miRNA-10a对小鼠肝纤维化细胞增殖及TGFβ1/Smads信号转导通路表达的影响
邓国孙,李镇伽
·基础医学· ·论著·
miRNA-10a对小鼠肝纤维化细胞增殖及TGFβ1/Smads信号转导通路表达的影响
邓国孙,李镇伽
目的 通过观察肝纤维化(hepatic fibrosis,HF)小鼠肝组织TGFβ1/Smads信号转导通路的表达与HF进展的关系,探讨微小RNA(microRNA,miRNA)-10a对于HF的作用机制。方法 选用9周龄健康雄性小鼠(C57BL6/J)40只,按照数字表法随机分为对照组和HF模型组(观察组),对照组生理盐水5 μl/g腹腔注射,每周2次,注射8周;观察组10% CCL4橄榄油5 μl/g腹腔注射,每周2次,注射8周,制作小鼠HF模型。RT-PCR法检测HF细胞中miRNA-10a表达后,将观察组HF细胞进行培养及miRNA-10a模拟物转染(转染组),CCK-8法检测HF细胞增殖能力,Western blotting检测HF细胞中TGFβ1、Smad7的表达水平。结果 与对照组比较,观察组miRNA-10a表达水平明显增加(P<0.05);与对照组比较,转染组肝细胞miRNA-10a表达水平明显降低(P<0.05);与对照组相比,低表达miRNA-10a明显降低观察组TGFβ1的表达水平,提高Smad7的表达水平(均P<0.05)。结论 小鼠HF细胞中低表达miRNA-10a,转染miRNA-10a模拟物可明显促进小鼠HF细胞增殖,其作用机制是通过miRNA-10a调控TGFβ1/Smads信号转导通路促进小鼠HF。
肝纤维化;转化生长因子β1;Smad7蛋白;TGFβ1/Smads信号转导通路
肝纤维化(hepatic fibrosis,HF)是机体对各种原因诱发的慢性肝细胞损伤后的一种自我修复反应。有研究[1]显示,HF是一个可逆的病理生理过程,消除相关致病因素或有效的早期干预治疗可改善HF程度,反之则可进展为终末失代偿期肝硬化。微小RNA(microRNA,miRNA)是一类大小为18~22个核苷酸的内源性非编码单链小分子RNA,通过与靶mRNA完全或不完全互补配对,引起mRNA降解或翻译抑制,从而能在转录后水平调控机体基因的表达[2]。有研究[3]显示,一些miRNA在肝细胞内表达相对丰富,并在疾病的进展中表达有明显差异,从而影响肝脏疾病的发生发展。也有研究[4-5]表明,miRNA-10a参与造血细胞的分化、肿瘤的发生发展、免疫功能的调节等多个病理生理过程。在博莱霉素所致的肺纤维化小鼠肺组织中,发现了161个差异表达的miRNA,其中miRNA-10a通过调节TGF-β信号通路参与调控纤维母细胞激活和胶原沉着[6]。有文献[7]报道,肝星状细胞(hepatic stellate cell,HSC)的激活或表型转化为成纤维细胞是HF形成的中心环节,转化生长因子β1(transforming growth factor beta 1,TGFβ1)是公认的最强致HF的细胞因子之一[8],Smad蛋白是TGFβ1关键的作用底物[9],可诱发HSC激活,启动胶原基因表达,从而导致HF的发生。TGFβ1/Smads信号转导通路的激活及引起的相应病理变化, 在HF的发生发展中具有非常重要的意义[10]。本研究探讨miRNA-10a调控HF的分子机制,并将miRNA-10a作为HF药物靶点,从而指导HF的诊断与治疗。
