金属-空气电池阴极双功能催化剂研究进展
王 亚,来庆学,朱军杰,梁彦瑜
(南京航空航天大学 材料科学与技术学院,江苏省能量转换材料与技术重点实验室,江苏 南京 211106)
金属-空气电池阴极双功能催化剂研究进展
王 亚,来庆学,朱军杰,梁彦瑜*
(南京航空航天大学 材料科学与技术学院,江苏省能量转换材料与技术重点实验室,江苏 南京 211106)
可充电金属-空气电池因具有超高的能量密度被认为是最有发展前景的能源存储与转换装置之一.阴极电化学氧还原/生成反应缓慢动力学是影响金属-空气电池性能的关键因素,因此其充放电过程需要双功能催化剂进行催化.在此我们详细论述了近年来开发的新型双功能催化剂,包括贵金属、碳材料、过渡金属氧化物和复合材料.其中,强耦合过渡金属氧化物/纳米碳复合物成为新一代具有高催化活性的氧催化材料.最后,基于目前所存在的问题提出了几个未来可能的研究方向.
金属-空气电池;空气阴极;双功能催化剂;氧还原/生成反应
能源和环境是现代社会面临的两大问题[1-3],可再生能源转换是显著降低化石燃料依赖的有效解决方案.目前,各国致力于提高各式电化学能源存储与转换装置的容量和稳定性,可充电锂离子电池由于循环寿命长(>5 000圈)和能量转换效率高(>90%)等优点被认为是最有前途的存储技术,但是能量密度低(理论值约400 Wh·kg-1)限制其长期应用[4-5],因此,开发高能量密度的存储技术迫在眉睫.金属-空气电池由于能量密度高、成本低和环境友好受到广泛关注.金属-空气电池(图1)具有开放的电池结构[6],能持续供应阴极活性材料(氧气),因此具备超高的理论比能量密度(11 700 Wh·kg-1),是锂离子电池的几十倍,甚至能与汽油能源系统(13 000 Wh·kg-1)媲美[7-8],金属-空气电池是最有发展前景的能源存储装置之一.
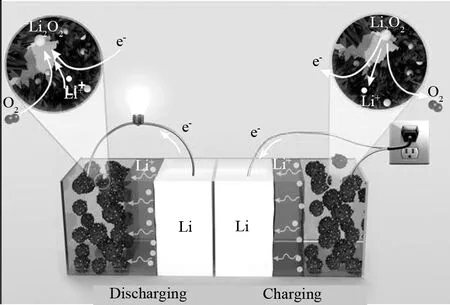
图1 可充电金属-空气电池示意图[6]Fig.1 Schematic illustrations of a typical regenerative metal-air battery[6]
尽管金属-空气电池的研究已取得较大进展,仍面临很多挑战,主要包括阳极的利用率低和阴极的动力学过程缓慢、过电势高和可逆性差,导致实际能量密度低[9],而且阴极反应机理仍不是很明晰.毫无疑问,空气电极是目前影响金属-空气电池性能的关键因素.图2所示是典型的金属-空气电池充放电循环示意图[10],氧还原反应(ORR)和氧生成反应(OER)的过电势严重降低了金属-空气电池的输出功率和循环效率.因此,想要发挥金属-空气电池的全部潜能,首先要阐明基础的氧化学过程,其次发展高性能、低成本的双效氧电极降低ORR和OER过电势.为此,研究者们致力于探究阴极反应机理,发展高效的双功能催化剂材料和设计合理的电极结构.
本文主要介绍金属-空气电池的原理以及近年来取得的重大进展,重点论述双功能催化剂材料的种类和发展.内容如下:1)阴极电化学反应的基本原理,2)双功能催化剂的种类和发展,3)未来的挑战和发展.本文的目的是对极具发展前景的金属-空气电池进行深入的理解.

图2 金属-空气电池充放电循环示意图[10]Fig.2 Discharge-charge loop for a metal-air battery[10]
1 电池构造和工作原理
金属-空气电池由金属阳极、空气阴极和电解液组成.不同种类的金属-空气电池涉及不同的电化学反应和产物,取决于选择的金属、电解质和催化材料[11-12].本节内容中,金属-空气电池基础的氧电化学反应机理分为两类:水系电解液体系和非水系电解液体系.
1.1 水系电解液体系
在酸性电解液中,金属(例如Li、Mg、Zn)会发生剧烈反应,产生氢气,同时释放大量的热,导致严重的阳极腐蚀和复杂的热管理.而且,有些电催化材料在强酸环境下不稳定,因此,金属-空气电池通常使用碱性电解液.在碱性电解液中,放电反应如下:



氧的电化学过程相当复杂,涉及一系列多步电子转移的电化学反应[13-16].目前普遍认为,金属表面进行的ORR一般有两种途径:四电子转移和二电子转移.当ORR按二电子转移途径反应时,产物中除了有氢氧根离子外,还产生大量过氧化物,降低ORR效率,而且过氧化物具有强氧化作用,损坏电池隔膜而影响循环寿命,因此对于水系电解液而言,四电子转移是实现高效ORR的主要途径.此外, ORR路径和机制会随使用的催化材料发生改变.金属-空气电池充电时空气电极发生的OER是ORR的逆过程,反应机制也取决于电极材料.典型的OER催化剂二氧化钌(RuO2)和二氧化铱(IrO2)具有相当高的活性,低的氧化电势(约1.39和1.35 Vvs.RHE)[17]和高的导电性.一些过渡金属氧化物也是高效的OER催化剂,尤其是尖晶石型氧化物(例如NiCo2O4),具体电化学活性将在第2部分讨论.
