MALDI-TOF MS磷脂质组学快速分析三文鱼肌肉组织
沈 清,冯俊丽,金仁耀,薛 静,郑振霄,戴志远
(浙江工商大学海洋食品研究院,浙江 杭州 310012)
MALDI-TOF MS磷脂质组学快速分析三文鱼肌肉组织
沈 清,冯俊丽,金仁耀,薛 静,郑振霄,戴志远
(浙江工商大学海洋食品研究院,浙江 杭州 310012)
建立了基质辅助激光解吸电离-飞行时间质谱(MALDI-TOF MS)快速分析三文鱼肌肉组织磷脂质组学的方法。以非自然磷脂酰胆碱DMPC(14∶0/14∶0)和磷脂酰乙醇胺DPPE(15∶0/15∶0)为标准品,分析其在正离子模式下的主要分子峰与干扰峰,并进行方法学验证。实验结果表明,各化合物平均加标回收率在74%~83%之间,相对标准偏差小于7%,日内和日间精密度的相对标准偏差均低于8.5%。将三文鱼磷脂提取物进样分析,共鉴定出28种磷脂分子 ,其中m/z806.40([PC38:6+H]+和[PE38:4+K]+)信号最强。三文鱼中多不饱和磷脂种类丰富,如m/z824.38([PC38:8+Na]+和[PE42:5+H]+),m/z832.41([PC40:7+H]+,[PC38:4+Na]+和[PE40:5+H]+),部分磷脂分子的脂肪酸链达到了满不饱和,如[PC42:11+H]+,其sn-1和sn-2上两条脂肪酸链分别为二十碳五烯酸链(EPA-)和二十二碳六烯酸链(DHA-)。该方法稳定可靠,可用于鉴定三文鱼肌肉组织中的磷脂分子结构,并从脂质组学角度为三文鱼营养评价提供理论依据。
三文鱼;脂质组学;基质辅助激光解吸电离-飞行时间质谱(MALDI-TOF MS);磷脂
三文鱼是生长在挪威、美国和加拿大等高纬度地区的鲑科鱼类,属冷水性洄游鱼类,其肉色一般呈红色或橘色,肉质细嫩、口感鲜美,是鱼类中的珍品。三文鱼富含脂溶性维他命A、维他命D以及水溶性维他命B12和维他命B6,是ω-3不饱和脂肪酸的重要来源,其蛋白质含量高,胆固醇和热量含量低,是营养价值较高的高档水产品之一[1]。三文鱼具有较高的食用价值和保健价值,能有效防治心脑血管、糖尿病等疾病,具有缓解血管炎症和安抚神经的功效[2]。目前,对于三文鱼脂类的研究主要集中在小分子脂肪酸[3],而分子水平磷脂质组学研究则尚未见报道。
脂质组学是继基因组学和蛋白质组学后又一迅速发展的学科,研究内容包括生物体内脂质分子的结构鉴定与定量,以及其在生物代谢、疾病、免疫等过程中所扮演的角色分析。自Han等[4]首次提出脂质组学这一概念以来,脂质组学于近几年迅猛发展,并得到了科学家们的广泛关注。然而,目前关于脂质组学的研究主要集中在生命科学与医学领域,而在食品脂质组学的研究报道较少。Dasila等[5]采用脂质组学分析了食物中EPA和DHA含量比值对代谢综合征的影响;Kim等[6]发现用磷脂标记物可区分人参不同种类、年份和部位;Shen等[7]用壳核材料提取并用亲水色谱从虾中分析得到了大量多不饱和磷脂。