1 材料与方法
1.1 主要材料与试剂 Pronase E购于美国Merck公司,Collagenase II购于美国Sigma公司,淋巴细胞分离液购自中科院生工所,DMEM培养液及干粉购于GIBCO公司,胎牛血清购于杭州四季青公司,Interferin siRNA转染剂购自Polypus公司,miRNA分析系统购自Applied 公司,PrimeScript RT-PCR反转录试剂盒购自TOYOBO公司,miRNA-10购于广东锐博公司,各类单抗均购于美国Abcam公司,细胞裂解液CLB购自Cell Signaling T公司。
1.2 建立HF小鼠模型 选用9周龄健康雄性小鼠(C57BL6/J)40只,体质量19~22 g,按照随机数字表法分为对照组和HF模型组(观察组),对照组生理盐水5 μl/g腹腔注射,每周2次,注射8周;观察组10% CCL4橄榄油5 μl/g腹腔注射,每周2次,注射8周,制作小鼠HF模型。
1.3 制备标本 实验小鼠禁食12 h,在乙醚麻醉下,断头取血,部分肝脏组织采用10%中性甲醛固定,备制病理切片,其余肝组织液氮快速冷冻,置于-80 ℃冰箱保存,备用。
1.4 HSC分离 75%酒精消毒腹部皮肤后,2%戊巴比妥钠(40 mg/kg)小鼠腹腔注射,十字切口开腹,充分暴露小鼠心脏和下腔静脉,18号套管针连接输液瓶,从左心室进针注入灌注液,下腔静脉放血,10 ml/min流速肝脏灌流,直至肝脏变为土黄色,随后采用酶灌注液继续灌流14~18 min,直至肝脏呈现烂泥状,取肝脏,生理盐水冲洗后,剔除肝脏包膜及结缔组织,剪碎后加入振荡消化液,37 ℃振荡20 min,200×g离心5 min,细胞悬液经300目钢网过滤后,收集于2个30 ml离心管中,1 400×g离心5 min,弃上清,D-Hanks液重悬沉淀,取上清进行梯度分离。采用淋巴细胞分离液铺梯度,上层加充分消化的单细胞悬液,25 ℃,1 500×g离心25 min,平抛离心后的分离细胞,小心吸出中层少量白色液体,即为HSC。DMEM制备,1 400 ×g离心10 min,洗涤2次,以1.0×106个细胞接种于100 ml塑料培养瓶中24 h,静置培养72 h后换成含10%胎牛血清DMEM培养液,每3 d更换培养液1次。采用台盼蓝进行染色,计算细胞成活率,以2组小鼠HSC成活率均达到90%以上表示分离成功。
1.5 Real-time PCR检测miRNA-10a表达 采用TRIzol试剂,提取2组肝组织总RNA,利用SYBR Premix ExTaq荧光定量PCR试剂盒及LightCycler仪器,进行操作及分析。miRNA-10a逆转录引物序列:5’-GTCGTATC-CAGCAGGGTCCGTATTCGCACTGGATACGACACA-3’;miRNA-10a定量引物上游序列:5’-ACGTACCTGTAGATCCG-3’,引物下游序列:5’-GTG-CAGGTCCGAGGT-3’。