1.2 非水系电解液体系
水系电解液体系具有廉价、来源广泛和高离子电导率等优点.但是,在水介质中发生的电化学过程受析氢和析氧的限制具有较窄的电位区间,导致金属-空气电池实际电压远低于高的理论电压.而且金属和水之间的反应很危险[18-22].因此,非水系电解液金属-空气电池成为研究热点.发生反应如下(以锂-空为例):

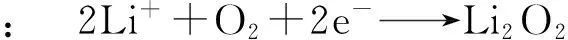

和水介质中发生的ORR一样,非质子电解液里发生的阴极反应也涉及多步反应.根据电化学分析,LAOIRE等提出以下可能的阴极反应[23-24]:
除了上述机理外,催化剂表面性质也对阴极反应路径产生很大的影响.通过比较有机电解液中玻碳电极、多晶Au和Pt电极的循环伏安曲线,LU等[25]表明还原机理和放电产物可能与催化剂有关,认为氧分子首先接受一个电子形成超氧自由基,该自由基与Li+结合生成表面吸附的LiO2.当催化剂表面的吸附氧键能较弱时(例如碳),LiO2能快速得到电子还原为Li2O2;而当吸附氧键能较强时(例如Pt),电子转移受到阻碍,LiO2更易还原为Li2O.因此,催化剂的选择对反应历程起着非常重要的作用.催化剂依赖的ORR活性[26-28]和路径表明复合催化材料电极机制的研究可能比较复杂,催化剂的筛选应予以更多关注.
金属-空气电池的氧电化学是极其复杂的过程,ORR和OER反应受电解液、电极材料等影响.深入了解反应机理、机理/活性与催化剂间的关系是理解金属-空气电池充放电起源的关键,对发展高活性空气电极也具有重要的指导意义.
2 双功能催化剂
众所周知,双功能催化剂可以降低充放电反应的过电势,从而提高循环性能.双功能催化剂通过两种方式催化ORR和OER,一种是组合多功能组分,通过协同作用降低过电势;另一种是对ORR和OER起到双催化作用的单组分催化剂[29].近年来,发展双功能催化剂已取得重大成就,大致分为以下4种:1)贵金属及其合金,2)碳材料,3)过渡金属氧化物,4)复合材料.
2.1 贵金属及其合金
贵金属催化剂Pt、Au、Ag、Pd等应用的较多.贵金属的d电子轨道通常都未填满,表面易吸附反应物且强度适中,因而具有很高的催化活性,同时还具有抗氧化、耐腐蚀和耐高温等优良特性,被认为是最好的化学反应催化剂.但贵金属成本高且资源短缺,阻碍金属-空气电池产业化,这就要求在降低贵金属用量的同时保证高活性和稳定性,因此国内外在贵金属合金方面进行了大量研究.
YANG课题组系统研究贵金属在非水系锂-空电池的应用,发现Au/C能有效催化ORR而Pt/C能有效催化OER(图3).有趣的是,PtAu/C展现出优异的双功能活性,以及在可充电锂-氧电池中具有高的循环性能[30].KO等[31]合成各种碳支撑的金属及其合金(Pt、Pd、Ir、Ru、Pt-Pd、Pd-Ir和Pt-Ru),并将其作为锂-空电池阴极催化剂.Pt-Ru、Pt-Pd和Pd-Ir电流密度为0.2 mA·cm-2时的初始放电容量分别是346、153和135 mAh·g-1,因此,他们研究表明不同金属合金催化剂产生与纯金属催化剂不同的行为特征.为了降低贵金属成本,还可以用便宜的过渡金属修饰Pt.例如:KIM等[32]合成碳支撑的Pt3Co合金纳米材料,电流密度为100 mA·g-1时,Pt3Co/KB、Pt/KB和 KB的过电势分别为135、635和1 085 mV.作者认为提升的性能与Pt催化位点最外层LiO2减小的吸附能有关,同时,合金催化剂趋于产生无定型Li2O2,在充电过程中更易分解.LEE等[33]基于密度泛函理论研究Pt-Cu合金催化剂的电化学性能,与Pt (111)和Pt3Cu (111)相比,PtCu (111)显著降低锂氧电池ORR/OER过电势,由于PtCu (111)表面带较多的负电荷,与Li-O中间体的结合力较弱.这是首次表明充电过电势受合金催化剂表面电荷特征影响,为设计高效的锂氧电池催化剂提供新思路.
尽管贵金属及其合金在电化学活性和稳定性方面都是最高效的催化剂,成本高和资源稀缺阻碍其商业化.因此,需要探索和研究具有低成本、高活性和稳定性的电催化材料.为此提出两种不同的方案:通过增加贵金属的利用率减少催化剂载量;发展非贵金属电催化剂.与贵金属催化剂相比,非贵金属催化剂由于高丰度和低成本广受关注.

