低剂量邻苯二甲酸二(2-乙基)己酯对老年大鼠前列腺的促增生作用
黄冬妍,吴双双,朱婧,毛晓燕,李雷,吴建辉,孙祖越
(1.上海市计划生育科学研究所药理毒理学研究室,中国生育调节药物毒理学检测中心,上海200032;2.国家人口和计划生育委员会计划生育药具重点实验室,上海200032;3.复旦大学生殖与发育研究院,上海200032)
低剂量邻苯二甲酸二(2-乙基)己酯对老年大鼠前列腺的促增生作用
黄冬妍1,2,3,吴双双1,3,朱婧1,3,毛晓燕1,2,3,李雷1,2,3,吴建辉1,2,3,孙祖越1,2,3
(1.上海市计划生育科学研究所药理毒理学研究室,中国生育调节药物毒理学检测中心,上海200032;2.国家人口和计划生育委员会计划生育药具重点实验室,上海200032;3.复旦大学生殖与发育研究院,上海200032)
目的 评价环境暴露剂量的邻苯二甲酸二(2-乙基)己酯(DEHP)对老年大鼠前列腺的促增生作用及机制。方法32只1.5岁龄老年雄性SD大鼠随机分成4组,每组8只,分别ig给予DEHP(30,90和270 μg·kg-1)或溶媒,每天1次,连续4周。于末次给药24 h后将大鼠麻醉,而后①腹主动脉采血,采用ELISA检测血清中睾酮(T)、雌二醇(E2)和泌乳素(PRL)水平;②处死后取前列腺,分叶,称重、测量体积,计算脏器系数;③制作前列腺组织病理切片,HE染色后用显微镜观察组织形态改变,并利用显微图像分析软件分析前列腺上皮高度变化。结果 与溶媒对照组比较,DEHP 270 μg·kg-1组前列腺系数、背侧前列腺质量和背侧前列腺系数均显著增加(P<0.05);DEPH各剂量组腹侧前列腺上皮高度明显升高(P<0.01);DEHP 270 μg·kg-1组背侧前列腺上皮高度明显升高(P<0.01);DEHP各剂量组E2,PRL和T水平均无显著改变,但DEHP 30和270 μg·kg-1组E2/T比值显著增加(P<0.05)。结论低剂量DEHP对老年大鼠前列腺具有促增生作用,该作用可能与其影响内源性激素的相对水平有关。
邻苯二甲酸二(2-乙基)己酯;环境内分泌干扰物;前列腺增生;老年大鼠;雌激素;雄激素
DOl:10.3867/j.issn.1000-3002.2017.06.021
良性前列腺增生症(benign prostatic hyperplasia,BPH)多发于中老年男性,随着人们寿命的日益延长,社会人口老龄化问题日益突出,老年男性人口数量持续增加,BPH发病率不断上升[1-2]。BPH在临床上主要表现为逼尿肌功能障碍,膀胱出口梗阻,以及尿频、尿急和急性尿潴留等症状,严重者甚至会出现泌尿系统结石以及肾功能衰竭等[3],严重影响患者的生活质量。前列腺是雄激素依赖性器官,其结构和功能的维持都与雄激素密不可分。随着研究的深入,人们发现雌激素能调节雄激素活性并影响前列腺生长,在一定范围内,提高雌激素水平将刺激前列腺细胞生长[4-5]。除了内源性雌激素,越来越多的学者将目光聚焦在具有雌激素活性的环境内分泌干扰物(environmental endocrine disruptors,EDC)上。