U6逆转录引物序列:5’-CGCTCACGAATTTGCGTGTAT-3’;U6定量引物上游序列:5’-CTCGCTTCGCAGCACA-3’,引物下游序列:5’-AACGCTTCACGAATTTCGT-3’。按3步法进行DNA扩增,反应条件:95 ℃、20 s;94 ℃、25 s;55 ℃、15 s;75 ℃、10 s;40次循环条件:72 ℃、10 min。每次Real-timePCR被测标本的Ct值减去对应样品中U6Ct值,即为ΔCt,HF细胞中miRNA-10a的表达量采用2-ΔΔCt计算,HF组织标本中miRNA-10a表达量采用log22-ΔΔCt计算。
1.6 HF细胞培养和miRNA-10a模拟物转染 小鼠HF细胞置于含10%胎牛血清的DMEM培养液中,37 ℃、5%CO2饱和湿度培养箱进行传代培养。将处于对数期生长的HF细胞接种于12孔板,每孔细胞密度为3×105个,当细胞生长融合度至50%时,再将其分为2组,一组为miRNA-10a模拟物转染组,一组为对照组,分别用含有miRNA-10a模拟物和对照miRNAOpti-MEM培养基转染细胞,同时加入Interferin提高转染率。转染时每孔miRNA-10a模拟物或对照miRNAOpti-MEM的终浓度均为20 nmol/L,Interferin为4 μl,转染72 h。本实验重复3次。
1.6 CCK-8法检测HF细胞的增殖能力 根据CCK-8试剂盒说明书操作,HF细胞转染miRNA-10a模拟物或对照miRNA Opti-MEM 72 h后,将2组细胞根据每孔2×104个,接种于96孔培养板中,并各设置3个平行孔,3~5 h后细胞贴壁,加入100 μl 1640培养液、10 μl CCK-8,置于37 ℃、5%CO2培养箱培养2 h,酶标仪测定光密度(D)值。本试验重复3次。
1.7 Western blotting检测HF细胞中TGFβ1、Smad 7蛋白的表达 HF细胞转染72 h后,收集转染组和对照组待测细胞,细胞裂解液裂解,BCA法测定蛋白浓度,取50 μg蛋白经8% SDS-PAGE分离后,转至PVDF膜,25 ℃封闭1 h,分别加入TGFβ1抗体(1∶2 000)、Smad 7抗体(1∶2 000)、β-actin抗体(1∶1 000),4 ℃孵育过夜,洗膜后加过氧化物酶标记的二抗,25 ℃孵育1h,洗膜后ECL法显影,Gene gnome采集图片,利用Image J软件进行灰度分析。本试验重复3次。
1.8 统计学处理 采用SPSS 18.0统计学软件,数据以均数±标准差(x±s)表示,组间比较采用t检验,P<0.05表示差异有统计学意义。
2 结果
2.1 miRNA-10a在小鼠HF中高表达 Real-time PCR检测观察与对照组中miRNA-10a相对表达量,结果显示,观察组HF组织中miRNA-10a表达量明显高于对照组[(-7.84±1.38)vs(-9.97±1.59),P<0.05]。
