠1,3
(1福建省农业科学院畜牧兽医研究所,福州 350013;2福建昌龙集团,福建漳州 363000;3福建农林大学食品科学学院,福州351100)
【目的】研究半番鸭与番鸭精巢组织转录组差异表达基因,为进一步阐明半番鸭不育的遗传机制奠定理论基础。【方法】利用转录组测序方法对半番鸭和番鸭的精巢组织进行研究,筛选其差异表达基因,并对其功能进行注释和荧光定量PCR(quantitative real-time PCR, QRT-PCR)验证。【结果】测序共获得43.84Gb Clean Data,组装后共获得193 535条Unigene。DESeq分析发现3 597个基因在两个鸭品种间差异表达,其中上调基因1 194个和下调基因2 403个,包括与生殖功能相关的基因,如成纤维细胞生长因子(fibroblast growth factor, FGF)、蛋白激酶A(protein kinaseA, PKA)、丝裂原活化蛋白激酶7(mitogen-activated protein kinase 7-like, partial, BMK)、生长因子受体结合蛋白2(growth factor receptor-bound protein 2, GRB2)、c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)和肿瘤坏死因子受体超家族成员6(tumor necrosis factor receptor superfamily member 6, FAS)等。GO (gene Ontology)分析发现382个差异基因获得功能注释,其中97个基因涉及发育繁殖生物学过程。KEGG (kyoto Encyclopedia of Genes and Genomes)通路分析表明差异表达基因共富集到 50 条信号通路中,其中 17个通路显著富集,包括丝裂原活化蛋白激酶信号转导通路(mitogen-activated protein kinase signaling pathway, MAPK)、甘油酯代谢(glycerolipid metabolism)以及钙信号途径 (calcium signaling pathway)信号通路等,与生殖功能密切相关的有促性腺激素释放激素信号通路(gonadotropin releasing hormone (GnRH) signaling pathway)和MAPK信号转导通路。经QRT-PCR验证,差异基因表达变化模式与转录组测序结果一致,测序结果可靠。【结论】在转录组水平上筛选出半番鸭和番鸭精巢组织差异表达基因,揭示了GnRH和MAPK信号通路在鸭的生殖活动中发挥了重要作用,为进一步探索半番鸭生殖系统的分化机理提供可靠的参考依据。
半番鸭;番鸭;精巢组织;差异表达基因;转录组
Abstract:【Objective】 The purpose of this study is to analyze the transcriptome differential gene expression of mule duck and muscovy duck testis, results of the study will lay a theoretical foundation for the further elucidation of the mechanisms of genetic sterility of mule duck. 【Method】 Transcriptome sequencing of testis from mule duck and muscovy duck was performed using the Illumina HiSeq 2500 platform with 2 biological replicates per duck breed, and verified by quantitative real-time PCR(QRT-PCR). 【Result】 After removing sequencing adaptors and the low-quality reads, a total of 43.84 Gb clean reads wereobtained, the Q30 base percentage at 91.36% and above, and clean reads were assembled into 193 535 Unigene. The r2Differential expression analysis showed that 3 597 differentially expressed genes were found between two duck breeds, including 1 194 up regulated genes and 2 403 down-regulated genes. Several genes were related with reproductive function, such as fibroblast growth factor, FGF, protein kinase A (PKA), mitogen-activated protein kinase 7-like, partial (BMK),growth factor receptor-bound protein 2 (GRB2), c-Jun N-terminal kinase (JNK )and tumor necrosis factor receptor superfamily member 6(FAS),and so on. With Gene Ontology (GO) annotation, 382 differentially expressed genes were identified including 97 related annotation genes involving development and reproduction biological process. Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis showed that differentially expression genes annotated to 50 metabolic pathway, and 17 pathways were enriched significantly, such as calcium signaling pathway, glycerolipid metabolism and MAPK signaling pathway, and gonadotropin releasing hormone (GnRH)signaling pathway and MAPK signaling pathway associated with testis physiology and reproduction activities. Verified by QRT-PCR, the pattern of differential gene expression was consistent with the results of transcriptome sequencing, which showed the sequencing results were reliable. 【Conclusion】 Differentially expressed genes of mule duck and muscovy duck testis were screened by transcriptional analysis, revealed that the GnRH and MAPK signaling pathway play an important role in duck reproductive activities, which provide reliable reference for exploring the differentiation mechanism of reproductive system in mule duck.
Key words:mule duck; muscovy duck; testis tissue; differentially expressed genes; transcriptome
0 引言
【研究意义】番鸭属的公番鸭与河鸭属的母家鸭亲缘关系远,其属间杂交后代为无繁殖能力的骡鸭,俗称半番鸭。半番鸭抗逆性强,饲料报酬高,肉质细腻,具有很强的杂种优势,但其公母外型差异不显著,性器官、性行为分化不明显,无法正常繁育后代,无实际的种用价值。实践研究发现,半番鸭并非完全意义上的不育,它们拥有精巢组织,极少数可产生少量精液,少数可正常交配,雄性偶见爬跨,雌性偶见产蛋,这可能涉及到基因表达、调控以及性细胞形成方式等。【前人研究进展】远缘杂交不育是一个很复杂的生物学现象,其遗传基础较复杂,可能受众多的基因及其产物的调控。关于鸭类属间杂交不育的原因,檀俊秩和陈晖等[1]认为半番鸭父母本的染色体核型不一致,减数分裂受到破坏,进而影响性腺轴发育,最终导致其后代不育。刘军须等[2]研究认为大鼠不育性状由常染色体上单一隐性基因控制,呈隐性遗传。张庆波等[3]认为 DAZL(Deleted in AZoospermia-like)基因是牛精子发生的重要调控因子,其突变或表达缺乏将导致雄性不育。以 Illumina为基础的转录组测序(RNA-Seq)技术被广泛的应用到差异基因的检测及功能注释,在畜禽方面也应用广泛,如鉴定鸭黑、白羽相关基因[4],筛选野鸡和家鸡肉质基因[5],分析绍兴鸭青壳性状相关基因[6]等研究。生殖、发育机制等一直是生物学热点,BAUERSACHS等[7]通过RNA-Seq技术筛选到不同物种间繁殖相关差异基因,FIEDLER等[8]对海兔不同发育阶段进行转录组测序,张伟[9]构建了中华绒螯蟹精巢组织文库,筛选雄性生殖调控关键基因,钟志君[10]研究比较了成年藏猪的精巢和卵巢转录组表达谱,张升利等[11]分析了长尾草金鱼成熟期精巢和卵巢转录组差异表达基因,XU等[12]利用转录组测序方法挖掘出与卵泡发育相关基因,朱志明等[13]探明了山麻鸭开产期和产蛋高峰期卵巢组织的转录组差异。这些成果为研究鸭不育性状和繁殖性能的遗传机制奠定了基础。【本研究切入点】虽然 RNA-Seq技术已被应用到鸭的性状研究,但由于半番鸭不育性状的特殊性,其与番鸭或家鸭精巢组织的转录组比较分析尚未有报道,其不育遗传基础需进一步深入研究。【拟解决的关键问题】本研究通过对半番鸭和番鸭个体精巢组织的转录组比较,对其差异表达基因进行筛选,并进行GO(gene ontology)与 KEGG(kyoto encyclopediaof genes and genomes)功能富集,分析通路功能并探索精巢分化相关的差异表达基因,通过转录组数据挖掘半番鸭雄性不育相关的基因,为后续半番鸭雄性不育形成的遗传机制研究提供线索。