EDC又称环境激素,是指可通过干扰生物或人体内保持自身平衡和调节发育过程天然激素的合成、分泌、运输、结合、反应和代谢等,从而对生物或人体的生殖、神经和免疫系统等的功能产生影响的外源性化学物质[6]。EDC在体内富集后,一般以类激素的形式产生生物学效应,使体内原有的内分泌功能出现紊乱[7]。邻苯二甲酸二(2-乙基)己酯〔di(2-ethylhexyl)phthalate,DEHP〕是常见的树脂原料,广泛存在于填充剂原料、高分子材料、化妆品以及增塑剂中,是EDC中较为重要的一类。总结以往的研究发现,DEHP具有的生殖毒性主要体现在2方面。一方面,亲代暴露在DEHP中,会对子代雄性个体生殖器官和生育力产生影响;另一方面,雄性动物直接暴露在DEHP中,其生殖系统也会受到影响,如精子发生异常、睾丸萎缩和隐睾症等[8-9]。但在DEHP生殖毒性研究中,尚无关于对前列腺毒性的报道,为数不多的研究也仅限于较高的暴露剂量。如张鸿毅等[10]报道,分别每天给予雄性SD大鼠DEHP 50,200和500 mg·kg-1,连续8周,大鼠前列腺腺上皮细胞呈柱状或立方状,腺腔增大,间充质增生,符合前列腺增生的病理改变特征。
据文献报道,在典型的饮食方式前提下,育龄妇女、青少年和婴儿的DEHP暴露剂量分别为每天5.7,8.1和42.1 μg·kg-1,较高者暴露剂量可达每天2 mg[11-12]。因此,如能明确环境暴露剂量下DEHP对前列腺增生的作用,将是对DEHP生殖毒性研究的一个重要补充,也更具实际意义。前列腺增生是明确与年龄有关的疾病,故选用中老年动物作为研究对象。本研究探讨环境暴露剂量的DEHP对老年SD大鼠前列腺的促增生作用及其可能的机制,旨在为探索前列腺增生的发生机制及寻找更好的治疗药物提供实验依据。
1 材料与方法
1.1 药物、试剂及主要仪器
DEHP,纯度≥99.5%,批号D201154,美国Sigma-Aldrich公司;羧甲基纤维素钠(CMC-Na),批号20140520,国药集团化学试剂有限公司。大鼠雌二醇(estradiol,E2,编号NB-E30608)和大鼠睾酮(testosterone,T,编号NB-E90064)ELISA检测试剂盒,美国诺泰生物科技有限公司;大鼠泌乳素(prolactin,PRL,编号55-PRLRT-E01)ELISA检测试剂盒,美国ALPCO公司。ML-104型电子天平和PL-203型电子太平(瑞士MettlerToledo公司);Eclipse 50i显微镜和NIS-Elements BR 3.1显微图像分析软件(日本Nikon公司);ST5010全自动染色机(德国Leica公司)。
1.2 动物和分组
5~7周龄雄性SD大鼠32只,体质量200~220 g,由上海西普尔-必凯实验动物有限公司提供,许可证编号SCXK(沪)2013-0016。大鼠在上海市计划生育科学研究所屏障系统内饲养,每笼2只,室温20~26℃,相对湿度40%~70%,保持12/12 h昼夜节律,自由饮水摄食。大鼠饲养至1.5岁龄,按体质量随机分为4组,依次为溶媒对照组、DEHP 30,90和270 μg·kg-1剂量组,每组8只,分别ig给予不同剂量DEHP或同体积溶媒(0.5%CMC-Na水溶液),给药容积为10 mL·kg-1,每天1次,连续4周。末次给药24 h后,腹腔注射3%戊巴比妥钠麻醉大鼠,腹主动脉采血,而后处死。
1.3 大鼠前列腺取材及脏器系数计算
大鼠处死后,取前列腺组织,称量其总质量,并用排水法测量其体积。将前列腺分为腹侧叶和背侧叶,分别测量其质量。计算前列腺及其各叶的脏器系数,脏器系数=脏器质量(g)/体质量(g)×100。
1.4 HE染色法分析前列腺病理变化