2.2 转染miRNA-10a模拟物后HF细胞miRNA-10a的表达 Real-time PCR检测转染组及对照组中miRNA-10a的相对表达量,结果(图1)显示,转染组(miRNA-10a mimics)HF细胞miRNA-10a表达量明显低于对照组(P<0.01)。
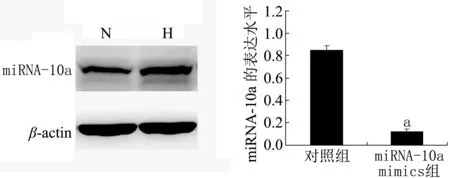
注:与对照组比较aP<0.01图1 Real-time PCR检测2组肝细胞miRNA-10a表达水平
2.3 低表达miRNA-10a促进HF细胞增殖 为了研究低表达miRNA-10a对HF细胞增殖的影响,利用CCK-8法检测转染组及对照组HF细胞的增殖水平,结果(图2)显示,与对照组相比,低表达miRNA-10a的HF细胞增殖水平明显提高(P<0.05)。
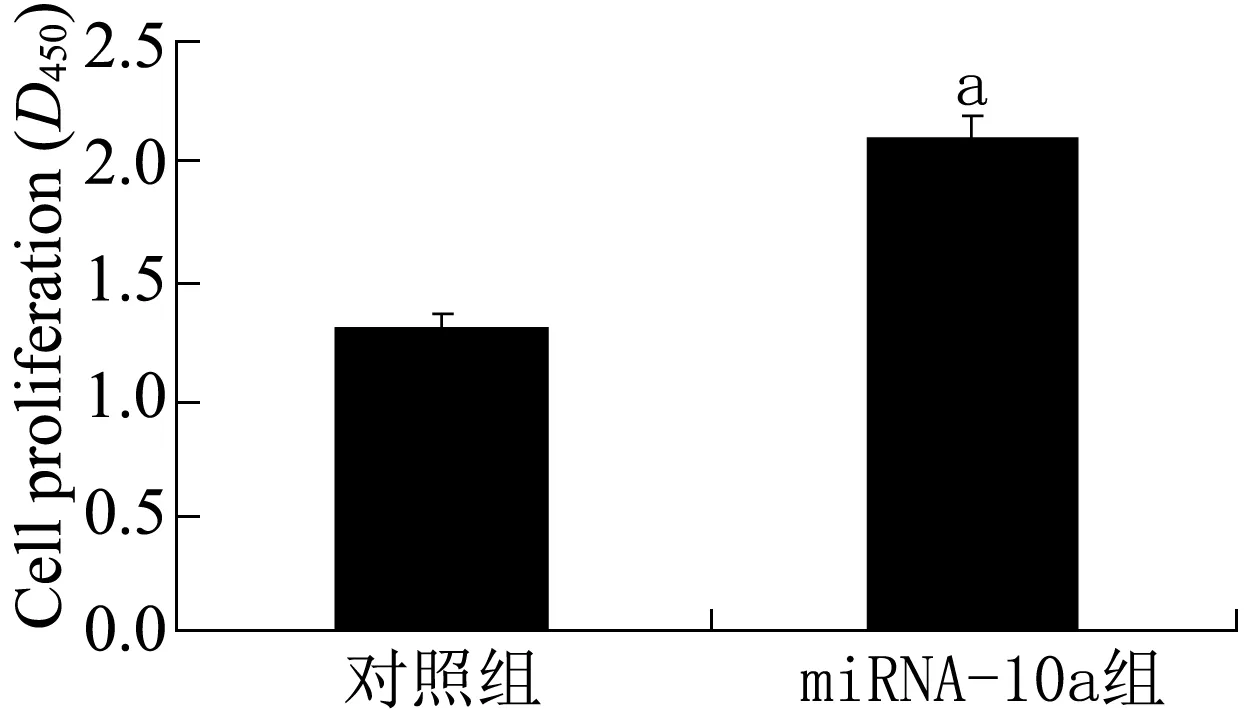
注:与对照组比较aP<0.05图2 CCK-8法检测转染组及对照组HF细胞的增殖情况
2.4 miRNA-10a调控TGFβ1/Smads信号转导通路 Western blotting检测转染miRNA-10a模拟物后HF细胞中TGFβ1、Smad 7蛋白的表达,结果(图3)显示,与对照组比较,转染组TGFβ1蛋白的表达量明显降低(P<0.05),显示miRNA-10a上调HF细胞中TGFβl表达,而转染组Smad 7蛋白的表达量显著增多(P<0.05),显示miRNA-10a下调Smad 7蛋白的表达。
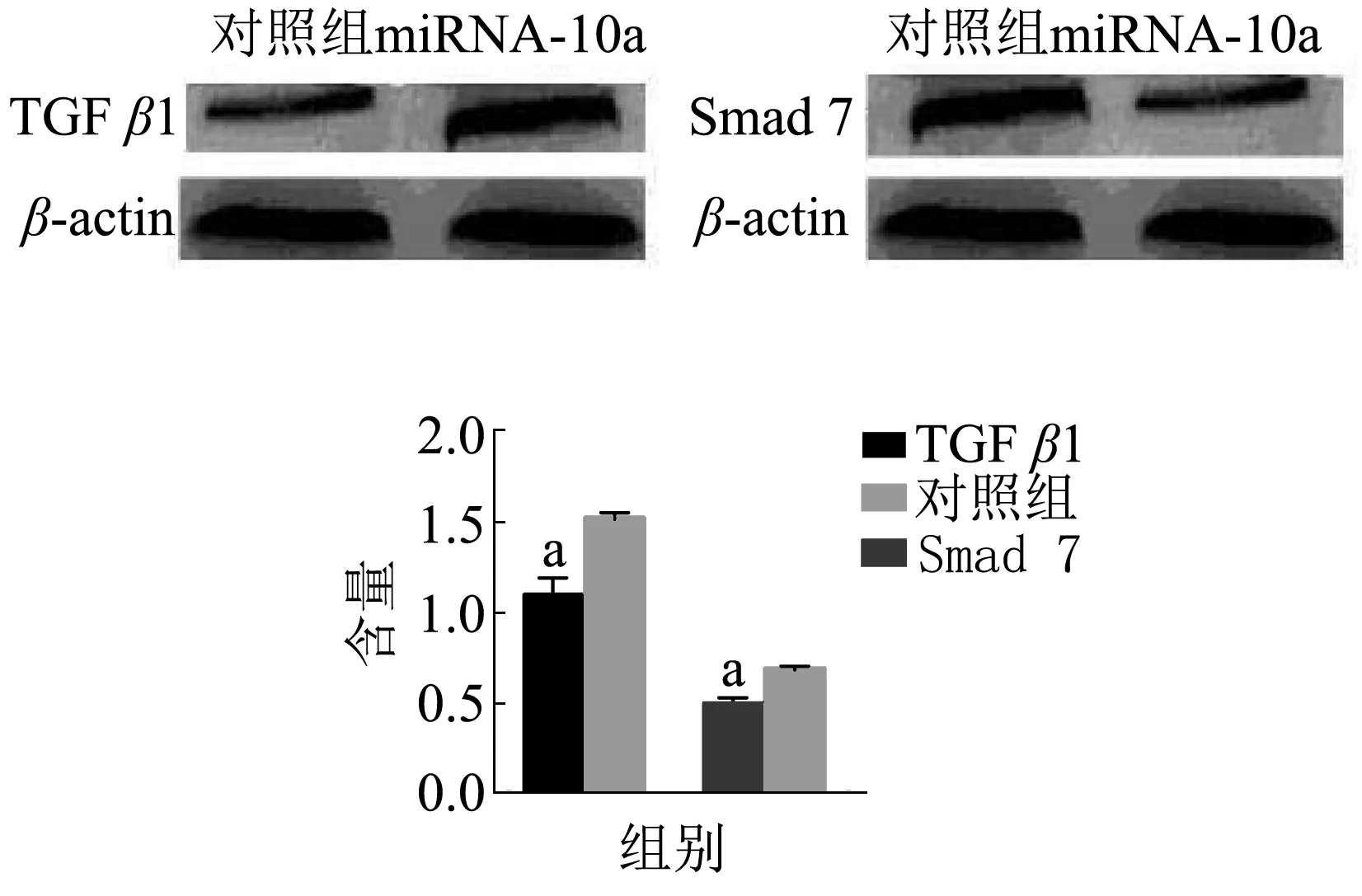
注:与对照组比较aP<0.05图3 Western blotting检测miRNA-10a对TGFβ1/Smads表达的影响
3 讨论
有研究显示[11-12],TGFβ1及其Smad信号转导通路与HF的发生发展密切相关,其中TGFβ1为双硫键结构的二聚体碱性蛋白,是激活HF细胞的主要细胞因子,也是最强的HF促进剂[13];Smad蛋白是目前发现的TGFβ家族受体激酶的关键作用底物之一,根据其结构和功能的不同分为3类,其中抑制型Smad蛋白中的Smad 7主要功能为抑制TGFβ的转导通路。Smad 7可与活化的TGFβ1受体结合,抑制Smad蛋白磷酸化;进入胞浆Smad 7,使Smad蛋白不能与其受体相结合,在TGFβ信号转导中形成负反馈环路,从而发挥抗HF作用[14]。
研究[15-17]显示,miRNA参与HF的发生发展,但关于miRNA调控HF的分子机制还不是很清楚。miRNA-10a作为miRNA家族中成员之一,本研究显示,miRNA-10a在小鼠HF组织中高表达,并且低表达miRNA-10a能够促进HF细胞增殖,进一步表明miRNA-10a在HF中发挥着促纤维化因子的作用。本研究同时发现,与对照组相比,转染组TGFβ1蛋白表达明显降低,表明miRNA-10a上调TGFβ1的表达;而转染组Smad 7蛋白表达显著增多,表明miRNA-10a能够下调Smad 7表达,说明miRNA-10a通过调控TGFβ1/Smads信号转导通路发挥促HF作用,这为HF的治疗提供了潜在的治疗靶点。