图3 (a) C和PtAu/C在0.04 mA·cm-2下第三圈充放电曲线,(b) C (电流密度85 mA·g-1) 和Au/C、Pt/C和PtAu/C (电流密度100 mA·g-1) 首次充放电曲线[30]Fig.3 (a) Charge/discharge profiles of carbon and PtAu/C in the third cycle at 0.04 mA·cm-2,(b) First charge/discharge profiles of carbon at 85 mA·g-1 and of Au/C,Pt/C,and PtAu/C at 100 mA·g-1 [30]
2.2 碳材料
碳材料具有高导电性和大比表面积,已广泛应用在金属-空气电池,通常作为电极材料构造有孔的电极,也可以作为催化ORR/OER双功能催化剂[34].与ORR不同,碳表面OER具体的反应机理尚不明确,而且碳材料在OER高压下电化学性能不稳定,但碳基催化剂的发展持续顺利推进.碳材料可以划分为以下3类:商业碳材料、功能化碳材料和掺杂的碳,下面将给予详细的讨论.
2.2.1 商业碳材料
商业碳材料(例如导电炭黑、科琴黑KB、Vulcan XC-72和BP 2000等)已被研究为非水系金属-空气电池的阴极材料[35-37].MEINI等[38]报道了碳材料的表面积与放电容量有着密切联系.例如:碳材料Vulcan XC72 和 BP 2000的表面积依次为240 和1 509 m2·g-1,对应的放电容量分别是183和 517 mAh·g-1.
商业碳材料作为金属-空气电池的阴极具有可行性,但在投入实际应用前也面临许多问题,例如:低的放电电压、高的充电电压、差的倍率性能和循环性能[39-41].因此,商业碳材料通常作为非水系金属-空气电池阴极导电剂或者催化剂载体,而不是反应位点[42-44].
2.2.2 功能化碳材料
不同于商业碳材料高OER电位下发生碳腐蚀导致催化活性衰减,功能化碳材料在非水系金属-空气电池阴极反应中展现出优异的性能,由于其独特的结构和大量的缺陷/空位.功能化碳材料包括一维(1D)纳米管、2D石墨和石墨烯、3D纳米多孔结构碳.
碳纳米管(CNTs)包括单壁碳纳米管[45]和多壁碳纳米管[46],已被研究为非水系金属-空气电池阴极材料,具有高化学和热稳定性、高强度和高导电性等优点.TIAN等[47]报道CNT@NCNT作为高效的无金属纳米碳电催化剂,碳纳米管由于高比表面作为全面暴露表面活性位的平台,褶皱的掺氮碳层外延生长在圆柱形CNT外表面,此独特的结构使其表面聚集活性位,降低ORR/OER间过电势,成为有前景的双功能电催化剂.
石墨烯是由碳原子构成的单层二维蜂窝状晶格结构的一种新型碳材料,具有大表面积(理论上单层是2 630 m2·g-1)、高导电性以及热和化学稳定性等优点[48-50],近年来石墨烯由于放电容量高和循环效率高已成为有前景的金属-空气电池阴极材料.YOO和ZHOU[51]以无金属石墨烯纳米片(GNS)作为混合可充电锂-氧电池的催化剂,其充放电间过电势只有0.56 V,表明GNSs能有效降低ORR和OER过电势,此高性能来源于边缘和表面缺陷位引起的sp3杂化,这些边缘和表面缺陷利于空气中的氧气分解为氧原子,然后迁移到GNS表面,与H2O分子结合形成OH-.
对于非水系锂-空电池而言,不溶性放电产物Li2O2堆积在空气电极活性位处会堵塞孔,从而降低电池性能.因此,研究者们致力于优化非水系锂-空电池空气电极的微观结构[52].SAKAUSHI等[53]用SiO2模板法制备介孔掺氮的碳,具有高的双功能ORR/OER活性和稳定性,在锂-空电池中展现出极低的充电过电势(0.45 V),是目前锂-空电池非贵金属催化剂中过电势最低值.其实,电极结构的设计对于提高能量转换过程尤为重要[54-56].多孔碳材料通常用粘合剂紧密聚集在电极上,导致低的O2扩散率和有限的Li2O2沉积空间,最终引发碳材料低的利用率以及锂-氧电池低的容量和倍率性能.为此,ZHANG课题组[56]原位溶胶凝胶法构造自支撑分级多孔的碳(FHPC),最大化多孔碳材料的利用率和反应物的传输.图4a展示最初泡沫镍的大孔骨架,原位合成后碳薄片垂直于骨架表面(图4b),形成相互连接的通道贯穿整个电极,高倍放大图观察到碳薄片由无数小的纳米孔组成(图4c和4d).FHPC作为锂-氧电池阴极展现出高的比容量和优异的倍率性能.电流密度为0.2 mA·cm-2时,容量高达11 060 mAh·g-1,是商业KB碳的两倍;甚至当电流密度为2 mA·cm-2时,容量达到2 020 mAh·g-1(图4e).FHPC如此卓越的性能来源于自支撑结构疏松填充的碳,为Li2O2沉积提供充足的空隙容积且增加碳的有效利用.同时,分级多孔结构加速O2扩散、电解液的浸润和反应物的物质传输.类似的,ZHU等[57]用酚醛树脂作为碳源,通过简单碳化法在泡沫镍上直接生长碳纳米管(CNT/NF),具有高的倍率性能和杰出的循环稳定性.