1 材料与方法
1.1 试验材料
本研究所用的半番鸭和番鸭公鸭由福建省农业科学院畜牧兽医研究所动物房提供,试验于3—6月进行,各试验鸭饲养管理条件一致。180日龄性成熟时,对公鸭进行采精训练。210日龄禁饲12 h后,每个品种分别取2 只个体用于转录组测序,其中半番鸭公鸭为无爬跨行为个体,番鸭公鸭为正常个体。按照国家实验动物处理行为准则屠宰,取精巢组织,置于-80℃备用。
1.2 RNA 提取及转录组测序
利用RNA easy Lipid Tissue Mini Kit(QIAGEN,Germany)提取每个个体总 RNA,单个建池。采用Nanodrop、Qubit 2.0和Aglient 2100方法检测各RNA样品的纯度、浓度及完整性等。构建文库,Qubit2.0和 Agilent 2100分别对文库的浓度和插入片段大小(Insert Size)进行检测,QRT-PCR对文库的有效浓度进行准确定量,以保证文库质量。基于边合成边测序(Sequencing By Synthesis,SBS)技术,利用 Illumina HiSeq 2500(Illumina, America)平台进行高通量测序,测序读长为PE150。
1.3 测序数据处理
测序数据去除接头及低质量数据后,利用Trinity软件将数据组装成转录本,进行转录注释及表达量的计算。利用DESeq进行基因的差异表达分析,绘制差异表达基因火山图,并进行聚类分析。
1.4 GO注释、KEGG通路分析
利用GO数据库对差异表达基因进行功能注释,采用COG(cluster of orthologous groups of proteins)对差异表达基因进行分类统计,运用KEGG数据库进行通路分析,均以P<0.05作为显著性富集标准。
1.5 Real-time PCR检测
采用Primer Premier 6.0和Beacon designer 7.8软件设计荧光定量PCR引物,然后由生物工程(上海)股份有限公司负责合成,引物序列如表1,QRT-PCR扩增体系和反应条件如表2。以甘油醛-3-磷酸脱氢酶基因(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)做内参,采用2-ΔΔCt法计算基因的相对表达量。以P<0.05作为显著性标准。
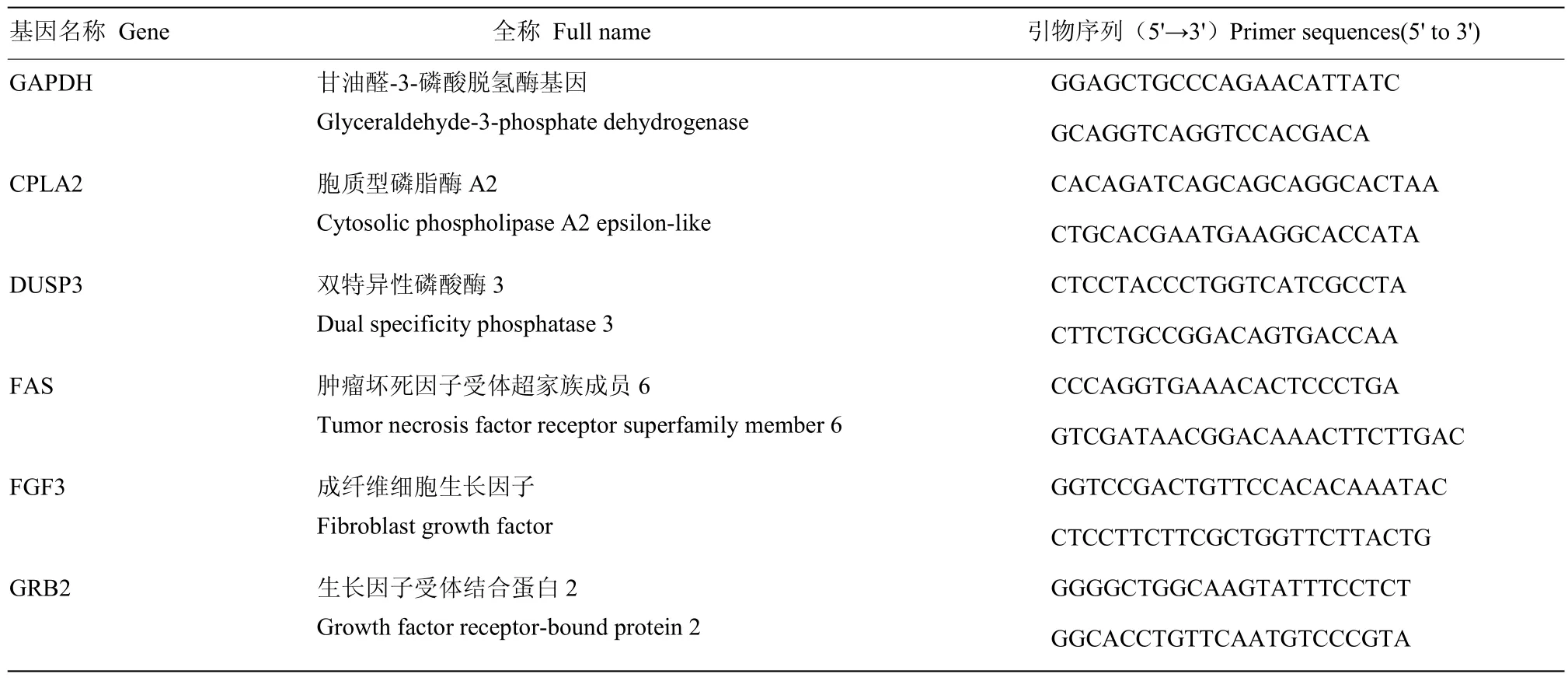
表1 荧光定量引物序列Table 1 Real-time PCR primers and conditions

表2 荧光定量PCR反应体系及条件Table 2 Real-time PCR reaction system and conditions
2 结果
2.1 半番鸭与番鸭精巢组织转录组测序
本试验共构建半番鸭和番鸭4个精巢组织的转录组文库。测序数据去除接头以及低质量数据后共获得288 008 582个高质量数据。从表3中可以看出,本试验共获得43.84Gb的Clean Data,各样品Clean Data均达到6.29Gb。另外,各样品GC含量均不小于51.88%,Q30(Clean Data质量不小于30的碱基所占的百分比)全在 91.36%以上,该结果表明测序质量可靠,构建文库可用于后续分析。数据组装后共获得193 535条Unigene,其中长度在1kb以上的Unigene有46 175条。
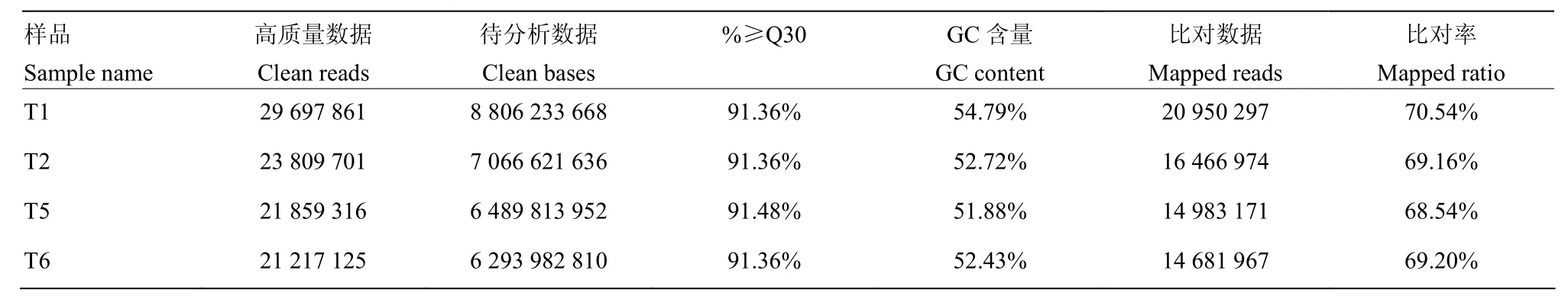
表3 转录组数据组装情况Table 3 Summary of the sequencing data assembly
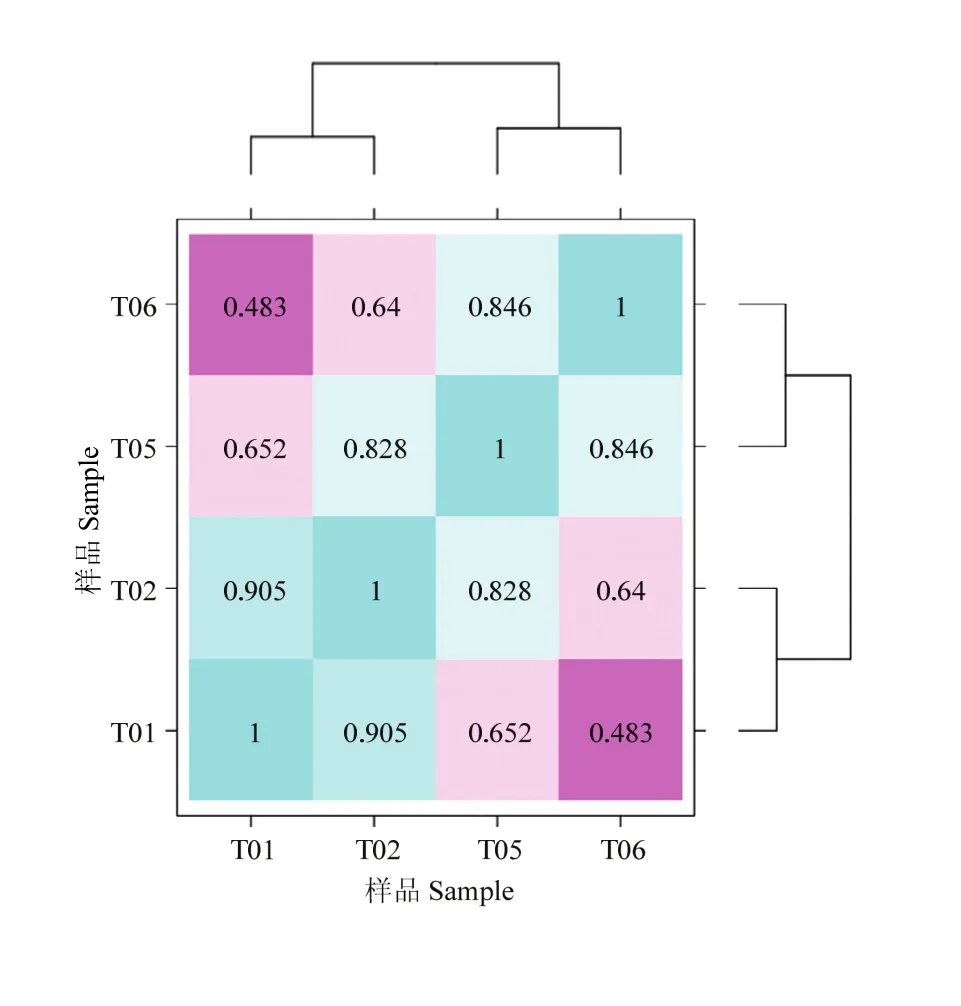
图1 四样品基因表达量相关性图Fig. 1 Correlation heat map of gene expression level in 4 samples
2.2 差异表达基因的筛选及聚类分析
FPKM(Fragments Per Kilobase of transcript per Million mapped reads)是每百万Reads中来自比对到某一基因每千碱基长度的Reads数目,是转录组测序数据分析中常用的基因表达水平估算方法。将皮尔逊相关系数r(Pearson’s Correlation Coefficient)作为生物学重复相关性的评估指标[14]。r2越接近1,说明两个重复样品相关性越强。对同一条件的每一对生物学重复样品的基因表达量做相关性图,相关性图见图 1。半番鸭组内 2个不同重复样品和番鸭组内 2个不同重复样品的 r2值均大于 0.9,而两组间 r2值均小于 0.6,说明了试验的可靠性和可重复性都很高。