[1] Lee YA, Friedman SL. Reversal, maintenance or progression: what happens to the liver after a virologic cure of hepatitis C[J]. Antiviral Res, 2014, 107(1): 23-30. DOI:10.1016/j.antiviral.2014.03.012.
[2] van der Ree MH, de Bruijne J, Kootstra NA, et al. MicroRNAs: role and therapeutic targets in viral hepatitis[J]. Antivir Ther (Lond), 2014, 19(6): 533-541. DOI:10.3851/IMP2766.
[3] Kim KM, Kim SG. Autophagy and microRNA dysregulation in liver diseases[J]. Arch Pharm Res, 2014, 37(9): 1097-1116. DOI:10.1007/s12272-014-0439-9.
[4] Havelange V, Ranganathan P, Geyer S, et al. Implications of the miR-10 family in chemotherapy response of NPM1-mutated AML[J]. Blood, 2014, 123(15): 2412-2415. DOI:10.1182/blood-2013-10-532374.
[5] Ohuchida K, Mizumoto K, Lin C, et al. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene[J]. Ann Surg Oncol, 2012, 19(7): 2394-2402. DOI:10.1245/s10434-012-2252-3.
[6] Xie T, Liang J, Guo R, et al. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation[J]. Physiol Genomics, 2011, 43(9): 479-487. DOI:10.1152/physiolgenomics.00222.2010.
[7] Uemura M, Swenson ES, Gamna MD, et al. Smad 2 and Smad 3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization[J]. Mol Biol Cell, 2005, 16(9): 4214-4224. DOI:10.1091/mbc.E05-02-0149.
[8] Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice[J]. Gut, 2006, 55(3): 415-424. DOI:10.1136/gut.2005.071118.