脂质组学的发展得益于近几年分析技术的进步,如高效液相色谱(HPLC)、核磁共振波谱(NMR)、荧光光谱(FLR)、串联质谱(MS/MS)、基质辅助激光解析电离(MALDI)成像技术等。其中质谱已经成为当前脂质组学研究的主流技术手段,主要分析方法是采用三重四极杆质谱(QqQ)、四极杆-飞行时间串联质谱(Q-TOF)、飞行时间-飞行时间串联质谱(TOF-TOF)、静电场轨道阱质谱(Orbitrap)等检测器,配以电喷雾电离(ESI)、大气压化学电离(APCI)、基质辅助激光解吸电离(MALDI)等离子源。不同结构的质谱在检测灵敏度和工作效率方面各有优缺点,如QqQ在多反应监测模式下的定量能力强,但分辨率较低,且检测分子质量上限约为2 000 u;而TOF-TOF分离能力更高,适合分析大分子蛋白,但定量能力稍弱[8-9]。基质辅助激光解吸电离-飞行时间质谱(MALDI-TOF MS)最早用于蛋白质、糖类及核酸等分析,已被证实是一种快速有效的分析手段,每个样品的分析可以控制在几秒钟以内。Shen等[10-12]建立了MALDI-TOF MS快速分析橄榄、杏仁、牛油果的脂质组学方法。
在水产品科学领域内开展脂质组学的研究不仅对水产品的高效综合利用有重要意义,还可以为水产品鉴伪、溯源、污染监测等提供科学依据,但我国脂质组学研究尚属初级阶段。因此,本研究拟采用MALDI-TOF MS法快速分析三文鱼肌肉组织中磷脂酰胆碱和磷脂酰乙醇胺的分子种类,并根据磷脂脂肪酸链长度与不饱和度对三文鱼进行营养评价。
1 实验部分
1.1 主要仪器与装置
AB4800串联飞行时间质谱仪:美国AB Sciex公司产品,配有基质辅助激光解吸离子源、200 Hz三倍频Nd:YAG脉冲355 nm激光及4000 Series Explorer数据处理系统;Milliplus 2150超纯水处理系统:美国Millipore公司产品。
1.2 主要材料与试剂
磷脂酰胆碱(DMPC,14∶0/14∶0)和磷脂酰乙醇胺(DPPE,15∶0/15∶0)标准品:美国Avanti公司产品;甲醇、乙腈及甲酸:色谱纯,美国Sigma-Aldrich公司产品;2,5-二羟基苯甲酸(DHB):质谱纯,美国Sigma-Aldrich公司产品;挪威三文鱼样品:购自杭州物美超市,新鲜度佳。
分别准确称取0.01 g DMPC和DPPE标准品,用甲醇-氯仿溶液(1∶1,V/V)溶解并定容至10 mL棕色容量瓶中,分别配制成1.0 g/L储备液,于4~6 ℃冷藏,备用;称取30 mg DHB,溶解于70%甲醇水溶液中,并定容至1 mL,备用。
1.3 样品处理
取0.1 g均质后的三文鱼肌肉组织样品于5 mL离心管中,加入1.75 mL氯仿-甲醇溶液(1∶2,V/V),振荡混匀。于探头式超声(25 kHz,60%变幅)冰浴中提取10 min,然后向离心管中加入1.25 mL纯水,振荡混匀。混合物用冷冻离心机以8 000 r/min离心15 min,待离心管内溶液上下分层,三文鱼肌肉组织样品则呈饼状居于两相中间。用200 μL移液枪将下相溶液转移到另一新的移液管中,继续向上相和样品中加入2 mL氯仿,重复上述操作2次。合并3次提取的有机相,氮气低温吹干,甲醇水溶液复溶。