以FDR(False Discovery Rate)作为差异表达基因筛选的关键指标, 将FDR小于0.001且差异倍数FC(Fold Change)大于等于2作为两品种鸭个体间差异表达基因显著的筛选标准,共鉴定出3 597个基因,其中包括1 194个表达上调的差异基因,主要有(FDR由小到大排列)c86758.graph_c1(NADH脱氢酶亚基4),c86758.graph_c0(外周型苯二氮卓受体相关蛋白1)等差异基因,以及2 403个表达下调基因,包含c243330.graph_c0(DnaJ同源B亚家族成员8),c277017.graph_c0(凋亡因子BCL-2蛋白14)等。进一步分析发现,显著差异基因中含有一些与繁殖性能相关的基因(表 4),例如,成纤维细胞生长因子(fibroblast growth factor,FGF)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)、细胞外信号调控的蛋白激酶5(ERK5)、蛋白激酶A(protein kinase A,PKA)、生长因子受体结合蛋白 2(growth factor receptor-bound protein 2,Grb2)、丝裂原活化蛋白激酶 7,部分(mitogen-activated protein kinase 7-like,partial,BMK)等下调基因,肿瘤坏死因子受体超家族成员 6(tumor necrosis factor receptor superfamily member 6, FAS)、双特异性磷酸酶3(dual specificity phosphatase 3, DUSP3)、L型电压依赖性钙通道α1c亚单位(alpha-1c-like voltage-dependent L-type calcium channel subunit alpha-1C-like, CACNA1C)、胞质型磷脂酶 A2(cytosolic phospholipase A2 epsilon-like,CPLA2)等上调基因。从火山图(图 2)能够快速查看两组样品间表达的差异水表平分布情况。通过 MA图(图 3)可以直观地查看两组样品中基因的表达丰度和差异倍数的整体分布。
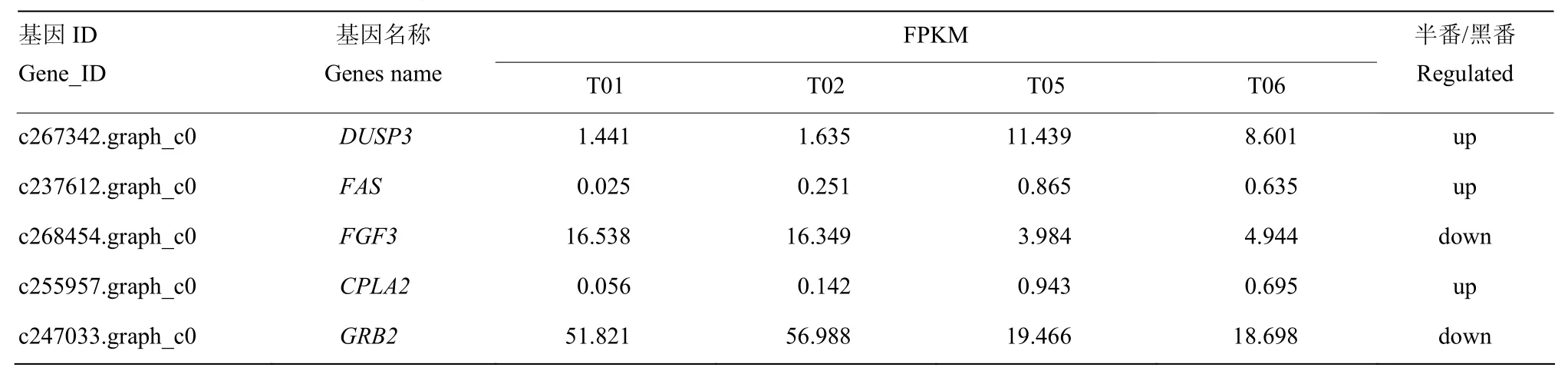
表4 差异表达基因(部分)Table 4 Differentially expressed genes (parts)
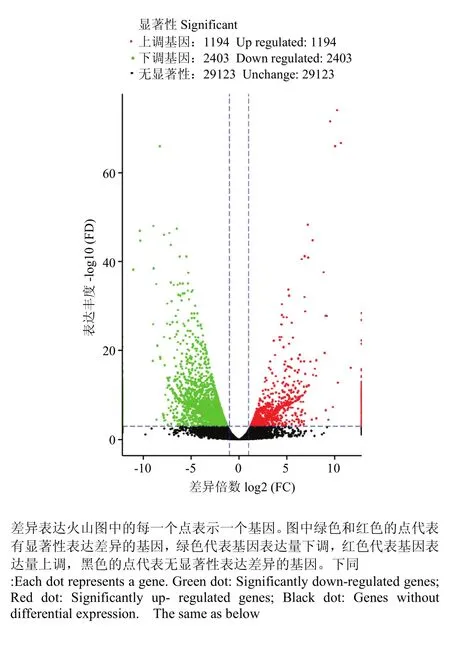
图2 差异表达基因火山图Fig. 2 Volcano plot of differentially expressed genes
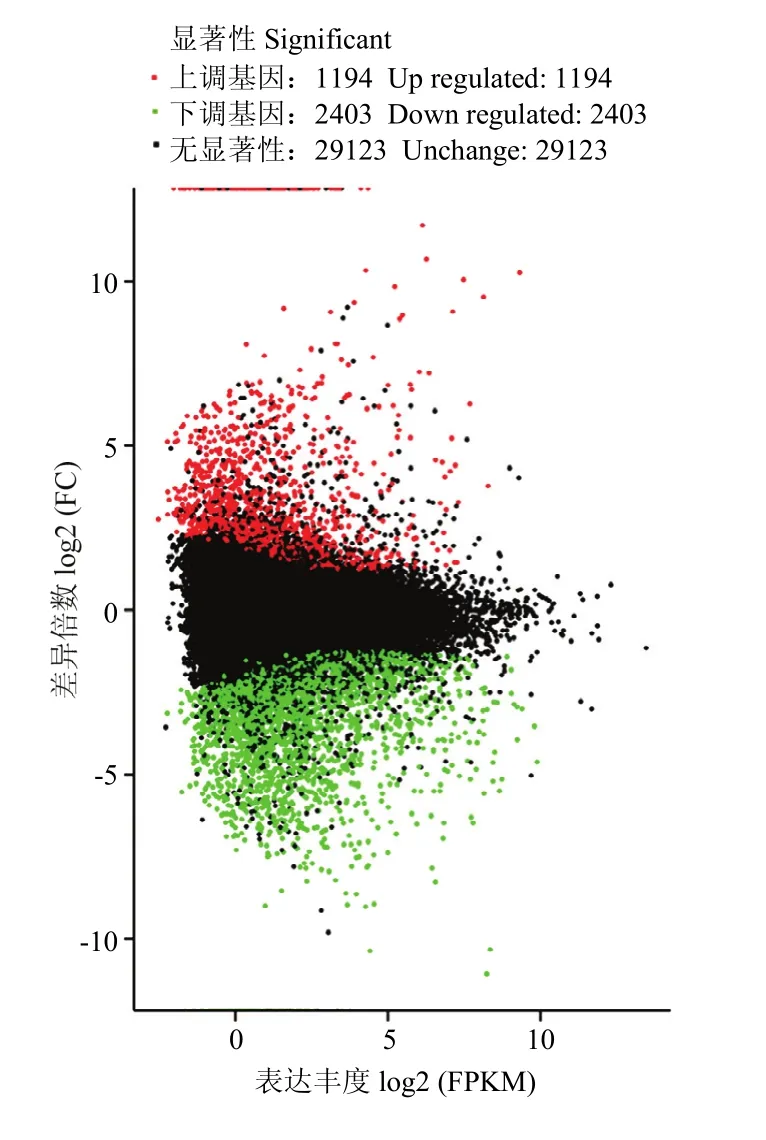
图3 差异表达基因MA图Fig. 3 MA plot of differentially expressed genes
对筛选出来的差异表达基因进行聚类分析(图4),发现同一鸭品种的2 个生物学重复聚到了一起,而不同鸭品种间基因表达模式则出现分离,表明本研究所用样本生物学重复性较好,且样本分组较合理。
2.3 差异表达基因GO功能富集
本研究运用GO数据库对具有同源比对的差异基因进行生物学过程(biological process)、分子功能(molecular function)与细胞组分(cellular component)三方面的注释。将比对得到的1 381个显著差异基因进行功能注释,382个差异基因在GO分类中有功能意义。上述3个功能被区分为更具体的61个类别,分别包括了22、19和20个功能亚分类。由图5可知,在生物功能的组分中,两组间的差异表达基因在细胞过程(cellular process, GO: 0009987)与单一的生物过程(single-organism process, GO: 0044699)中数目比例最大。在细胞功能中,细胞(cell, GO: 0005623)与细胞部分(cell part, GO: 0044464)数目最多。在分子功能分类中,差异基因在结合(binding,GO: 0005488)中所占的比例最高,催化活性(catalytic activity, GO:0003824)次之。由图5可知,与发育繁殖相关的生物学过程有繁殖(reproduction, GO:0000003),发育过程(developmental process, GO:0048589)和繁殖过程(reproductive process, GO:0022414),涉及97个相关基因。
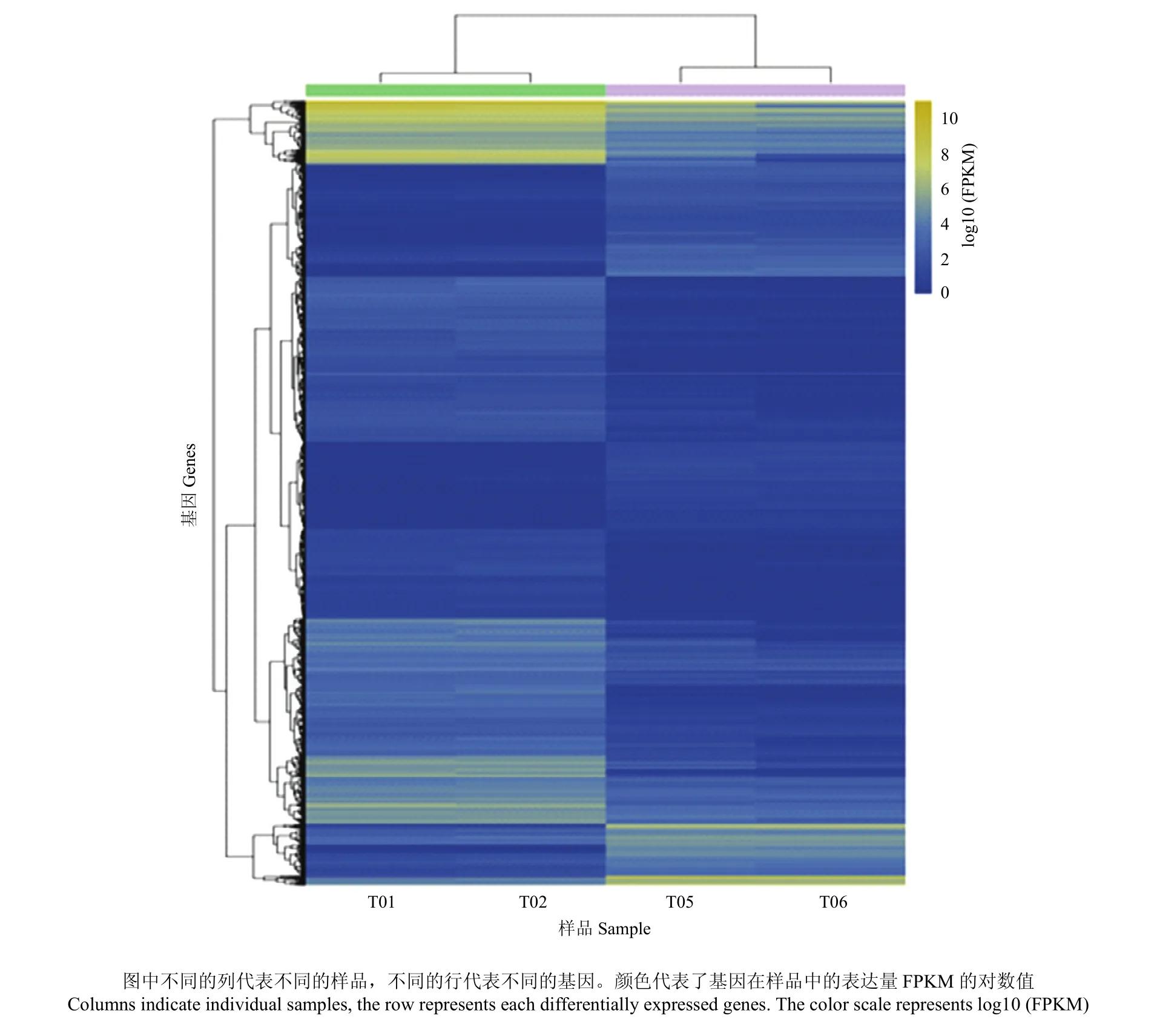
图4 显著差异表达基因聚类分析Fig. 4 Heat map of the differentially expressed genes
2.4 差异表达基因COG分类
统计显著富集的Go term中包含的基因数,分析结果见图6。其中一般的功能预测(general function prediction only)基因数目最多,其次为信号传导机制(signal transduction mechanism),转录(Transcription)和复制、重组与修复(replication, recombination and repair)。
2.5 差异表达基因KEGG注释