图4 SEM图:(a) 初始泡沫镍,(b-d) FHPC电极,(e) FHPC电极电流密度从0.2 mA·cm-2到2 mA·cm-2的放电曲线[56]Fig.4 SEM images of (a) the pristine nickel foam,(b-d) the FHPC electrode,(e) Discharge curves of FHPC electrode at different current densities ranging from 0.2 mA·cm-2 to 2 mA·cm-2 [56]
2.2.3 掺杂碳
碳材料掺杂定量的非金属元素(例如N、B、S、P)可以提升电化学性能,因为异原子掺杂会改变碳材料的化学和电子性质[58-60],形成缺陷和官能团[61].
碳纳米管具有高比表面积,能充分地暴露活性位点,且嵌入氮会引入活性位点,参与氧分子O-O键的断裂.YADAV等[62]通过改变前驱体和生长条件制备不同直径的竹状碳氮纳米管(CNNTs),发现ORR/OER性能与纳米管直径和氮官能团都密切相关,掺氮降低CNNTs氧吸附能且增强导电性.因此,需协调纳米管直径和氮官能团两种因素以获得高效的双功能催化剂.LI等[63]通过氨气热处理化妆棉合成3D弯曲多孔的掺氮碳微管海绵(NCMT),由中空多孔的石墨化碳微管相互交错而成,如此独特的结构提供高密度活性位和快速电子转移能力,因此NCMT是迄今为止最杰出的ORR/OER催化剂,在柔性能源存储与转换领域有巨大的应用前景.
异原子掺杂能有效提升石墨烯作为双功能催化剂的电化学稳定性.LI等[61]首次介绍掺氮的石墨烯纳米片(N-GNSs)应用于锂-空电池,电流密度为75、150 和300 mA·g-1时的放电容量分别为11 660、6 640 和 3 960 mAh·g-1,远超越纯GNSs电极.掺杂其他元素(例如B、P和S)也可以增强电催化活性.VINEESH等[64]用B4C作为前驱体制备高产量的掺硼石墨烯(B-G),展现出优异的双功能催化活性.B4C结构转化为石墨烯的过程还伴随着原位掺杂B,形成来源于非电催化活性材料的电催化活性材料,扩展在各种能源相关技术的应用前景.近来,共掺杂已成为提高碳活性的研究方案.QU等[65]简单热解氧化石墨烯、聚多巴胺和2-巯基乙醇混合物制备N、S共掺杂介孔碳纳米片,展现出优异的双功能活性.DAI等[66]在植酸存在下热解聚苯胺气凝胶大规模生产三维氮磷共掺介孔碳泡沫(NPMC),展现出优异的ORR和OER电催化性能.ORR和OER过电势分别为0.44 V和0.39 V,都低于最优的催化剂(图5a,5b),而且在可充电锌-空电池中展现出卓越的稳定性(循环600圈,图5d).通过密度泛函理论表明N、P共掺杂和石墨烯边缘缺陷对双功能电催化活性至关重要.XIA等[67]发现一个特性描述符可以准确地描述共掺杂碳纳米材料的ORR/OER性能,认为共掺杂碳基催化剂性能的提升来源于双掺杂物间的相互作用.当两个异原子掺杂在石墨结构里且彼此接近时,p电子云发生重叠且相互作用,在相邻碳原子上产生比单元素掺杂更多的活性位,从而降低ORR/OER过电势,表明共掺杂是发展高活性无金属碳基双功能催化剂的有效途径.
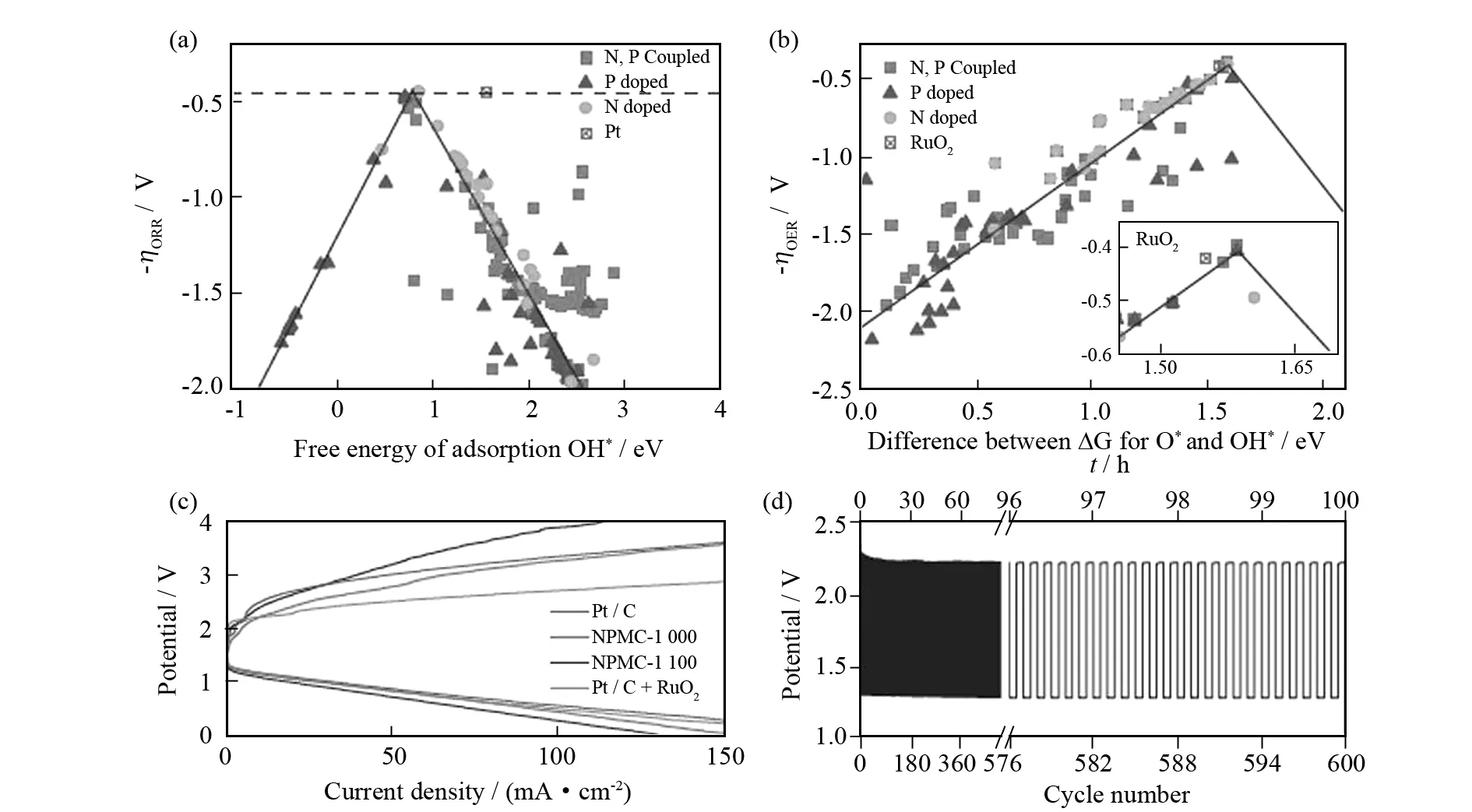