分别将100 μL磷脂提取液和100 μL DHB基质加入到1.5 mL离心管中,振荡混匀后静置;将0.5 μL溶液点在MALDI点样板上,于空气中干燥结晶后进行质谱分析。
1.4 质谱条件
正离子反射模式,加速电压20 kV,离子萃取时间延迟450 ns,质量扫描范围m/z450~950,激光能量调整至高于建议阈值的5%~10%。质谱数据采集使用4000 Series Explorer v3.5.2数据处理系统,所有谱图均经同位素校正,以确保微量磷脂的离子峰不受其他高浓度磷脂离子峰干扰。
2 结果与讨论
2.1 磷脂提取条件的优化
脂质的有效提取是影响脂质组学分析结果的重要因素之一。由于磷脂极性官能团的作用导致亲水性较强,实验分别采用Bligh&Dyer,Folch和Nicols方法从三文鱼脂质粗提物中提取磷脂。其中,Bligh&Dyer法的提取效率相对较高,达78%,且实验结果较为稳定;Folch法的提取效率相对较低,为60%,这可能是在提取过程中形成了乳浊液,导致分离过程不可控;Nicol法采用异丙醇作为提取液,尽管提取率达到82%,但是结果的稳定性较差,相对偏差大于10%。因此,本实验选取Bligh&Dyer法提取三文鱼肌肉组织中磷脂。
2.2 磷脂离子化分析
MALDI离子源用于小分子化合物检测时,离子峰成分较为复杂,这给后期数据解析造成一定的困难。因此,分析磷脂在激光诱导下的离子化规律有利于对复杂磷脂进行结构解析。在负离子模式下,磷脂酰胆碱(PC)极易失去[CH3]生成[PC-15]-,该产物易与磷脂酰乙醇胺[PE-H]-发生质谱峰重叠,从而影响后期磷脂结构解析,故实验采用正离子模式。对DMPC和DPPE进行离子化规律分析的结果示于图1。可见,DMPC在正离子模式下的质谱图较为干净,加氢峰[M+H]+信号最强,其次为加钠峰[M+Na]+;DPPE质谱图则相对较为复杂,[M+Na]+信号最强,[M+H]+次之,此外还有[M+K]+、[M+2Na-H]+、[M+Na+K-H]+等加合峰。因较多的加合峰不利于谱图解析,在分析三文鱼样品时,应排除[PC+Na]+和PE多加合峰的假阳性干扰。
2.3 方法学验证
分别取6份预处理后的空白样品,添加高、中、低3个水平的DMPC和DPPE标准溶液,每个加标水平做6次平行实验,计算方法的日内精密度和回收率;连续测定5天,计算日间精密度,结果列于表1。结果表明,各化合物的平均加标回收率在74%~83%之间,相对标准偏差小于7%,日内和日间精密度的相对标准偏差均不超过8.5%。

图1 正离子模式下,磷脂酰胆碱(a)和磷脂酰乙醇胺(b)标准品的质谱图Fig.1 Mass spectra of the standards of DMPC (a) and DPPE (b) in positiveion mode
表1 基质辅助激光解吸质谱磷脂质组学方法的精密度与回收率
Table 1 Precisions and recoveries of MALDI MS based phospholipidomics method