为确定差异基因参与的主要生化代谢途径和信号通路,对差异表达基因进行KEGG通路分析,结果显示差异表达基因共富集到50 条信号通路中。以P<0.05作为差异表达基因在该通路显著富集,共鉴定出17个通路显著富集:包括与丝裂原活化蛋白激酶(MAPK)信号转导通路(MAPK signaling pathway)、钙信号途径(calcium signaling pathway)、甘油酯代谢(glycerolipid metabolism)、紧密连接(gap junction)以及血管平滑肌收缩(Vascular smooth muscle contraction)等信号通路。对差异表达基因的注释结果按照 KEGG中通路类型进行分类,分类图如图7所示,其中与生长发育、生殖过程相关的有 MAPK信号转导通路和促性腺激素释放激素信号通路(gonadotropinreleasing hormone(GnRH)signaling pathway)等。
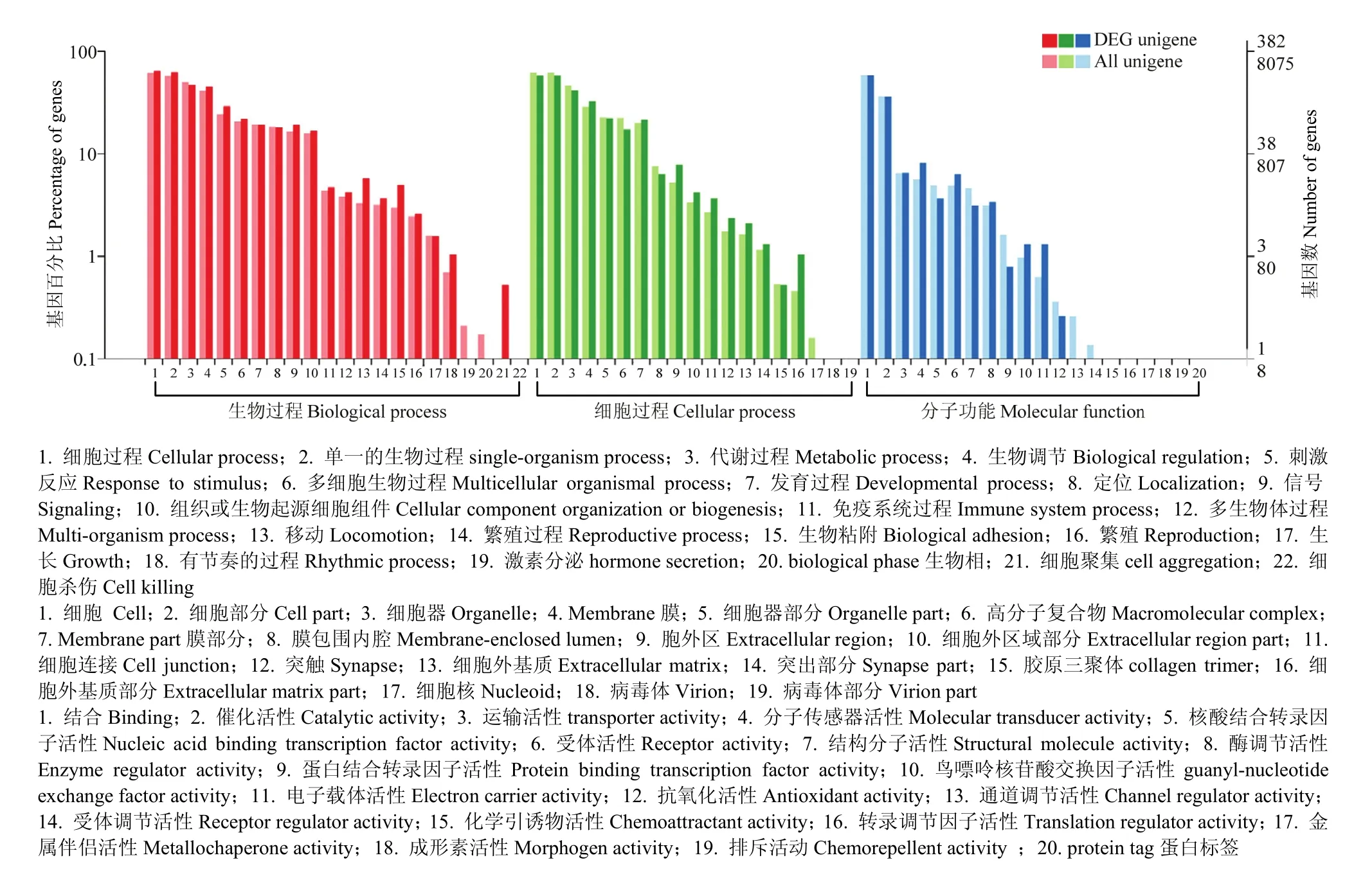
图5 差异表达基因GO注释Fig. 5 GO annotation of differentially expressed genes
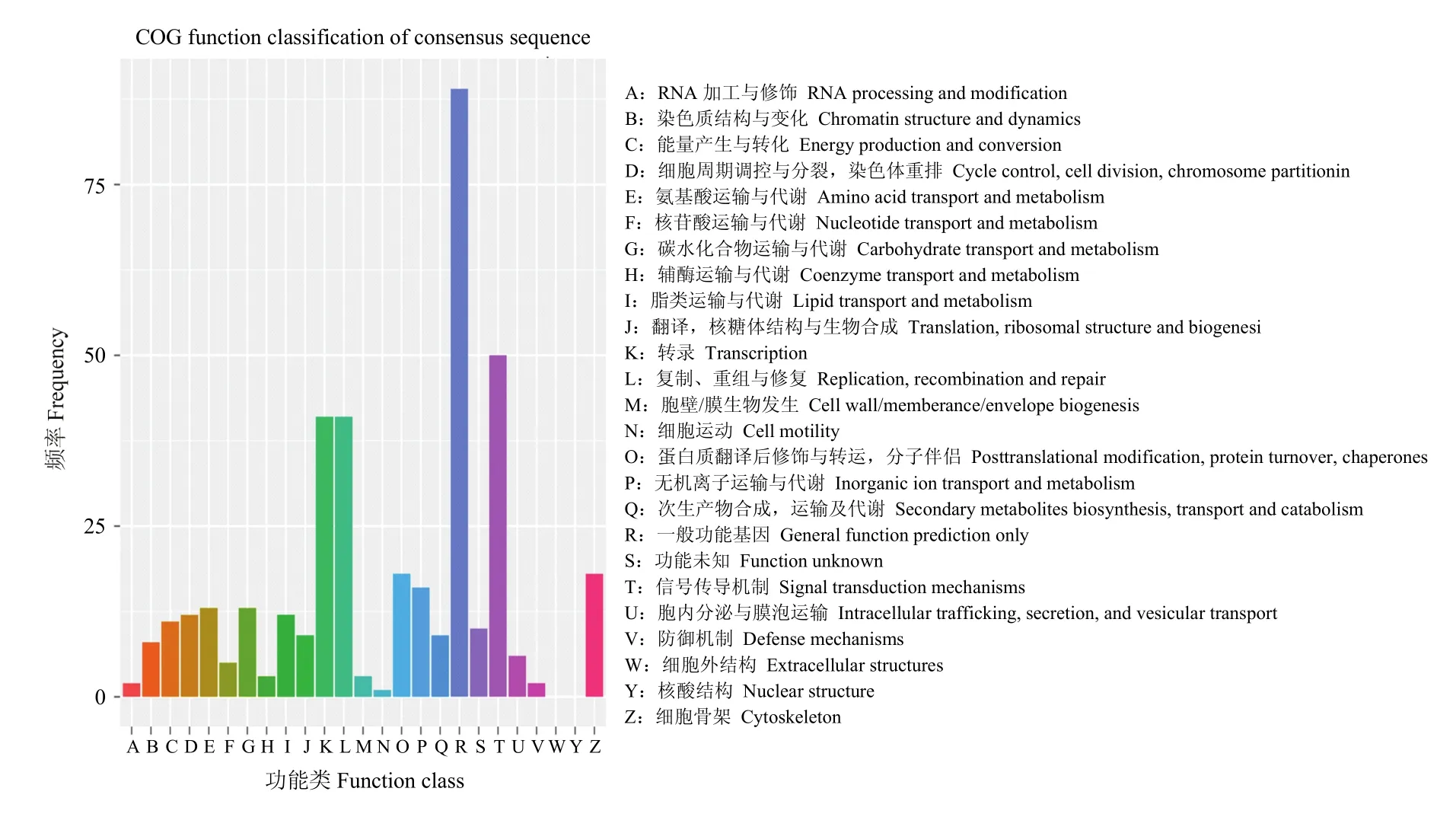
图6 差异表达基因COG注释分类统计图Fig. 6 COG annotation classification statistics of differentially expressed genes

图7 差异表达基因显著富集的KEGG 通路Fig. 7 List of KEGG pathway for differentially expressed genes
挑选富集显著性最可靠(即 Q值最小)的前 20个通路以散点图的形式展示(图 8),在该图中越靠近右上角的图形代表的通路,参考价值越大;反之亦然。由此可知甘油酯代谢(glycerolipid metabolism),钙信号途径(calcium signaling pathway)信号通路以及血管平滑肌收缩(vascular smooth muscle contraction)富集显著性较为可靠。
2.6 Real-time PCR验证转录组测序数据
为验证转录组测序结果,本研究选择了GRB2、CPLA2、FGF3、DUSP3及FAS基因进行Real-time PCR试验。结果表明除FAS基因整体表达低,数据不准确外,其他4个基因在番鸭和半番鸭个体间表达变化模式与转录组测序结果一致(表 5),表明本研究利用转录组测序获得的数据较为准确。
3 讨论
近来, 高通量转录组测序技术取得了较大的研究进展[15-20]。雄性生殖系统的发育、分化过程是一个复杂的生理过程,涉及多种代谢过程,目前已有研究表明p53信号通路[21]、Wnt代谢通路[22]以及TGFβ通路[23-24]在雄性生殖发育过程中起重要作用。为了解鸭精巢的系统机制和半番鸭不育的特性,本研究对半番鸭和番鸭精巢组织进行转录组学测序及比对分析,共筛选获得3 597个差异表达基因,同时为进一步确定差异基因参与的主要生化代谢途径和信号通路进行KEGG分析,结果发现MAPK、甘油酯代谢以及钙信号途径等17个通路富集显著,其中与生殖过程相关的有GnRH信号通路和MAPK信号转导通路。
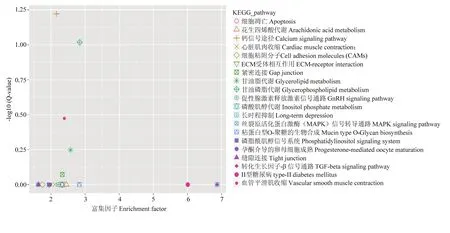
图8 差异表达基因KEGG通路富集散点图Fig. 8 Enriched scatter map of differential expression gene KEGG pathway

表5 Real-time PCR 验证转录组测序数据Table 5 Validation of the RNA-seq expression data by Real-time PCR for selected genes
MAPK信号通路是细胞间信号传递的重要通路[25],可参与细胞增殖、分化[26]、精子成熟[27]及凋亡[28-29]等动物生殖过程,被认为是精子发育的重要影响因素之一。本研究筛选到参与此通路的FGF、c- JNK、ERK5等10个下调基因,FAS、DUSP3等4个上调基因,以及CPLA2、CACN 2个混合型调节基因。在此通路筛选的差异表达基因中,FGF属于可促进成纤维细胞生长的多肽家族。VALVE等[30]研究发现 FGF8基因在成年鼠睾丸精子发生的特定阶段表达,在鼠、牛卵巢中卵子发生的特定阶段也有表达。DVORAK等[31]研究表明FGF信号参与包括细胞增殖、迁移、分化等多种细胞应答,调控广泛的生理和病理过程。JNK在细胞分化、凋亡和疾病的发生均有重要作用,是MAPK信号转导系统的重要效应因子[32]。目前发现的JNK的底物有转录因子c-jun、ATF-2及P53等。陈丽莉等[33]发现JNK可调控黄鳝卵巢发育、凋亡及雄性发育的启动。ERK基因则主要参与动物生殖细胞增殖、分化以及凋亡等多个发育过程[34]。
GnRH信号通路可调控动物体内性腺轴生殖激素的分泌,下丘脑分泌产生的神经激素能促进垂体分泌促性腺物质的释放,并参与动物生殖调控[35]。本研究筛选到了参与此通路上的PKA、Grb2和BMK等下调基因,以及CPLA2、CACNA1C 2个混合调节基因。在此通路筛选的差异表达基因中,已有研究证实Grb2是信号转导途径中的一个重要成分,它最初是作为表皮生长因子受体(epidermal growth factor receptor,EGFR)和MAPK通路之间的衔接蛋白被发现的[36]。Grb2作为一种信号接头蛋白,参与细胞信号转导过程,具有促进细胞增殖、细胞生长、细胞分化等功能[37]。PLA2参与雄性生殖过程, 其与精子获能、顶体反应以及精卵融合等过程密切相关,并受到各种信号通路的调节,不同通路相互协调。精子PLA2活性的激活及其调控机制受G蛋白受体可介导[38]。
