CPB期间血氧分压在60和170 mmHg的心内直视手术患者心肝肾损伤、炎症、氧化应激指标观察
柳磊,李召彬,李滨,徐贯杰,王园园,宋万军
(河北医科大学第三医院,石家庄 050051)
CPB期间血氧分压在60和170 mmHg的心内直视手术患者心肝肾损伤、炎症、氧化应激指标观察
柳磊,李召彬,李滨,徐贯杰,王园园,宋万军
(河北医科大学第三医院,石家庄 050051)
目的 比较体外循环(CPB)期间血氧分压控制在60、170 mmHg的心内直视手术患者心肝肾损伤指标、炎症因子、氧化应激指标变化。方法 80例择期行心内直视手术患者随机分为低氧组和高氧组各40例,低氧组、高氧组在CPB期间血氧分压分别控制在60、170 mmHg。分别于术前及开主动脉后30 min、2 h、6 h、24 h(T0~T4)抽取患者静脉血,采用全自动生化分析仪检测血清谷草转氨酶(AST)、谷丙转氨酶(ALT)、碱性磷酸酶(ALP)、肌酐(Cr)、尿素氮(UN)、尿酸(UA),ELISA法检测血清心肌肌钙蛋白(cTnI)、肌酸激酶同工酶(CK-MB)、C反应蛋白(CRP)、白细胞介素6(IL-6)、肿瘤坏死因子α(TNF-α),采用试剂盒检测血清活性氧(ROS)、丙二醛(MDA)。结果 与同组T0时点比较,两组T1~T4时点cTnI、CK-MB、AST、ALT、ALP、Cr、UN、UA水平升高(P均<0.05);与高氧组比较,低氧组T2~T4时点cTnI、CK-MB、AST、ALT、ALP、Cr、UN、UA水平降低(P均<0.05)。与同组T0时点比较,两组T1~T4时点CRP、TNF-α、IL-6水平升高(P均<0.05);与高氧组比较,低氧组T1~T4时点CRP、TNF-α、IL-6水平降低(P均<0.05)。与同组T0时点比较,两组T1~T4时点ROS、MDA水平升高(P均<0.05);与高氧组比较,低氧组T1~T4时点ROS、MDA水平降低(P均<0.05)。结论 相对于高血氧分压,心内直视手术患者CPB期间采用低血氧分压能够显著降低由CPB引发的心肝肾损伤、炎症反应及氧化应激。
体外循环;血氧分压;心内直视手术;心肝肾损伤;炎症因子;氧化应激
体外循环(CPB)是利用一系列特殊人工装置将回信静脉血引流到体外,经过人工进行温度调解、气体交换和过滤等处理后,输回体内动脉系统的生命支持技术[1]。CPB的目的是在实施心中直视手术时,维持全身组织器官的血液供应[2]。随着临床医学的发展,CPB应用范围不断扩展,在心、肝、肾、肺等大血管手术中获得应用。CPB技术大大降低了心脏病患者病死率,改善了患者的生活质量[3]。CPB过程中充足的氧合是患者生命安全的保证,但是过高的血氧分压可启动体内的再氧合损伤,而再氧合损伤可能诱发机体内心肝肾损伤、炎症反应、氧化应激[4~6]。本研究通过观察CPB期间不同血氧分压控制对心内直视手术患者心肝肾损伤指标、炎症因子及氧化应激指标的影响,旨在为临床上应用CPB行心内直视手术期间血氧分压的选择提供理论基础。
1 资料与方法
1.1 临床资料 选取2014年12月~2016年12月河北医科大学第三医院收治的择期行心脏病心内直视手术患者80例。纳入标准[7]:①符合心脏病诊断标准且为ASA Ⅱ~Ⅲ级;②术前肝功能正常;③无长期明显肝功能损害药物服用史。排除标准:①术前肝功能异常;②各种急性肝炎以及慢性乙型肝炎活动期的患者;③合并糖尿病、高血压、肿瘤等疾病的心脏病患者;④治疗过程中使用有明确肝功能损害的药物者。经医院伦理委员会批准及患者家属签字同意。所有患者随机分为两组各40例。低氧组男24例,女16例;年龄(47.53±17.67)岁;体质量(61.33±5.31)kg。高氧组男23例,女17例;年龄(46.71±18.41)岁;体质量(59.18±5.61)kg。
1.2 CPB及血氧分压控制 采用静吸复合气管插管麻醉,连接CPB管路,肝素化后采用体外循环机建立CPB。CPB过程两组均保持灌注流量(Q)2.2~2.4 L/(min·m2),维持MAP 50~80 mmHg,CVP<0 cmH20,血流降温使鼻咽温在32~35 ℃,保持降温速度0.5~1 ℃/min,稳态管理血气,动脉血pH值维持在7.35~7.45。低氧组在CPB转流中控制血氧分压在60 mmHg左右,高氧组在CPB转流中控制血氧分压在170 mmHg左右。
1.3 血清心肝肾损伤指标、炎症因子及氧化应激指标检测 分别于手术前(T0)及开主动脉后30 min(T1)、2 h(T2)、6 h(T3)、24 h(T4)抽取患者颈内静脉置管中的血浆,离心取血清。采用全自动生化分析仪检测血清中谷草转氨酶(AST)、谷丙转氨酶(ALT)、碱性磷酸酶(ALP)、肌酐(Cr)、尿素氮(UN)、尿酸(UA);采用ELISA法检测血清心肌肌钙蛋白(cTnI)、肌酸激酶同工酶(CK-MB)、C反应蛋白(CRP)、白细胞介素6(IL-6)、肿瘤坏死因子α(TNF-α);采用试剂盒检测血清活性氧(ROS)、丙二醛(MDA)。