将各叶前列腺组织用4%甲醛溶液固定48 h,依次经乙醇梯度脱水,二甲苯透明,常规石蜡包埋,并制成4 μm病理切片,经HE染色后,于光学显微镜下观察前列腺组织病理学变化。每张切片在同一视野下随机选取10个前列腺腺腔,并在对应的腺腔上选取2处上皮,利用显微图像分析软件,定量分析前列腺腺腔面积和上皮高度。
1.5 ELlSA法检测血清E2,T和PRL水平
腹主动脉采血后静置2 h,3000×g离心15 min,分离血清。按照ELISA试剂盒说明书步骤检测血清E2,T和PRL的水平。
1.6 统计学分析
实验结果 数据以x±s表示,使用SPSS 17.0软件进行统计分析,组间比较采用单因素方差分析,方差齐用LSD法进行组间两两比较,不齐则用Dunnett T3法进行组间两两比较,以P<0.05具有统计学差异。
2 结果
2.1 DEHP对老年大鼠体质量的影响
给予DEHP期间,DEHP 90 μg·kg-1组1只老年大鼠自然死亡,其余大鼠摄食、饮水正常,行为体征状况良好。各组大鼠体质量在给予DEHP 1周后有所下降,之后缓慢增长,在第4周时体质量与给药前相当。与同时段溶媒对照组相比,各组大鼠体质量无明显差异,表明在受试剂量范围内DEHP对老年大鼠的生长无明显影响(图1)。
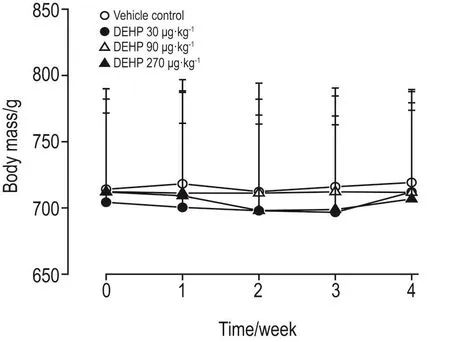
Fig.1 Mean body mass of aged rats treated with di(2-ethylhexyl)phthalate(DEHP).The 1.5-year-old SD rats were ig treated with DEHP 0,30,90 and 270 μg·kg-1,once daily for 4 weeks,respectively.The body mass was measured at the initiation of treatment and once per week untill the end of study. x±s,n=8.
2.2 DEHP对老年大鼠前列腺质量和脏器系数的影响
与溶媒对照组比较,DEHP各剂量组大鼠前列腺质量和腹侧叶前列腺质量均无显著差异,DEHP 270 μg·kg-1组背侧叶前列腺质量显著增加(P<0.05)。脏器系数随DEHP暴露剂量的增加而增加,与溶媒对照组比较,DEHP 270 μg·kg-1组前列腺系数和背侧叶前列腺系数均显著增加(P<0.05)(表1)。DEHP各剂量组大鼠前列腺体积与溶媒对照组均无显著差异(数据未列出)。
2.3 DEHP对老年大鼠前列腺组织形态的影响
病理结果 显示,给药4周后,DEHP各剂量组老年大鼠前列腺腺腔向内突出,褶皱增多(图2和图3)。各叶前列腺腺腔面积无明显变化(表2)。显微图像分析结果 显示,与溶媒对照组比较,DEHP各剂量组大鼠腹侧叶前列腺上皮高度显著性增加(P<0.01),DEHP 270 μg·kg-1组背侧叶前列腺上皮高度显著增加(P<0.01)(图3和图4)。
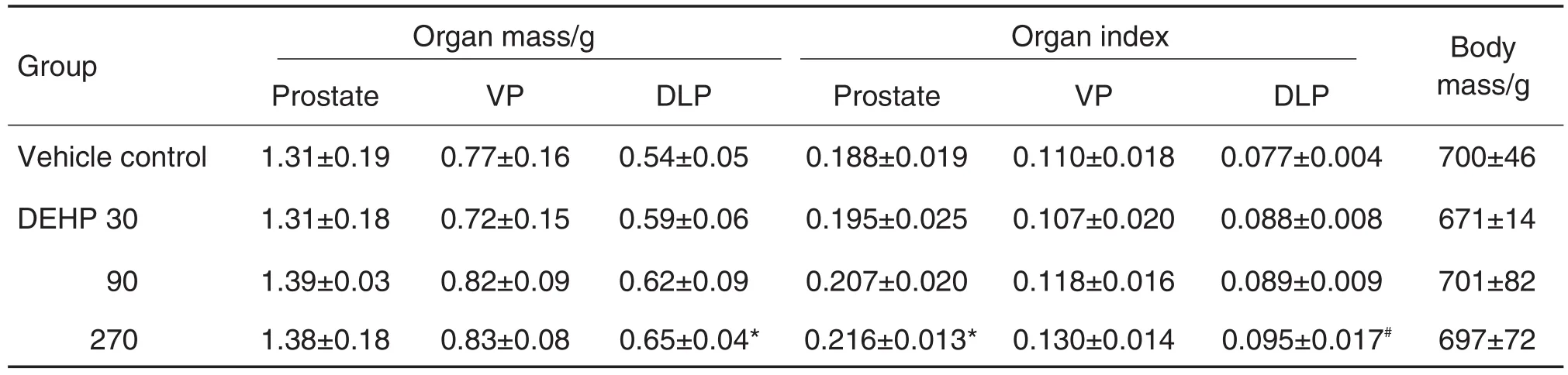
Tab.1 Effect of DEHP on prostate mass,prostate index and body mass of aged rats

Fig.2 Histological analysis of prostate in male aged rats treated with DEHP(HE staining×40).See Tab.1 for the rat treatment.