化合物添加浓度/(mg/L)日内精密度均值RSD/%日间精密度RSD/%回收率/%均值RSDDMPC21.84.97.3835.154.65.87.9796.3109.26.18.5746.9DPPE2.52.44.56.9814.954.76.17.7776.8109.36.98.1766.7
2.4 三文鱼磷脂检测
将三文鱼磷脂提取物进样分析,其质谱图示于图2。根据偶氮规则,PC和PE的质荷比为偶数,由此可初步判断磷脂峰主要分布在m/z750~900区域,而m/z500~600区域主要为溶血性磷脂酰胆碱(LPC),如m/z518.20、542.2、580.23分别为[LPC18:3+H]+、[LPC20:5+H]+和[LPC22:0+H]+。溶血性磷脂是磷脂被磷脂酶A水解生成的一种化合物,其含量变化通常与某些代谢疾病相关。磷脂是三文鱼的主要极性脂类,由Lipids_MS_Predictor共鉴定出28种磷脂分子,其中信号最强的是m/z806.40;经MS2检测到其PC特征峰m/z184,碎片峰m/z623.4([M+H-183]+)和m/z627.4([M+H-141]+),其中m/z183为胆碱,m/z141为乙醇胺,故推断离子峰m/z806.40是[PC38:6+H]+和[PE38:4+K]+的重叠。实验还发现,三文鱼中高不饱和磷脂种类丰富,如m/z824.38([PC38:8+Na]+和[PE42:5+H]+)、m/z832.41([PC40:7+H]+,[PC38:4+Na]+和[PE40:5+H]+)等。部分磷脂分子甚至达到了满不饱和度,如[PC42:11+H]+,其sn-1和sn-2上两条脂肪酸链分别为二十碳五烯酸链(EPA-)和二十二碳六烯酸链(DHA-)。
影响化合物离子化效率的主要因素是基团的偶极矩,磷脂的偶极矩主要存在于极性头,而两条脂肪酸链几乎不含偶极矩,同一类磷脂的离子化强度只受其浓度影响,而碳链长度与不饱和度的影响几乎可以忽略。因此,采用归一化法以DMPC对所有PC磷脂分子和以DPPE对所有PE磷脂分子进行定量分析,结果列于表2。可见,m/z806.40([PC38:6+H]+和[PE38:4+K]+)的总量占20%以上,m/z826.41、852.38、856.40和878.39双链均为EPA链或DHA链的磷脂占12.38%。实验结果表明,三文鱼磷脂种类丰富,多不饱和磷脂含量较高。
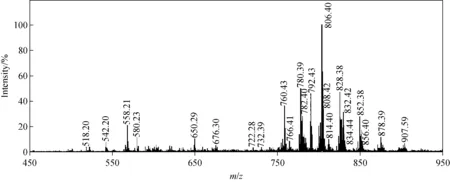
图2 正离子模式下,三文鱼肌肉组织脂质提取物的质谱图Fig.2 Mass spectrum of the phospholipids extracted from salmon muscle in positive ion mode
表2 三文鱼中主要磷脂及其丰度
Table 2 Molecular species and contents of phospholipids in salmon
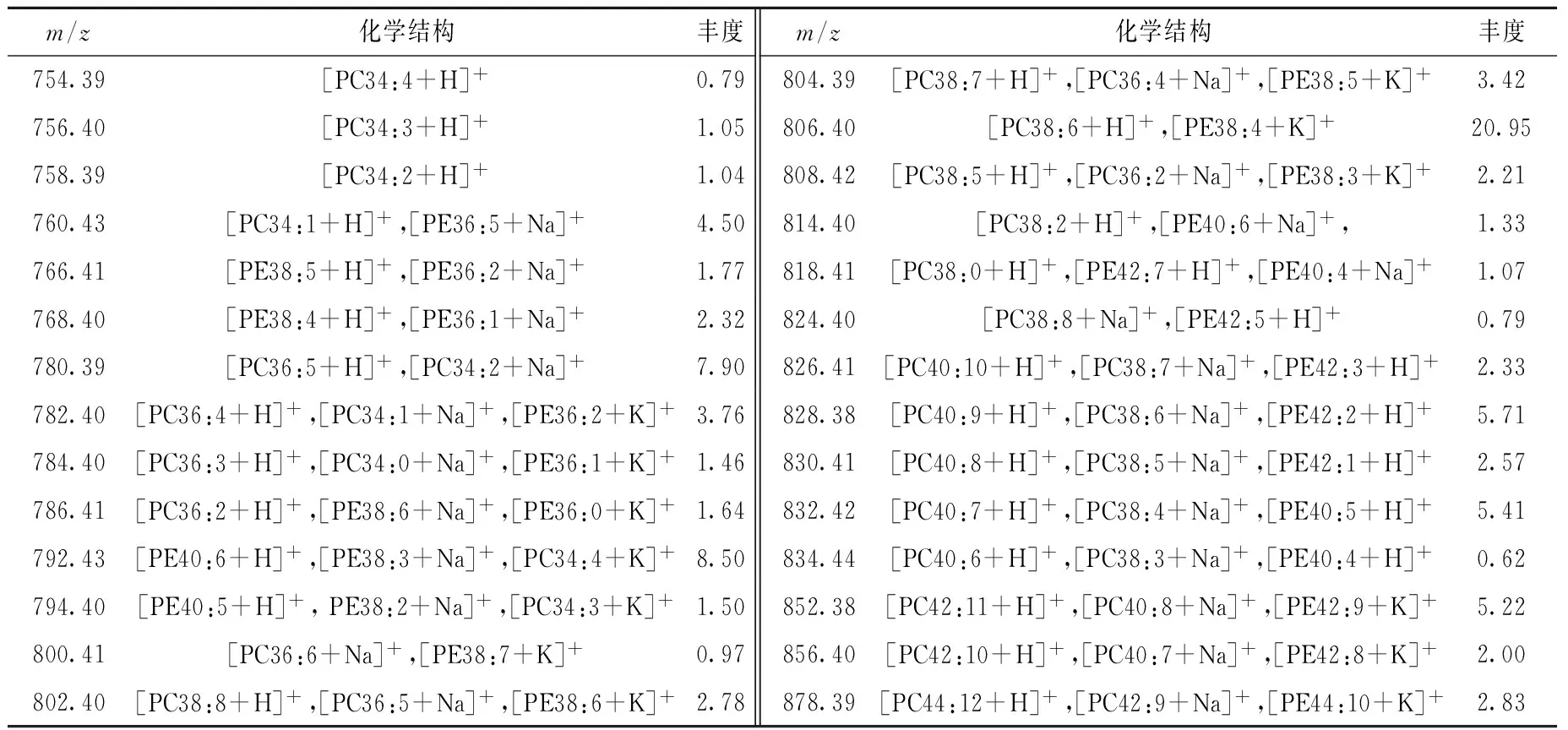