2 结果
2.1 两组不同时点血清心肌损伤指标比较 结果见表1。

表1 两组不同时点血清心肌损伤指标比较
注:与同组T0时点比较,*P<0.05;与高氧组比较,△P<0.05。
2.2 两组不同时点血清肝功能损伤指标比较 结果见表2。
2.3 两组不同时点血清肾功能损伤指标比较 结果见表3。
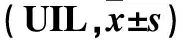
表2 两组不同时点血清肝功能损伤指标比较
注:与同组T0时点比较,*P<0.05;与高氧组比较,△P<0.05。

表3 两组不同时点血清肾功能损伤指标比较
注:与同组T0时点比较,*P<0.05;与高氧组比较,△P<0.05。
2.4 两组不同时点血清炎症因子水平比较 结果见表4。

表4 两组不同时点血清炎症因子水平比较
注:与同组T0时点比较,*P<0.05;与高氧组比较,△P<0.05。
2.5 两组不同时点血清氧化应激指标比较 结果见表5。
3 讨论
CPB技术在心脏手术中广泛应用,展现出较高的应用前景,但CPB会暂时阻断心脏冠状动脉循环、稀释血液,导致红细胞压积下降,降低血液温度,引起细胞肿胀及血管扩张,缺血再灌注,抑制血管分泌血管紧张素、肾素、醛固酮等物质,进而导致患者心、肝、肾等损伤,重者可引发心肝肾衰竭而危及生命安全[8~11]。

表5 两组不同时点血清氧化应激指标比较
注:与同组T0时点比较,*P<0.05;与高氧组比较,△P<0.05。
血清学有多种指标能够反映器官功能,其中CK-MB、cTnI是反映心脏功能的常用指标,血清cTnI水平与心肌缺血损伤的程度呈正相关;AST、ALT、ALP是临床上常用的反映肝功能的血清学指标,其水平能够反映肝细胞的完整性;Cr、UN、UA是临床常用的反映肾功能的血清学指标,其中Cr是肌酸的代谢产物,UN是蛋白质代谢产物,血清Cr、UN水平则反映肾小球的滤过功能,血清UA水平反映肾小管损伤程度[12~15]。本研究显示,与同组T0时点比较,两组T1~T4时点cTnI、CK-MB、AST、ALT、ALP、Cr、UN、UA水平升高;与高氧组比较,低氧组T2~T4时点cTnI、CK-MB、AST、ALT、ALP、Cr、UN、UA水平降低。上述结果表明,在心内直视手术上,CPB能够显著引发患者的心肝肾功能损伤,但低血氧分压较高血氧分压能够显著降低由CPB引发的心肝肾损伤。
CPB过程诱发这些功能损伤的因素有多种,如心肌缺氧、血液与人工物质的表面接触、手术本身创伤、再灌注损伤、高氧血症等[16,17],其中由于高血氧分压引发的高氧血是诱发机体器官损伤的主要因素。一方面,高氧血能够激活机体中性粒细胞、单核细胞,使其释放炎症细胞因子,进而引发全身性炎症反应综合征,其中炎症因子如CRP、IL-6、TNF-α是参与炎症反应的重要因子,其血清水平在CPB后显著升高。CRP是机体受到微生物入侵或组织损伤等炎症性刺激时肝细胞合成的急性相蛋白,其在炎症开始数小时就升高,是反映机体炎症反应的重要因子;IL-6能够作用于机体多种炎症细胞,促进炎症反应,是反映炎症反应强弱的最主要细胞因子;TNF-α也是重要的促炎因子,当机体发生炎症反应时,其水平显著升高,且CPB后其升高速度比其他炎症因子更快[18~20]。另一方面,高氧血由于氧气水平较高导致高氧自由基,高氧自由基能够引发缺血再灌注损伤;此外,氧自由基能够进一步血管内皮细胞等释放NO等氧化物质,引发机体的氧化应激。ROS、MDA水平是反映氧化应激水平的关键指标,研究[21,22]表明CPB后患者体内ROS、MDA水平升高。本研究显示,与同组T0时点比较,两组T1~T4时点CRP、TNF-α、IL-6水平升高;与高氧组比较,低氧组T1~T4时点CRP、TNF-α、IL-6水平降低。与同组T0时点比较,两组T1~T4时点ROS、MDA水平升高;与高氧组比较,低氧组T1~T4时点ROS、MDA水平降低。上述结果提示,在心内直视手术上,CPB能够显著引发患者的氧化应激及炎症反应,但低血氧分压较高血氧分压能够显著降低由CPB引发的氧化应激及炎症反应。
总之,CPB能够引发机体心肝肾损伤、炎症反应、氧化应激,但低血氧分压较高血氧分压能够显著降低CPB引发的心肝肾损伤、炎症反应、氧化应激。
[1] 胡彦艳,黄中华,黄爱兰,等.体外循环心脏手术后肝脏损害的研究进展[J].广东医学,2014,35(14):2282-2284.