Fig.3 Histological analysis of prostate in male aged rats treated with DEHP(×400).See Fig.2 for the rat treatment.
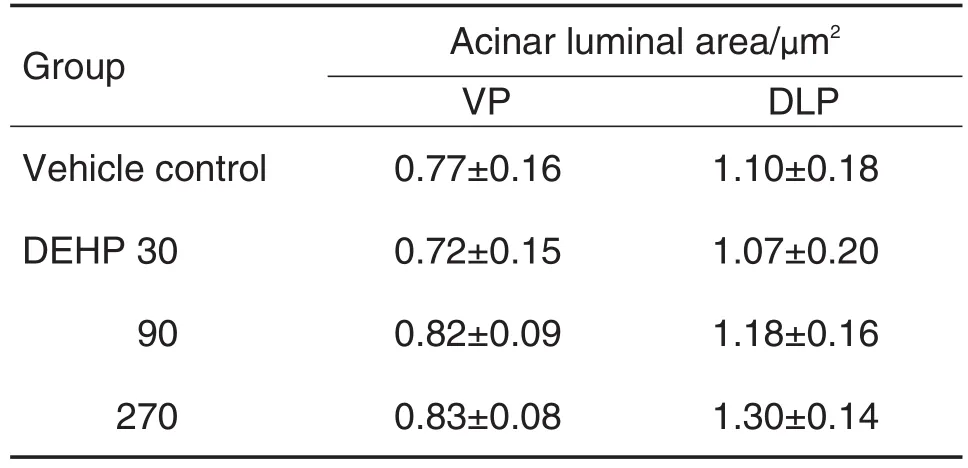
Tab.2 Effect of DEHP on acinar luminal area of prostate in aged rats
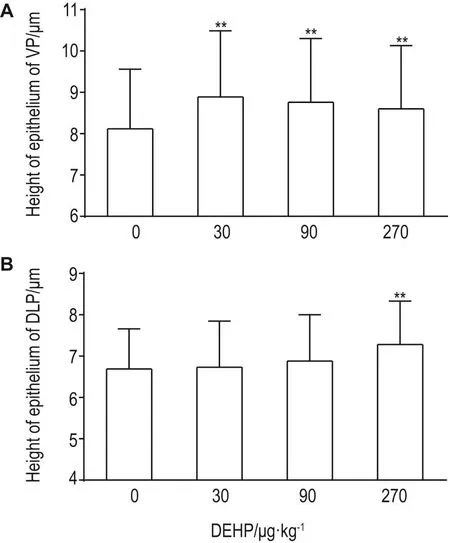
Fig.4 Effect of DEHP on height of prostatic epithelium in aged rats.See Fig.1 for the rat treatment.A:epithelium of VP;B:epithelium of DLP.x±s,n=8.**P<0.01,compared with vehicle control(0)group.