m/z化学结构丰度m/z化学结构丰度754.39[PC34:4+H]+0.79804.39[PC38:7+H]+,[PC36:4+Na]+,[PE38:5+K]+3.42756.40[PC34:3+H]+1.05806.40[PC38:6+H]+,[PE38:4+K]+20.95758.39[PC34:2+H]+1.04808.42[PC38:5+H]+,[PC36:2+Na]+,[PE38:3+K]+2.21760.43[PC34:1+H]+,[PE36:5+Na]+4.50814.40[PC38:2+H]+,[PE40:6+Na]+,1.33766.41[PE38:5+H]+,[PE36:2+Na]+1.77818.41[PC38:0+H]+,[PE42:7+H]+,[PE40:4+Na]+1.07768.40[PE38:4+H]+,[PE36:1+Na]+2.32824.40[PC38:8+Na]+,[PE42:5+H]+0.79780.39[PC36:5+H]+,[PC34:2+Na]+7.90826.41[PC40:10+H]+,[PC38:7+Na]+,[PE42:3+H]+2.33782.40[PC36:4+H]+,[PC34:1+Na]+,[PE36:2+K]+3.76828.38[PC40:9+H]+,[PC38:6+Na]+,[PE42:2+H]+5.71784.40[PC36:3+H]+,[PC34:0+Na]+,[PE36:1+K]+1.46830.41[PC40:8+H]+,[PC38:5+Na]+,[PE42:1+H]+2.57786.41[PC36:2+H]+,[PE38:6+Na]+,[PE36:0+K]+1.64832.42[PC40:7+H]+,[PC38:4+Na]+,[PE40:5+H]+5.41792.43[PE40:6+H]+,[PE38:3+Na]+,[PC34:4+K]+8.50834.44[PC40:6+H]+,[PC38:3+Na]+,[PE40:4+H]+0.62794.40[PE40:5+H]+,PE38:2+Na]+,[PC34:3+K]+1.50852.38[PC42:11+H]+,[PC40:8+Na]+,[PE42:9+K]+5.22800.41[PC36:6+Na]+,[PE38:7+K]+0.97856.40[PC42:10+H]+,[PC40:7+Na]+,[PE42:8+K]+2.00802.40[PC38:8+H]+,[PC36:5+Na]+,[PE38:6+K]+2.78878.39[PC44:12+H]+,[PC42:9+Na]+,[PE44:10+K]+2.83
3 结论
本研究建立了MALDI-TOF MS分析三文鱼肌肉组织磷脂质组学的方法。结果表明,各化合物的平均加标回收率在74%~83%之间,相对标准偏差小于7%,日内和日间精密度的相对标准偏差均不超过8.5%,该方法稳定可靠,可快速分析三文鱼中磷脂组成轮廓。实验鉴定了脂质提取物中28种磷脂分子,结果显示,三文鱼中多不饱和磷脂的种类丰富,含量较高,可作为人体摄取多不饱和脂质的重要来源。
[1] 邓林,李华,江建军. 挪威三文鱼的营养评价[J]. 食品工业科技,2012,33(8):377-379.