图5 掺N、P石墨烯 (a) ORR和 (b) OER过电势对OH* 吸附能和对 O*、OH* 吸附能差异的火山图, 锌-空电池空气电极NPMC-1000 (c) 充放电极化曲线和 (d) 充放电循环曲线 (电流密度2 mA·cm-2) [66]Fig.5 (a) ORR and (b) OER volcano plots of overpotential η versus adsorption energy of OH* and the difference between the adsorption energy of O* and OH* for N,P-doped graphene,(c) Charge and discharge polarization curves and (d) Discharge/charge cycling curves of Zn-air battery using NPMC-1000 for the air electrodes at a current density of 2 mA·cm-2[66]
2.3 过渡金属氧化物
过渡金属氧化物具有高丰度、低成本和环境友好等优点,已广泛应用为金属-空气电池阴极催化剂.过渡金属元素具有多价态,可以形成各种不同晶体结构的氧化物.本节中将根据过渡金属氧化物的组分和结构介绍4种氧化物电催化剂.
2.3.1 单金属氧化物
锰氧化物由于价态可变、结构丰富和环境友好已广受关注.OGASAWARA等[68]在2006年首次将MnO2引入锂-空电池阴极,自此以后许多研究致力于评估和优化MnOx作为空气电极催化剂[69-72].晶体结构、形貌和结构等影响锰氧化物催化性能的因素将在本节予以讨论.
MnO2由于具有多种晶相和形貌被广泛研究为阴极催化剂.MENG等[73]研究MnO2不同的晶体结构(α-MnO2、β-MnO2、δ-MnO2和无定型MnO2(AMO))对双功能氧催化活性的影响(图6a).此外,各种形貌的MnO2(例如纳米线、纳米片和纳米颗粒)在合成不同晶体结构MnO2时被合成(图6b-g).ORR/OER活性顺序:α-MnO2> AMO >β-MnO2>δ-MnO2(图6h和i),表明晶体结构和形貌是决定氧电化学整体活性的重要因素.为了进一步提高MnO2的催化性能,结构修饰已经被发展和探究.ZHANG等[74]以普鲁士蓝类配合物为前驱体合成分级多孔的δ-MnO2纳米盒,形成的电极具有低过电势、高倍率性能和优异循环稳定性,归因于分级多孔的结构和大比表面积.除此以外,ZHENG等[75]基于密度泛函理论研究α-MnO2表面,发现表面氧位点比暴露的金属位点发挥更重要的作用.表面氧密度越大,Li2O2分散越均匀,形成颗粒小,利于ORR和OER.此报道为非质子锂-氧电池ORR催化机制提供新视角.
其他形式的锰氧化物也被应用为金属-空气电池阴极催化剂.例如:GORLIN 等[76]制备纳米结构的锰氧化物(Ⅲ)薄膜,双功能性能与贵金属媲美,利用原位X射线吸收光谱研究MnxOy的活性位,发现无序相Mn3Ⅱ,Ⅲ,ⅢO4利于ORR,而混合的MnⅢ,Ⅳ氧化物与OER活性相关,表明催化剂表面的氧化态对于促进高活性至关重要.KUO等[77]强调表面结晶度对电催化活性的影响,制备八面体纳米颗粒、纳米花和纳米多豆荚结构的MnO.其中,更易暴露(100)面的纳米多豆荚的ORR/OER催化性能都优于纳米花结构的MnO.