2.4 DEHP对老年大鼠血清E2,T,PRL水平及E2/T比值的影响
与溶媒对照组比较,DEHP各剂量组大鼠血清E2,T和PRL水平均无显著差异;DEHP 30和270 μg·kg-1组E2/T比值显著升高(P<0.05)(图5)。
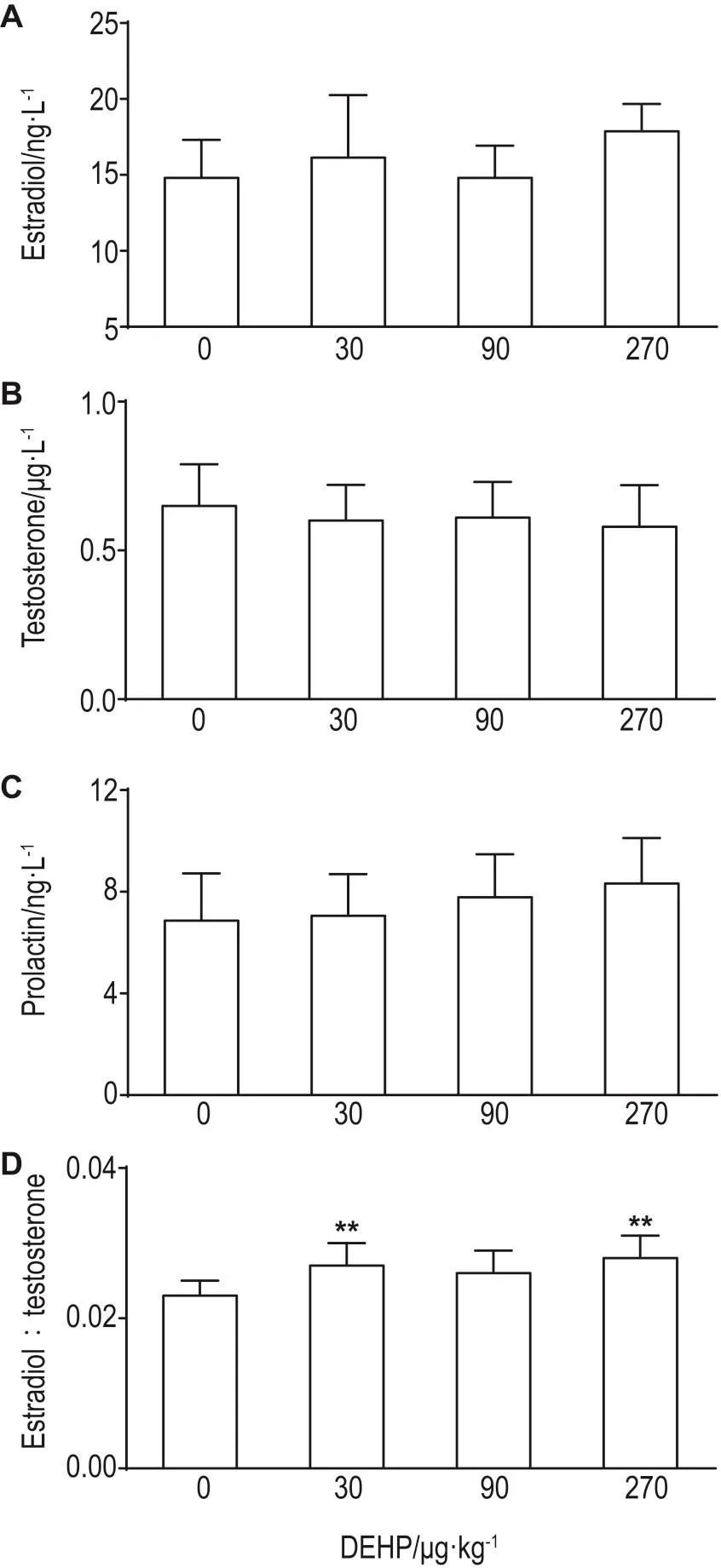
Fig.5 Effect of DEHP on serum estradiol(A),testosterone(B),prolactin(C)levels and ratio of estradiol/ testosterone(D)in aged rats.See Fig.1 for the rat treatment.Abdominal aortic blood samples were collected,then serum estradiol,testosterone,and prolactin levels were assayed by ELISA.x±s,n=8.*P<0.05,compared with vehicle control(0)group.