DENG Lin, LI Hua, JIANG Jianjun. Nutrition evaluation of Norway salmon[J]. Science and Technology of Food Industry, 2012, 33(8): 377-379(in Chinese).
[2] 刘延岭,邓林. 养殖三文鱼与挪威三文鱼营养成分的比较分析[J]. 食品与发酵科技,2011,47(6):84-86.
LIU Yanling, DENG Lin. Comparision of the nutritional components in muscles of Norway salmon and artificial breeding salmon[J]. Food and Fermentation Technology, 2011, 47(6): 84-86(in Chinese).
[3] 江建军,邓林,李华. 人工养殖三文鱼营养成分的分析[J]. 食品与机械,2011,(6):40-46.
JIANG Jianjun, DENG Lin, LI Hua. Nutrition evaluation of artificial breeding salmon[J]. Food & Machinery, 2011, (6): 40-46(in Chinese).
[4] HAN X, GROSS R W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry a bridge to lipidomics[J]. Journal of Lipid Research, 2003, 44(6): 1 071-1 079.
[5] DASILVA G, PAZOS M, GARCA-EGIDO E, et al. Lipidomics to analyze the influence of diets with different EPA:DHA ratios in the progression of metabolic syndrome using SHROB rats as a model[J]. Food Chemistry, 2016, 205: 196-203.
[6] KIM S H, SHIN Y S, CHOI H K. Nano ESI-MS-based lipidomics to discriminate between cultivars, cultivation ages, and parts of Panax ginseng[J]. Analytical & Bioanalytical Chemistry, 2016, 408(8): 2 109-2 121.
[7] SHEN Q, CHENG H Y. TiO2/SiO2core-shell composite-based sample preparation method for selective extraction of phospholipids from shrimp waste followed by hydrophilic interaction chromatography coupled with quadrupole time-of-flight/mass spectrometry analysis[J]. Journal of Agricultural & Food Chemistry, 2014, 62(36): 8 944-8 951.
[8] KHANDELWAL P, STRYKER S, CHAO H, et al. 1H NMR-based lipidomics of rodent fur: species-specific lipid profiles and SCD1 inhibitor-related dermal toxicity[J]. Journal of Lipid Research, 2014, 55(7): 1 366-1 374.
[9] HEBBAR S, SCHULZ W, SAUER U, et al. Laser capture microdissection coupled with on-column extraction LC-MS(n) enables lipidomics of fluorescently labeled Drosophila neurons[J]. Analytical Chemistry, 2014, 86(11): 5 345-5 352.
[10]SHEN Q, DONG W, YANG M, et al. Lipidomic study of olive fruit and oil using TiO2, nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS[J]. Food Research International, 2013, 54(2): 2 054-2 061.
[11]SHEN Q, DONG W, YANG M, et al. Lipidomic fingerprint of almonds (PrunusdulcisL. cvNonpareil) using TiO2nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS and its potential in geographical origin verification[J]. Journal of Agricultural & Food Chemistry, 2013, 61(32): 7 739-7 748.