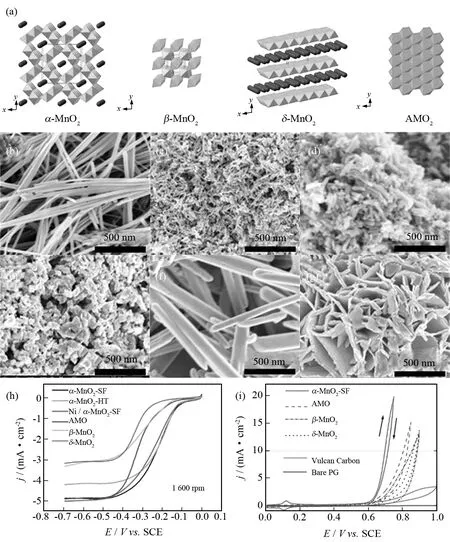
图6 (a) MnO2各种晶相示意图,MnO2纳米结构SEM图:(b) α-MnO2-HT (水热合成),(c) α-MnO2-SF (无溶剂合成), (d) 掺Ni的α-MnO2-HT (无溶剂合成),(e) AMO (无定型),(f) β-MnO2,(g) δ-MnO2,(h) ORR和 (i) OER极化曲线(在O2饱和的0.1 mol/L KOH溶液里,扫描速率5 mV·s-1和1 600 rpm转速下获得) [73]Fig.6 (a) Various crystal phases of manganese oxide,SEM images of manganese oxide nanostructures:(b) α-MnO2-HT (hydrothermal synthesis),(c) α-MnO2-SF (solvent-free synthesis),(d) Ni-doped α-MnO2-HT (solvent-free synthesis),(e) AMO (amorphous),(f) β-MnO2 and (g) δ-MnO2,(h) ORR and (i) OER polarization curves of MnO2 nanostructures obtained at 1 600 rpm with a scan rate of 5 mV·s-1 in O2-saturated 0.1 mol/L KOH solution[73]
2.3.2 尖晶石型金属氧化物
尖晶石型氧化物的通式是AB2O4,其中A是二价金属离子(例如Mg、Fe、Co、Ni、Mn、Zn),B是三价金属离子(例如Al、Fe、Co、Cr、Mn).尖晶石型氧化物由于制备简易、形貌多变和稳定性高成为最受关注的金属基双功能催化剂之一.
Co3O4作为高效ORR/OER双功能催化剂备受关注,由于多价态钴离子充当可逆吸-脱附氧过程中给-受体吸附位,具有双功能活性[78].RIAZ等[79]制备纳米片、纳米针和纳米花形貌的Co3O4电极,发现性能与Co3O4电极的结构密切相关.电流密度为20 mA·gcatalyst-1时的放电容量增加顺序依次为:纳米片(1 127 mAh·gcatalyst-1)< 纳米花(1 930 mAh·gcatalyst-1)< 纳米针(2 280 mAh·gcatalyst-1).ZHANG等[80]以普鲁士蓝类配合物纳米立方体为前驱体合成分级多孔的Co3O4纳米盒,在锂-氧电池中展现出低过电势、高倍率性能和优异循环稳定性.WU等[81]水热合成自支撑分级多孔Co3O4超薄纳米片,具有低过电势和优异的循环性能.纳米片排列形成的大孔使得活性物质与电解液充分接触以及提供足够的Li2O2存储空间,介孔提供充足的ORR/OER催化活性位.基于这些研究表明:具有特殊结构的单金属尖晶石氧化物展现出高效的双功能催化活性.
通常,用第二种金属阳离子对单金属尖晶石氧化物进行组分改性可以调整对氧催化起重要作用的性能(例如:晶体结构和导电性).例如MnxCo3-xO4[82-83]、NixCo3-xO4[84-85]、CuxCo3-xO4[86]和ZnxCo3-xO4[87]已被报道为双功能空气电极材料.除成分以外,催化剂的纳米结构也极大地影响活性.PENG等[83]溶剂热合成3D分级多孔NiCo2O4核壳微球,类似向日葵(图7a),由多孔纳米片构成(图7b).如此独特的结构提高催化位点的利用率,同时加快电子和反应物扩散.SP(导电炭黑)的充放电过电势为1.87 V,比NiCo2O4/SP高640 mV(图7c),而且NiCo2O4/SP电极在锂氧电池中展现出优异的循环稳定性.类似的,WANG等[88]用硬模板法制备分级NiCo2O4中空纳米球,由超薄纳米片构成,与普通刺猬状NiCo2O4相比,具有更低过电势和优异循环稳定性.此外,Mn基尖晶石氧化物也展现出卓越的ORR和OER双功能催化活性,由于锰元素高丰度、低成本和环境友好等诸多优点.CHEN等[89]分别用NaH2PO2和NaBH4作为还原剂,室温快速合成两种不同晶体结构的尖晶石(四方和立方CoxMn3-xO4).有趣的是,立方Co-Mn-O尖晶石的ORR催化能力比四方尖晶石强,而四方尖晶石的OER催化能力优于立方相,DFT理论计算表明相依赖的电催化行为源于两相表面不同的氧吸附结合能.这为合理设计尖晶石型ORR/OER双功能催化剂提供重要的指导.