[2] Barry AE, Chaney MA, London MJ. Anesthetic management during cardiopulmonary bypass: a systematic review[J]. Anesth Analg, 2015,120(4):749-769.
[3] Pekelharing J, Furck A, Banya W, et al. Comparison between thromboelastography and conventional coagulation tests after cardiopulmonary bypass surgery in the paediatric intensive care unit[J]. Int J Lab Hematol, 2014,36(4):465-471.
[4] 何苗.细胞凋亡在体外循环肺损伤中的研究进展[J].医学研究生学报,2015,28(10):1102-1106.
[5] 莫文魁,陈群清.体外循环对脑血管影响的研究进展[J].广东医学,2013,34(15):2422-2424.
[6] Liguori GR, Kanas AF, Moreira LF. Managing the inflammatory response after cardiopulmonary bypass: review of the studies in animal models[J]. Rev Bras Cir Cardiovasc, 2014,29(1):93-102.
[7] 徐网兰,方强.体外循环心脏手术患者围手术期肝功能的变化及相关危险因素分析[J]. 中国循环杂志,2015(11):1086-1089.
[8] Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery[J]. Circulation, 2007,116(22):2544-2552.
[9] Engelman R, Baker RA, Likosky DS, et al. The society of thoracic surgeons, the society of cardiovascular anesthesiologists, and the American society of extra corporeal technology: clinical practice guidelines for cardiopulmonary bypass-temperature management during cardiopulmonary bypass[J]. Ann Thorac Surg, 2015,100(2):748-757.
[10] Drenger B, Fontes ML, Miao Y, et al. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass effects on in-hospital morbidity and mortality[J]. Circulation, 2012,126(3):261-269.
[11] Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies[J]. Am J Kidney Dis, 2015,65(2):283-293.
[12] Safdar B, Bezek SK, Sinusas AJ, et al. Elevated CK-MB with a normal troponin does not predict 30-day adverse cardiac events in emergency department chest pain observation unit patients[J]. Crit Pathw Cardiol, 2014,13(1):14-19.
[13] Justinger C, Kouladouros K, Gärtner D, et al. Liver resection after selective internal radiotherapy (SIRT): proof of concept, initial survival, and safety[J]. J Surg Oncol, 2015,112(4):436-442.
[14] 姜晓芬,缪剑霞,池胜英,等.体外循环中白细胞滤除对心脏手术后患者肾功能的影响[J].中华麻醉学杂志,2007,27(4):301-304.
[15] Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives[J]. Lancet, 2013,382(9888):260-272.