[12]SHEN Q, MEI Y, LI L, et al. Graphene/TiO2, nanocomposite based solid-phase extraction and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for lipidomic profiling of avocado (PerseaamericanaMill.)[J]. Analytica Chimica Acta, 2014, 852: 153-161.
Phospholipidomics Profiling of Salmon Muscle by MALDI-TOF MS
SHEN Qing, FENG Jun-li, JIN Ren-yao, XUE Jing, ZHENG Zhen-xiao, DAI Zhi-yuan
(InstituteofSeafood,ZhejiangGongshangUniversity,Hangzhou310012,China)
Phospholipids are considered as nutrients with putative health benefits, which play critical roles in lipid digestion, transport, inflammatory processes and signaling pathways. As to the nutritive value for human body, salmon contains abundant components of polyunsaturated fatty acyl phospholipids, and it has the beneficial function of lowering the blood fat and cholesterol, reducing the risk of cardiovascular disease. However, lipidomics study of salmon is still missing due to the lack of efficient specific analytical method. In this study, a fast lipidomics method of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) was developed for the analysis of phospholipids from salmon muscle tissue. In brief, 0.1 g salmon sample was accurately weighted, placed in 5 mL polytetrafluoroethylene tube, and mixed with 1.75 mL of chloroform/methanol (2∶1,V/V) solution. After ultrasonic assisted extracting for 10 min, a potion of 1.25 mL water was added and the tube was centrifuged at 8 000 g for 15 min in order to separate the solvent phase. Then, the lower organic phase was recovered and transferred to a new glass tube by pipette. The aqueous phase was re-extracted with 2 mL chloroform for another two times and treated as described before. The collected organic phase were combined and evaporated under nitrogen flow. The sample spots under investigation was directly analyzed using MALDI-TOF MS in positive ion reflection mode at an accelerating potential of 20 kV, with a delayed ion extraction time of 450 ns according to the mass range under observation (m/z450-1 000) allowing for baseline isotopic mass resolution. The unnaturally occurred DMPC (14∶0/14∶0) and DPPE (15∶0/15∶0) were selected as standards for studying their ions in positive ion mode. The method was validated in terms of precision and recovery, and it was found that the recoveries of DMPC and DPPE were in the range of 74%-83%, and relative standard derivation (RSD) was lower than 7%. The RSDs of intra-day and inter-day precision were both lower than 8.5%. After analyzing the lipid extract of salmon, a total of 28 phospholipid molecular species was identified, among which the peak atm/z806.40 ([PC38:6+H]+and [PE38:4+K]+) showed the highest abundance. There is a variety of polyunsaturated phospholipids in salmon, for examples, the peak atm/z824.38 ([PC38:8+Na]+and [PE42:5+H]+),m/z832.41 ([PC40:7+H]+, [PC38:4+Na]+and [PE40:5+H]+). Part of the phospholipids were fully unsaturated, for example, the [PC42:11+H]+was composed with eicosapentaenoic acyl chain (EPA-) and docosahexaenoic acyl chain (DHA-) at sn-1 and sn-2 position, respectively. The method is precise and robust, which can provide theoretical support for the nutritional study of salmon in view of lipidomics.
salmon; lipidomics; matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS); phospholipids
2016-04-13;
2016-07-22
国家自然科学青年基金(31601542);国家国际科技合作专项(2014DFA32880);浙江省自然科学基金(LQ16C200001);浙江工商大学高等教育研究课题(xgy16075)资助
沈 清(1986—),男(汉族),浙江杭州人,博士研究生,从事水产品加工与贮藏研究。E-mail: leonqshen@163.com
戴志远(1958—),男(汉族),浙江杭州人,研究员,从事水产品加工与贮藏研究。E-mail: dzy@zjgsu.edu.cn
时间:2016-12-28;
http:∥www.cnki.net/kcms/detail/11.2979.TH.20161228.0937.026.html
O657.63
A
1004-2997(2017)02-0211-06
10.7538/zpxb.youxian.2016.0071