2.3.3 钙钛矿型金属氧化物
BRUCE等[26]首次使用La0.8Sr0.2MnO3作为非水系锂-氧电池的催化剂,但效能不理想.后来, XU等[41]结合电纺和热处理制备多孔的La0.75Sr0.25MnO3纳米管(PNT-LSM),展现出优异的往返效率、倍率性能和循环稳定性.在锂-氧电池中,PNT-LSM/KB的充电电压比KB低200 mV,库仑效率约100%,而且在1 000 mAh·g-1容量限制下维持124圈.如此杰出的性能来源于PNT-LSM特殊多孔的结构,多孔的管状结构提供更多传输氧和电解质的通道,加速放电产物的分解,因此提高O2电极的可逆性.YANG等报道许多钙钛矿型氧化物作为双功能催化剂[96-98].其中,电子和离子导体Ba0.5Sr0.5Co0.8Fe0.2O3-δ备受关注,掺杂或混合可以进一步提高其ORR/OER活性.JUNG等[99]报道一种新型结构掺La的Ba0.5Sr0.5Co0.8Fe0.2O3-δ催化剂(La0.3(Ba0.5Sr0.5)0.7Co0.8Fe0.2O3-δ)(图9a),菱形的LaCoO3纳米颗粒(~10 nm)分布在立方Ba0.5Sr0.5Co0.8Fe0.2O3-δ表面,展现出与RuO2可比的ORR活性(图9b)以及优于IrO2的OER活性(图9c).此后JUNG等[100]开创性研究纳米钙钛矿Lax(Ba0.5Sr0.5)1-xCo0.8Fe0.2O3-δ氧化物,以纳米粒子方式在可充电金属-空气电池领域寻求突破.众所周知,钙钛矿氧化物的电化学氧催化程度与表面阳离子密切相关,其实这取决于氧化物中氧原子的缺陷程度.在此方面,CHEN等[101]结合溶胶凝胶法和1 300 ℃真空热处理制备具有氧缺陷的六边形晶体结构BaTiO3-x(h-BaTiO3-x),与900 ℃ 空气热处理制备的四方相t-BaTiO3相比,h-BaTiO3-x具有部分占据的氧位点,展现出卓越的双功能活性.中子分析表明:BaTiO3-x的真实化学式是BaTiO2.76,双功能催化活性来源于氧缺陷,加速反应物吸附和电荷转移.这篇文章强调钙钛矿氧化物结构和氧含量的重要性,其可以通过热处理温度和氛围参数来控制.

图7 多孔的NiCo2O4核壳微球 (a) 低倍和 (b) 高倍FESEM图,(c) NiCo2O4/SP和纯碳电极在锂氧电池中首次充放电曲线 (电流密度为200 mA·gcarbon-1)[83]Fig.7 (a) Low-magnified and (b) high-magnified FESEM images of porous NiCo2O4 core-shell microspheres,(c) First charge-discharge curves of Li-O2 cells with NiCo2O4/SP and bare carbon electrodes at a current density of 200 mA·gcarbon-1[83]
尽管钙钛矿氧化物在金属-空气电池的应用备受关注,阳离子部分取代效应的机理尚不明确.除ABO3以外,双钙钛矿氧化物(A2B2O6,8b)[102]和层状钙钛矿氧化物(图8c)[103]很少被研究为金属-空气电池阴极催化剂.因此,未来需要更系统地研究钙钛矿型催化剂来提高ORR/OER的催化性能和促进金属-空气电池的发展[104].