[9] Heldin CH, Miyazono K, Dijke P. TGF-beta signalling from cell membrane to nucleus through Smad proteins[J]. Nature, 1997, 390(6659): 465-471. DOI:10.1038/37284.
[10] Schnabl B, Kweon YO, Frederick JP, et al. The role of Smad3 in mediating mouse hepatic stellate cell activation[J]. Hepatology, 2001, 34(1): 89-100. DOI:10.1053/jhep.2001.25349.
[11] Martinez FE, Lopez CL, Regordan C, et al. Meningitis by Cryptococcus neoformans in patients with HIV infection[J]. Neurologia, 1999,14(5):218-223.
[12] Paradis V, Dargere D, Bonvoust F, et al. Effects and regulation of connective tissue growth factor on hepatic stellate cells[J]. Lab Invest, 2002, 82(6): 767-774. DOI:10.1097/01.lab.0000017365.18894.d3.
[13] Long J, Wang G, Matsuura I, et al. Activation of Smad transcriptional activity by protein inhibitor of activated STAT3 (PIAS3)[J]. Proc Natl Acad Sci USA, 2004, 101(1): 99-104. DOI:10.1073/pnas.0307598100.
[14] Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf 2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation[J]. Mol Cell, 2000, 6(6): 1365-1375. DOI:10.1016/s1097-2765(00)00134-9.
[15] Roderburg C, Luedde M, Vargas CD, et al. miR-133a mediates TGF dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis[J]. J Hepatol, 2013, 58(4): 736-742. DOI:10.1016/j.jhep.2012.11.022.
[16] Kumar V, Mahato RI. Delivery and targeting of miRNAs for treating liver fibrosis[J]. Pharm Res, 2015, 32(2): 341-361. DOI:10.1007/s11095-014-1497-x.
[17] Chen SL, Zheng MH, Shi KQ, et al. A new strategy for treatment of liver fibrosis: letting microRNA do the job[J]. Bio Drugs, 2013, 27(1): 25-34. DOI:10.1007/s40259-012-0005-2.
(本文编辑:王映红)
Effects of miRNA-10a on the proliferation of hepatic fibrosis cells and the expression of TGF beta 1/Smads signal transduction pathway in mice
DengGuosun,LiZhen′ga
(DepartmentofGastroenterology,ChongmingBranch,XinhuaHospitalAffiliatedtoShanghaiJiaotongUniversity,Shanghai202150,China)
Objective To investigate the possible mechanism involved in the effect of micro RNA-10a(miRNA)on hepatic fibrosis (HF), through the observation on the expression of TGFβ1/Smads signal transduction pathway in mice with HF and its correlation with the development of hepatic fibrosis.Methods Forty healthy male mice with an age of 9 weeks (C57BL6/J) were randomly divided into the control group and the HF model group (or the observation group). The animals in the control group were given normal saline abdominally at a dosage of 5 μl/g, twice a week, for a succession of 8 weeks, while the animals in the observation group received 10% CCL4 olive oil also at a dosage of 5 μl/g with the same frequency and for the same length of time to develop the model of hepatic fibrosis. The expression of miRNA-10a was detected by RT-PCR. HF cells were cultured in the animals of the observation group and miRNA-10a mimics were transfected (the transfection group). CCK-8 method was used to detect the proliferation of HF cells, and the expression levels of TGFβ1 and Smad 7 in the HF cells were detected by Western-blotting. Results As compared with that of the control group, the expression level of miRNA-10a in the observation group was increased significantly (P<0.05); and as compared with that of the control group, the expression level of miRNA-10a in the transfection group were significantly decreased (P<0.05); and furthermore, as compared with that of the control group, the lower expression of miRNA-10a significantly decreased the expression level of TGFβ1 and increased the expression level of Smad 7(P<0.05).Conclusion The expression of miRNA-10a in the HF cells was lower, and the transfected miRNA-10a mimics could significantly promote the proliferation of HF cells, the possible mechanism of which might be associated with the regulation of TGFβ1/Smads signal transduction pathway in HF promotion.
Hepatic fibrosis; Transforming growth factor β1; Smad 7 protein; TGFβ1/Smads signal pathway
202150 上海,上海交通大学附属新华医院崇明分院消化内科(邓国孙);南昌大学医学院第二附属医院普外科(李镇伽)
R575.3
A
10.3969/j.issn.1009-0754.2017.03.013
2016-10-27)