另外,JNK、Crb2、CACN等基因同时参与上述两个通路,如PKA、蛋白激酶C(protein kinase C, PKC)及 Ca2+也在生理性顶体反应的精子信号转导中调节PLA2 的活性,此外,钙信号途径以及甘油酯代谢、紧密连接以及血管平滑肌收缩等信号通路也富集显著,说明各种发挥不同作用的信号传导通路交织在一起, 组成一个复杂庞大的细胞信号传导网络系统。关于钙信号途径,Ca2+作为细胞内重要第二信使,通过精子内Ca2+浓度调节精子生理活动。SANTI等[39]也发现 Ca2+信号通路与精子的形成有着密切的关系。甘油酯代谢、紧密连接以及血管平滑肌等信号通路在鸭精巢组织中的作用及其相互调节机制将进一步探究。
4 结论
本研究通过半番鸭和番鸭转录组比较,首次在转录组水平上筛选出与繁殖性能相关的差异表达基因,如PKA、Grb2、BMK、CPLA2、FGF、JNK及ERK5等,并进一步证实了GnRH 信号通路和MAPK信号通路在公鸭生殖活动中发挥了重要作用。信号通路的分析为鸭精巢组织的信号调控提供了线索,但还需对涉及这些通路的相关基因进行深入的生物信息学分析和验证,进一步明确繁殖性状的基因表达谱和调控模式,该研究结果可为今后探索半番鸭生殖系统的分化机理提供可靠的参考依据。
[1]檀俊秩, 陈晖, 宋建捷, 刘玉涛. 半番鸭繁殖性状的研究. 福建农业学报, 1998, 13(2): 41-45.TAN J Z, CHEN H, SONG J J, LIU Y T. Studies on reproductive character of mule duck. Journal of Fujian Agricultural Sciences, 1998,13(2):41-45. (in Chinese)
[2]刘军须, 蔡月花, 张敬各, 张福英. 雄性不育大鼠近交系MIJ的建立及其遗传特征观察. 动物学杂志, 2008, 43(5) :37-44.LIU J X, CAI Y H, ZHANG J G, ZHANG F Y. Establishment and genetic characteristics of rat inbred strain MIJ with spontaneous male infertility. Journal of Animal Science, 2008, 43(5):37-44. (in Chinese)
[3]张庆波, 李齐发, 李家璜, 李新福, 刘振山, 潘增祥, 宋大伟, 谢庄.牛精子发生相关新基因 b-DAZL的克隆、生物信息学分析与组织表达研究. 自然科学进展, 2008, 18(5):493-504.ZHANG Q B, LI Q F, LI J H, LI X F, LIU Z S, PAN Z X, SONG D W,XIE Z. Cloning, bioinformatics analysis and tissue expression of a novel b-DAZL gene related to spermatogenesis in cattle. Progress in Natural Science, 2008, 18(5):493-504. (in Chinese)
[4]LI S J, WANG C, YU W H, ZHAO S H, GONG Y Z. Identification of genes related to white and black plumage formation by RNA-Seq from white and black feather bulbs in ducks. PloS One, 2012, 7:e36592.
[5]LI Q H, WANG N, DU Z, HUX X, CHENL, FEI J, WANGY Y, LI.N. Gastrocnemius transcriptome analysis reveals domestication induced gene expression changes between wild and domestic chickens.Genomics, 2012, 100: 314-319.
[6]陈黎, 黄学涛, 田勇, 陶争荣, 卢立志. 利用转录组测序筛选与鸭青壳性状形成相关的基因. 农业生物技术学报, 2016, 24(7):1064-1072.CHEN L, HUANG X T, TIAN Y, TAO Z R, LU L Z. Identifying genes associated with blue eggshell in ducks (Anasplatyrhynchos domesticus) by transcriptome analysis. Journal of Agricultural Biotechnology, 2016, 24(7): 1064-1072. (in Chinese)
[7]BAUERSACHS S, WOLF E. Transcriptome analyses of bovine,porcine and equine endometrium during the pre-implantation phase[J].Animal reproduction science. 2012,134:84-94.