3 讨论
由于DEHP应用广泛,人们可通过摄取、吸入和皮肤接触等多种途径暴露在DEHP中。欧洲食品安全局规定DEHP日耐受摄入剂量为50 μg·kg-1,而美国环境保护署则将每天20 μg·kg-1作为安全参考剂量[13-14]。DEHP在生物体内主要被分解为邻苯二酸-单-2-乙基己酯〔mono-(2-ethylhexyl)phthalate〕、单(2-乙基-5氧乙基)邻苯二甲酸酯〔mono-(2-ethyl-5-oxohexyl)phthalate〕、单(2-乙基-5羟基乙基)邻苯二甲酸酯〔mono(2-ethyl-5-hydroxyhexyl)phthalate〕和单(2-乙基-5-羧基戊基)邻苯二甲酸酯〔mono(2-ethyl-5-carboxypentyl)phthalate〕,其中前三者占绝大部分,DEHP的毒性可能取决于由化合物到代谢物的转变,而转变又与摄入途径、种属和年龄等因素相关[15-16]。本研究发现,低剂量DEHP(30~270 μg·kg-1)能显著增加老年大鼠前列腺系数和背侧叶前列腺系数,以及腹侧叶和背侧叶前列腺上皮高度。据文献报道,在表达FGF-2的转基因小鼠中,背侧叶前列腺上皮增生要比腹侧叶明显,小鼠背侧叶前列腺可能与人前列腺周围带同源,而前列腺增生和前列腺癌几乎都源于移行带和周围带[17]。本研究结果 显示,老年大鼠背侧叶前列腺增生对DEHP更为敏感,提示大鼠前列腺背侧叶可能也与人前列腺移行带或周围带同源。
文献报道,每天给予E210 μg·kg-1可以促进前列腺上皮增生,并导致前列腺体积的整体增加,可能是通过直接刺激前列腺基质细胞,或间接调节上皮细胞实现的[18]。作为环境激素,DEHP可模拟内源性雌激素,与雌激素受体(estrogen receptor,ER)结合产生雌激素效应[19]。也有研究表明,DEHP能提高人子宫内膜基质细胞ERα mRNA和蛋白质的表达[20],推测DEHP也可能通过提高组织中ER的表达,实现促进前列腺基质细胞增生的作用。
前列腺是睾酮依赖性器官,睾酮在前列腺生长发育中起到重要的作用。许多研究发现,啮齿类动物青春期暴露于较高剂量DEHP(>100 mg·kg-1)中,雄激素调节基因的表达显著性下调,出现比较明显的抗雄激素作用[21-21]。DEHP可直接降低睾丸中睾酮的合成,也可通过其代谢物促进睾酮16α-羟化酶(CYP2C11)和睾酮6β-羟化酶(CYP3A2)的活性,加速睾酮的分解[24-25],从而降低睾酮的水平。在本次研究中,DEHP在促进前列腺增生的同时,睾酮水平仅有下降的趋势,这可能由于暴露水平太低,还不足以启动抗雄激素活性。
雌、雄激素平衡,在维持雄性生理特征和生殖功能中发挥重要作用,随年龄的增加,雄激素水平降低,雌激素水平相对升高,雌、雄激素比例失衡,是BPH病因学说之一[26]。在雄性体内,PRL由前列腺上皮细胞合成,在雄性生殖腺体内协同雄激素增强雄激素效应,并在前列腺增生发生中起作用[27]。本次研究暴露对象为老年大鼠,体内E2/T比值的变化对外源性激素更为敏感。本研究结果 也表明,DEHP使E2/T比值明显升高,提示DEHP可能通过升高雌、雄激素比例促进BPH发生。
综上所述,低剂量DEHP对老年大鼠前列腺具有促增生作用,该作用可能通过改变内源性激素的相对水平而实现。但是,DEHP究竟是通过介导哪种信号通路引起激素水平的改变还需要进一步研究。
[1]Liao LM,Schaefer W.Cross-sectional and longitudinal studies on interaction between bladder compliance and outflow obstruction in men with benign prostatic hyperplasia[J].Asian J Androl,2007,9(1):51-56.
[2]Wu Y,Pan H,Wang WM,Xu D,Zhang L,Gu ZQ,et al.A possible relationship between serum sex hormones and benign prostatic hyperplasia/lower urinary tract symptoms in men who underwent transurethral prostate resection[J].Asian J Androl,2017,19(2):230-233.
[3]Huang DY,Wu JH,Sun ZY.Categories and characteristics of BPH drug evaluation models:a comparative study[J].Natl J Androl(中华男科学杂志),2014,20(2):181-185.
[4]Roehrborn CG.Pathology of benign prostatic hyperplasia[J].Int J Impot Res,2008,20(Suppl 3):S11-S18.
[5]Liu XY.The study of the relation between estrogen or androgen and mechanism of benign prostatic hyperplasia(雌、雄激素引发前列腺增生的机制研究)[D].Shanghai:Fudan University(复旦大学),2008.