[16] 刘建华,沈金美,李李,等.体外循环期间不同氧分压对瓣膜置换患者围术期炎性细胞因子及心肌损伤的影响[J].中国现代医学杂志,2005,15(15):2356-2358.
[17] Kleinbongard P, Neuhäuser M, Thielmann M, et al. Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting[J]. Cardiology, 2016,133(2):128-133.
[18] Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients[J]. N Engl J Med, 2013,368(13):1189-1198.
[19] Engels M, Bilgic E, Pinto A, et al. A cardiopulmonary bypass with deep hypothermic circulatory arrest rat model for the investigation of the systemic inflammation response and induced organ damage[J]. J Inflamm(Lond), 2014,11:26.
[20] Svensson AS, Kvitting JP, Kovesdy CP, et al. Changes in serum cystatin C, creatinine, and C-reactive protein after cardiopulmonary bypass in patients with normal preoperative kidney function[J]. Nephrology (Carlton), 2016,21(6):519-525.
[21] Matyal R, Sakamuri S, Huang T, et al. Oxidative stress and nerve function after cardiopulmonary bypass in patients with diabetes[J]. Ann Thorac Surg, 2014,98(5):1635-1644.
[22] McDonald CI, Fraser JF, Coombes JS, et al. Oxidative stress during extracorporeal circulation[J]. Eur J Cardiothoracic Surg, 2014,46(6):937-943.
Observation of parameters of organ damage, inflammation, and oxidative stress in patients undergoing open-heart surgery during cardiopulmonary bypass with oxygen tension controlled at 60 and 170 mmHg
LIULei,LIZhaobin,LIBin,XUGuanjie,WANGYuanyuan,SONGWanjun
(TheThirdHospitalofHebeiMedicalUniversity,Shijiazhuang050051,China)
Objective To compare the parameter changes of organ damage, inflammation, and oxidative stress in patients undergoing open-heart surgery during cardiopulmonary bypass with oxygen tension controlled at 60 and 170 mmHg. Methods Eighty patients undergoing open-heart surgery were randomly divided into the low oxygen tension group and high oxygen tension group with 40 patients in each. The oxygen tension in the low oxygen tension group and high oxygen tension group was 60 mmHg and 170 mmHg, respectively. The plasma was collected before surgery and at 30 min, 2 h, 6 h, 24 h after aortic declamping (T0-T4). The levels of glutamic-pyruvic transaminase (ALT), glutamic oxalacetic transaminase (AST), alkaline phosphatase (ALP), creatinine (Cr), urea nitrogen (UN), uric acid (UA) in the plasma were measured by fully automatic biochemical analyser; the levels of cardiac troponin (cTnI), creatine kinase isoenzyme (CK-MB), C-reactive protein (CRP), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α) in the plasma were measured by ELISA; reactive oxygen (ROS), malonaldehyde (MDA) in the plasma were measured by kits.Results The levels of cTnI, CK-MB, ALT, AST, ALP, Cr, UN, and UA in the plasma of both groups increased significantly at T1-T4as compared with those of the same group at T0(allP<0.05); compared with the high oxygen tension group, the levels of cTnI, CK-MB, AST, ALT, ALP, Cr, UN, and UA in the low oxygen tension group at T2-T4decreased significantly (allP<0.05). The levels of CRP, TNF-α and IL-6 increased significantly at T1-T4as compared with those of the same group at T0(allP<0.05); compared with the high oxygen tension group, the levels of CRP, TNF-α and IL-6 in the low oxygen tension group at T1-T4decreased significantly (allP<0.05). The levels of ROS and MDA in both groups at T1-T4increased significantly as compared with those of the same group at T0(allP<0.05); compared with the high oxygen tension group, the levels of ROS and MDA in the low oxygen tension group at T1-T4decreased significantly (allP<0.05). Conclusion Compared with the high oxygen tension, using low oxygen tension during CPB in open-heart surgery can reduce CPB-induced organ damage, inflammation, and oxidative stress significantly.
cardiopulmonary bypass; oxygen tension; open-heart surgery; Heart, liver and kidney damage; inflammation; oxidative stress
河北省医学科学研究重点课题(20150731)。
柳磊(1981-),男,副主任医师,博士,主要研究方向为心血管外科基础与临床。E-mail: keu2239@163.com
李召彬(1984-),男,硕士,主要研究方向为心血管外科基础与临床。E-mail: 1059988676@qq.com
10.3969/j.issn.1002-266X.2017.31.006
R54
A
1002-266X(2017)31-0021-04
2017-03-28)