[8]FIEDLER T J, HUDDER A, MCKAY S J, SHIVKUMAR S, CAPO T R, SCHMALE M C, WALSH P J. The transcriptome of the early life history stages of the California Sea Hare Aplysia californica,comparative biochemistry and physiology Part D. Genomics &Proteomics, 2010, 5:165-170.
[9]张伟. 中华绒螯蟹精巢组织文库构建和基因的克隆与序列分析[D].上海: 华东师范大学, 2012.ZHANG W. Construction of cDNA library of Chinese mitten crab,Eriocheir sinensis and cloeing and molecular characterization ofgene[D]. Shanghai:East China Normal University, 2012. (in Chinese)
[10]钟志君. 猪睾丸和卵巢组织转录组的差异分析[D]. 雅安:四川农业大学, 2012 .ZHONG Z J. Repertoire of porcine microRNA in adult ovary and testis by deep sequencing[D]. Sichuan: Sichuan Agricultural University, 2012 (in Chinese)
[11]张升利, 付成东, 梁拥军, 李文通, 孙砚胜, 史东杰, 张 欣. 长尾草金鱼成熟期雌雄性腺 RNA-Seq转录组分析. 水产科学, 2014,33(12): 750-753.ZHANG S L, FU C D, LIANG Y J, LI W T, SUN Y S, SHI D J,ZHANG X. The RNA-Seq transcriptome analysis in male gonads of Long-tailed goldfish Rassius auratus. Fisheries Science, 2014, 33(12):750-753. (in Chinese)
[12]XU Q, ZHAO W M, CHEN Y, TONG Y Y, RONG G H, HUANG Z Y,ZHANG Y, CHANG G B, WU X S, CHEN G H. Transcriptome profiling of the goose (Anser cygnoides) ovaries identify laying and broodiness phenotypes. PloS One, 2013, 8:e55496.
[13]朱志明, 陈红萍, 林如龙, 缪中纬, 辛清武, 李丽, 张丹青, 郑嫩珠.山麻鸭开产期和产蛋高峰期卵巢组织转录组分析. 中国农业科学,2016, 49(5):998-1007.ZHU Z M, CHEN H P, LIN R L, MIAO Z W, XIN Q W, LI L,ZHANG D Q, ZHENG N Z. Transcriptome analysis of ovary tissue in early laying period and egg laying peak period of shanma Ducks.Scientia Agricultura Sinica, 2016, 49(5):998-1007. (in Chinese)
[14]SCHULZE S K, KANWAR R, GÖLZENLEUCHTER M,THERNEAU T M, BEUTLER A S. SERE: Single-parameter quality control and sample comparison for RNA-Seq. [BMC genomics Italic],2012, 13(1): 524.
[15]XIANG L X, HE D, DONG W R, ZHANG Y W, SHAO J Z.Deep sequencing-based transcriptome profiling analysis of bacteria challenged Lateolabrax japonicus reveals insight into the immune-relevant genes in marine fish. BMC Genomics, 2010, 11: 472.
[16]WANG B, GUO G W, WANG C, LIN Y, WANG X M, ZHAO M M,GUO Y, HE M H, ZHANG Y, PAN L. Survey of the transcriptome of Aspergillus oryzae via massively parallel mRNA sequencing. Nucleic Acids Research, 2010, 38(15): 5075-5087.