[6]Diamanti-Kandarakis E,Bourguignon JP,Giudice LC,Hauser R,Prins GS,Soto AM,et al.Endocrinedisrupting chemicals:an endocrine society scientific statement[J].Endocr Rev,2009,30(4):293-342.
[7]Huang DY,Wu JH,Sun ZY.Progress in toxicological studies in environmental endocrine disruptors on prostate[J].J Environ Health(环境与健康杂志),2014,31(9):837-840.
[8]Stenz L,Escoffier J,Rahban R,Nef S,Paoloni-Giacobino A.Testicular dysgenesis syndrome and long-lasting epigenetic silencing of mouse sperm genes involved in the reproductive system after prenatal exposure to DEHP[J].PLoS One,2017,12(1):e0170441.
[9]Erkekoglu P,Rachidi W,Yuzugullu OG,Giray B,Favier A,Ozturk M,et al.Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate(DEHP)and mono(2-ethylhexyl)-phthalate(MEHP)on MA-10 Leydig cells and protection by selenium[J].Toxicol Appl Pharmacol,2010,248(1):52-62.
[10]Zhang HY,Zhang W,Bao TY,Bai AS,Gao JX,Zhang Y,et al.Relationship between environmental endocrine disruptor DEHP and prostatic hyperplasia in rats[J].J Mod Urol(现代泌尿外科杂志),2009,14(5):347-349.
[11]Serrano SE,Braun J,Trasande L,Dills R,Sathyanarayana S.Phthalates and diet:a review of the food monitoring and epidemiology data[J].Environ Health,2014,13(1):43.
[12]Sui HX,Zhang L,Wu PG,Song Y,Yong L,Yang DJ,et al.Concentration of di(2-ethylhexyl)phthalate(DEHP)in foods and its dietary exposure in China[J].Int J Hyg Environ Health,2014,217(6):695-701.
[13]Wittassek M,Heger W,Koch HM,Becker K,Angerer J,Kolossa-Gehring M.Daily intake of di(2-ethylhexyl)phthalate(DEHP)by German children-A comparison of two estimation models based on urinary DEHP metabolite levels[J].Int J Hyg Environ Health,2007,210(1):35-42.
[14]Koch HM,Preuss R,Angerer J.Di(2-ethylhexyl)phthalate(DEHP):human metabolism and internal exposure-an update and latest results[J].Int J Androl,2006,29(1):155-165.
[15]Christensen KL,Makris SL,Lorber M.Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment[J].Regul Toxicol Pharmacol,2014,69(3):380-389.
[16]Huang LP,Lee CC,Fan JP,Kuo PH,Shih TS,Hsu PC.Urinary metabolites of di(2-ethylhexyl)phthalate:relation to sperm motility,reactive oxygen species generation,and apoptosis in polyvinyl chloride workers[J].Int Arch Occup Environ Health,2014,87(6):635-646.
[17]Konno-Takahashi N,Takeuchi T,Nishimatsu H,Kamijo T,Tomita K,Schalken JA,et al.Engineered FGF-2 expression induces glandular epithelial hyperplasia in the murine prostatic dorsal lobe[J]. Eur Urol,2004,46(1):126-132.
[18]Wu JH,Jiang XR,Liu GM,Liu XY,He GL,Sun ZY. Oral exposure to low-dose bisphenol A aggravates testosterone-induced benign hyperplasia prostate in rats[J].Toxicol Ind Health,2011,27(9):810-819.
[19]Mu X,Liao X,Chen X,Li Y,Wang M,Shen C,et al.DEHP Exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms[J].J Hazard Mater,2015,298:232-240.
[20]Cho YJ,Park SB,Han M.Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro[J].Mol Cell Endocrinol,2015,407:9-17.
[21]Dobrzyńska MM.Phthalates-widespread occurrence and the effect on male gametes.Part 2.The effects of phthalates on male gametes and on the offspring[J].Rocz Panstw Zakl Hig,2016,67(3):209-221.
[22]Christiansen S,Boberg J,Axelstad M,Dalgaard M,Vinggaard AM,Metzdorff SB,et al.Low-dose perinatal exposure to di(2-ethylhexyl)phthalate induces anti-androgenic effects in male rats[J]. Reprod Toxicol,2010,30(2):313-321.
[23]Jarfelt K,Dalgaard M,Hass U,Borch J,Jacobsen H,Ladefoged O.Antiandrogenic effects in male rats perinatally exposed to a mixture of di(2-ethylhexyl)phthalate and di(2-ethylhexyl)adipate[J].Reprod Toxicol,2005,19(4):505-515.
[24]Borch J,Axelstad M,Vinggaard AM,Dalgaard M. Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis[J].Toxicol Lett,2006,163(3):183-190.
[25]Stroheker T,Cabaton N,Nourdin G,Régnier JF,Lhuguenot JC,Chagnon MC.Evaluation of antiandrogenic activity of di-(2-ethylhexyl)phthalate[J].Toxicology,2005,208(1):115-121.
[26]Lee CH,Akin-Olugbade O,Kirschenbaum A. Overview of prostate anatomy,histology,and pathology[J].Endocrinol Metab Clin North Am,2011,40(3):565-575.
[27]Janulis L,Grayhack JT,Lee C.Prostatic diseases[M]//Lepor H.Endocrinology of the Prostate.Saunders WB,Orlando:Science Press and Harcourt Asia Pte.Ltd.2001,58-74.
Promotion of proliferation of prostate in aged rats by low-dose di(2-ethylhexyl)phthalate
HUANG Dong-yan1,2,3,WU Shuang-shuang1,3,ZHU Jing1,3,MAO Xiao-yan1,2,3,Li Lei1,2,3, WU Jian-hui1,2,3,SUN Zu-yue1,2,3
(1.Shanghai Institute of Planned Parenthood Research,National Evaluation Center for Toxicology of Fertility Regulating Drugs,Shanghai 200032,China;2.National Population and Family Planning Key Laboratory of Contraceptive Drugs and Devices,Shanghai 200032,China;3.Reproductive and Developmental Research Institute,Fudan University,Shanghai 200032,China)
OBJECTlVETo investigate the proliferation effect of di(2-ethylhexyl)phthalate(DEHP) on prostate in aged rats at the environmental exposure dose and the possible mechanism.METHODSThirty-two male Sprague-Dawley rats,aged 1.5 years,were randomly divided into 4 groups(8 rats per group)and treated with DEHP(30,90 and 270 μg·kg-1,ig)and vehicle once daily respectively for 4 weeks.All the animals were anesthetized with pentobarbital sodium and sacrificed on the day subsequent to the last treatment.①Abdominal aortic blood samples were collected,and serum estradiol(E2), testosterone(T)and prolactin(PRL)levels were assayed by ELISA.②The prostate tissues were dissected and categorized into different lobes,weighed and measured.The prostate relative mass was calculated.③The morphological changes were detected by HE staining and prostate epithelial height was analyzed with microscopic image analysis software.RESULTSCompared with vehicle control group,the prostate relative mass,dorsolateral prostate mass,and dorsolateral prostate index in DEHP 270 μg·kg-1group were significantly higher(P<0.05).The height of the ventral prostate epithelium in DEHP 30,90 and 270 μg·kg-1groups was increased significantly(P<0.01),so was the height of dorsal prostate epithelium in DEHP 270 μg·kg-1group(P<0.01).There were no significant changes in levels of E2,PRL or T in DEHP 30,90 and 270 μg·kg-1groups,but the ratios of E2/T in DEHP 30 and 270 μg·kg-1groups were increased significantly(P<0.05).CONCLUSlONLow-dose DEHP could promote the proliferation of prostatic hyperplasia in the aged rats,which might be associated with the relative levels of endogenous hormone.
di(2-ethylhexyl)phthalate;environmental endocrine disruptor;benign prostatic hyperplasia;aged rats;estrogen;androgen
The project supported by Talents Developmental Fund of Shanghai City(201372);and Professional Technical Service Platform of Shanghai City(15DZ2290400)
WU Jian-hui,E-mail:wujh_731@163.com,Tel:(021)64438949
R99
A
1000-3002-(2017)06-0642-07
2017-03-30接受日期:2017-06-02)
(本文编辑:赵楠)
上海市人才发展基金项目(201372);上海市科委研发公共服务平台项目(15DZ2290400)
黄冬妍,研究实习员,主要从事生殖药理毒理学研究,E-mail:hdy043@163.com
吴建辉,E-mail:wujh_731@163.com,Tel:(021)64438949