[17]LI M Y, TAN H W, WANG F, JIANG Q, XU Z S, TIAN C, XIONG A S. De novo transcriptome sequence assembly and identification of AP2/ERF transcription factor related to abiotic stress in parsley(Petroselinum crispum). PLoS One, 2014, 9(9): e108977.
[18]ZHU S Y, TANG S M, TANG Q M, LIU T M. Genome-wide transcriptional changes of ramie (Boehmeria nivea L. Gaud) in response to root-lesion nematode infection. Gene, 2014, 552: 67-74.
[19]CONG F, LIU X L, HAN Z X, SHAO Y H, KONG X P, LIU S W.Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. Genomics, 2013, 14:743-756.
[20]FRASER B A, WEADICK C J, JANOWITZ I, RODD F H, HUGHES K A. Sequencing and characterization of the guppy (Poecilia reticulata) transcriptome. BMC Genomics, 2011, 12:202.
[21]LI G Y, XIE P, LI H Y, HAO L, XIONG Q, QIU T. Involment of p53,Bax, and Bcl-2 pathway in microcystins-induced apoptosis in rat testis.Environmental Toxicology, 2011, 26(2):111-117.
[22]郑炜, 赵宗胜, 李青峰, 班谦, 李洪涛, 梁耀伟. Solexa测序技术分析鸡与鹌鹑属间杂交雌性和雄性胚胎的差异 microRNAs. 中国兽医学报, 2014, 34(1):116-122.ZHENG W, ZHAO Z S, LI Q F, BAN Q, LI H T, LIANG H T.Analysis of difference microRNAs in female and male embryos of chicken-quail hybrid by Solexa sequencing. Chinese Journal of Veterinary Science, 2014, 34(1):116-122. (in Chinese)
[23]DRUMMOND A E. TGFβ signaling in the development of ovarian function. Cell and Tissue Research, 2005, 322(l): 107-115.
[24]KONRAD L, KEILANI M M, LAIBLE L, NOTTELMANN U,HOFMANN R. Effects of TGF-betas and a specific antagonist on apoptosis of immature rat male germ cells in vitro. Apoptosis, 2006,11(5): 739-748.
[25]SUN Q Y, BREITBART H, SCHATTEN H. Role of the MAPK cascade in mammalian germ cells. Reproduction, Fertility, and Development, 1999, 11(8):443-450.
[26]HARRIS V K, COTICCHIA C M, KAGAN B L, AHMAD S,WELLSTEIN A, RIEGE A T, HARRIS V K, COTICCHIA C M.Induction of the angiogenic modulator fibroblast growth factorbinding protein by epidermal growth factor is mediated through both MEK/ERK and p38 signal transduction path ways. Journal Biology Chemistry, 2000, 275(15): 10802-10811.
[27]ALMOG T, NAOR Z. Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Molecular and Cellular Endocrinology, 2008, 282:39-44.
[28]JOHNSON C, JIA Y, WANG C, LUE Y H, SWERDLOFF R S,ZHANG X S, HU Z Y, LI Y C, LIU Y X, AMIYA P. Sinha Hikim.Role of Caspase 2 in apoptotic signaling in primate and murine germ cells. Biology of Reproduction, 2008, 79 (5): 806.
[29]SHOW M D, HILL C M, ANWAY M D, WRIGHT W W, ZIRKIN B R. Phosphorylation of mitogen-activated protein kinase 8 (MAPK8) is associated with germ cell apoptosis and redistribution of the Bcl2-Modifying Factor (BMF). Journal of Andrology, 2008,29(3): 338-344.
[30]VALVE E, PENTTILA T L, PARANKO J, HA¨RKO¨NEN P. FGF-8 is expressed during specific phases of rodent oocyte and spermatogonium development. Biochemical and Biophysical Research Communications, 1997, 232(1) :173-177.
[31]DVORAK P, DVORAKOV D, HAMPL A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Letters , 2006,580: 2869-2874.
[32]MIALON A, SANKINEN M, SO¨DERSTRO¨M H, JUNTTILA T T,HOLMSTRO¨M T, KOIVUSALO R, PAPAGEORGIOU A C,JOHNSON R S, HIETANEN S, ELENIUS K, WESTERMARCK J.DNA topoisomerase I is a cofactor for c-Jun in the regulation of epidermal growth factor receptor expression and cancer cell proliferation.Molecular and Cellular Biology, 2005, 12(25): 5040-5051.
[33]陈丽莉,肖亚梅,刘文彬,赵如榕,刘姣,刘筠,李万程.ERK/JNK 在黄鳝雌、雄发育阶段生殖腺中的表达和定位.动物学报,2007, 53(2):325-331.CHEN L L, XIAO Y M, LIU W B, ZHAO R R, LIU J, LI W C.Expression and location of ERK/JNK in ovary and spermary in rice field eels Monopterus albus. Journal of Animal Science, 2007,53(2):325-331. (in Chinese)
[34]SHEIKH A Q, TAGHIAN T, HEMINGWAY B, CHO H, KOGAN A B,NARMONEVA1 D A. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field.Journal of the Royal Society Interface, 2013, 10(78): 1-13.
[35]Vien H Y Lee, Leo T O Lee, Billy K C Chow. Gonadotropin- releasing hormone: regulation of the GnRH gene. FEBS Journal, 2008, 275:5458-5478.
[36]BELOV A A, MOHAMMADI M. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Science Signaling, 2012, 5(249):49.
[37]HAINES E, MINOO P, FENG Z Q, RESALATPANAH N, NIE X M,CAMPIGLIO M, ALVAREZ L, COCOLAKIS E, RIDHA M,FULVIO M D, GOMEZ-CAMBRONERO J, LEBRUN J J, ALI S.Tyrosine phosphorylation of Grb2:Role in prolactin /epidermal growth factor cross talk in mammary epithelial cell growth and differentiation.Molecular and Cellular Biology, 2009, 29(10):2505-2520.
[38]YUAN Y Y, CHEN W Y, SHI Q X, MAO L Z,YU S Q, FANG X,ROLDAN E R S. Zona pellucida induces activation of phospholipase A2 during acrosomal exocytosis in guinea pig spermatozoa. Biology of Reproduction, 2003, 68(3): 904-913.
[39]SANTI C M, SANTOS T, HERNÁNDEZ-CRUZ A, DARSZON A.Properties of a novel pH-dependent Ca2+permeation pathway present in male germ cells with possible roles in spermatogenesis and mature sperm function. The Journal of General Physiology, 1998,112: 33-53.
(责任编辑 林鉴非)
Transcriptome Analysis of Differential Gene Expression Associated with Testis Tissue in Mule Duck and Muscovy Duck
LI Li1, MIAO ZhongWei1, XIN QingWu1, ZHU ZhiMing1, ZHANG LinLi1,ZHUANG XiaoDong2, ZHENG NenZhu1,3
(1Institute of Animal Science and Veterinary Medicine, Fujian Academy of Agricultural Sciences, Fuzhou 350013;2Fujian Changlong Group, Zhangzhou 363000, Fujian;3Food College of Fujian Agriculture and Forestry University, Fuzhou 350002)
2017-02-04;接受日期:2017-06-13
福建省农科院青年人才创新基金(YC2017-7)、福建省省属公益类科研院所基本科研专项(2017R1023-5)、福建省农科院所青年基金(MYQJ2015-5)
联系方式:李丽,Tel:13960985616;E-mail:576801792@qq.com。通信作者郑嫩珠,Tel:0591-83815170;E-mail:zhengnz@163.com