图8 (a) 立方钙钛矿结构[90],(b) 双钙钛矿结构[102],(c) 层状钙钛矿结构[103]Fig.8 (a) Cubic perovskite structure[90],(b) Double perovskite structure[102],(c) Layered perovskite structure[103]
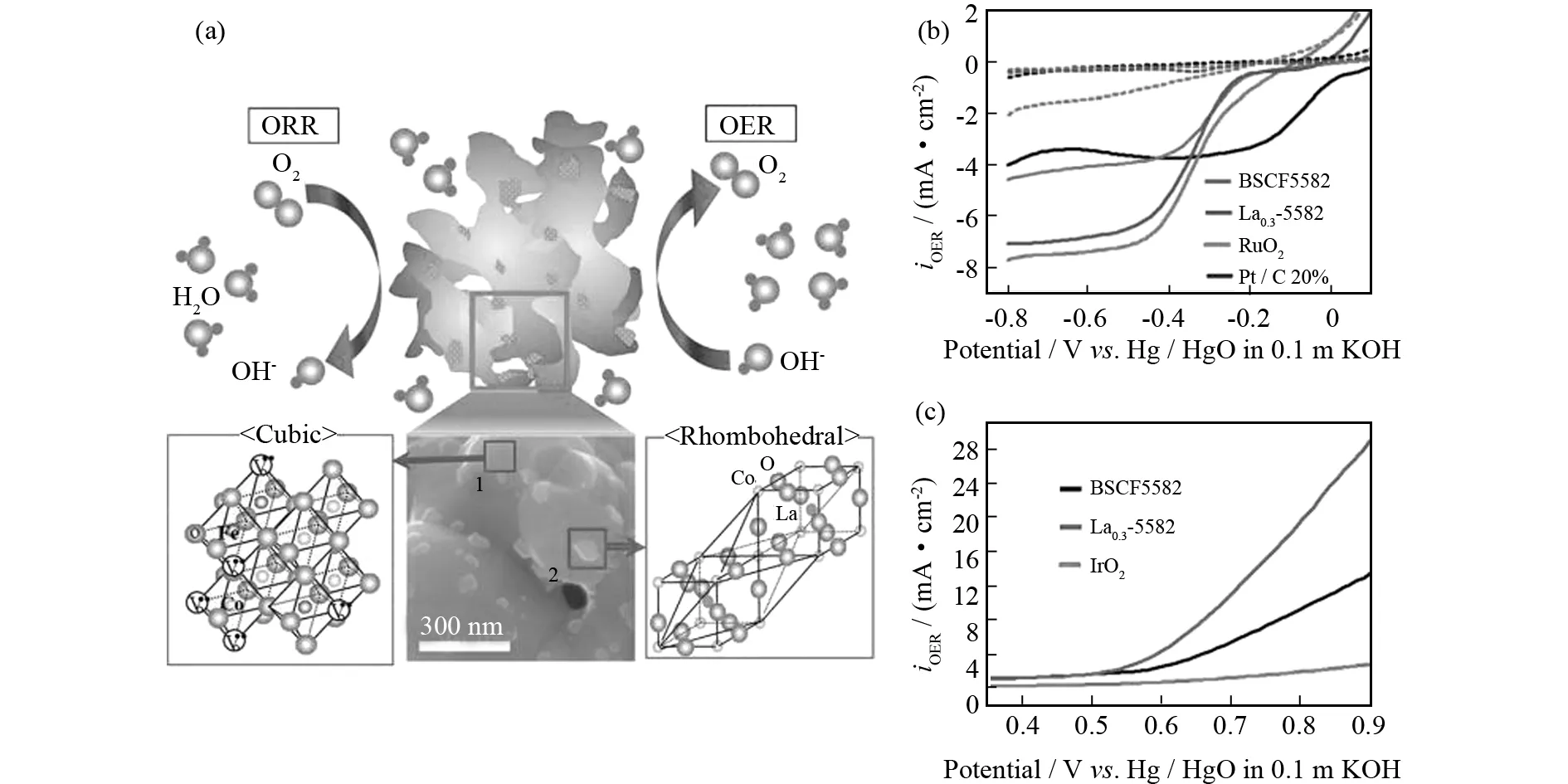
图9 (a) 双功能催化剂La0.3(Ba0.5Sr0.5)0.7Co0.8Fe0.2O3-δ (La0.3-5582)示意图,(b,c) 80% La0.3-5582与20% KB复合物和其他对比催化剂:(b) ORR和 (c) OER活性[99]Fig.9 (a) Illustration of the bifunctional catalyst:La0.3(Ba0.5Sr0.5)0.7Co0.8Fe0.2O3-δ (La0.3-5582),(b) ORR and (c) OER activ-ities of 80% La0.3-5582 on 20% Ketjen Black (KB) composite and other typical catalysts for comparison[99]
2.4 复合材料
如上所述,阴极材料需要催化ORR/OER的高效催化剂,还要具备大比表面积和大孔容量来储存更多的放电产物.但是单材料很难完全满足以上要求,因此,含复合材料的混合电极已成为提高金属-空气电池性能的趋势[105].
2.4.1 贵金属及其氧化物/纳米碳复合物
贵金属及其氧化物与纳米碳复合满足金属-空气电池阴极材料要求[106].功能化的碳在复合物中起重要作用,不仅提供大比表面积,而且促进表面催化剂材料的分散,从而增强催化性能.而且,功能化的碳具有空位和缺陷促进阴极反应.
碳纳米管作为典型的功能化碳材料,已广泛应用于混合电极.LI等[107]用化学湿选法合成多壁碳纳米管纸支撑的Ru催化剂(Ru/MWCNTP),与MWCNTP电极相比,Ru/MWCNTP降低充电电压约0.68 V.MA等[108]用测控溅射法制备贵金属(Ru和Pd)催化的碳纳米管,在锂-氧电池中展现出超高的循环效率和持久的循环寿命.但原位电化学质谱和非原位光谱分析表明:催化的锂-氧电池并没有按照预想的锂-氧反应进行操作,充电过程中伴随着CO2的产生,而且可逆性低于CNT.这篇报道为使用贵金属催化剂的非质子锂-氧电池的氧电化学提供新的视角,同时例证定量测定氧电化学对于追求真正可充电锂-氧电池的重要性.此外,钛的碳化物和氮化物由于超强的耐腐蚀性和高导电性被研究为催化载体.ROCA-AYATS等[109]用乙二醇法合成碳氮化钛担载的 Pt3M(M = Ru、Ir、Ta)纳米材料, Pt3Ru/TiCN 展现出最优异的ORR/OER活性和稳定性,归因于支撑物的促进作用,TiCN稳定活性相和抑制钌溶解.Pt3Ru/TiCN 在一体式可再生燃料电池中具有很大的应用潜力.
YILMAZ等[110]合成多壁碳纳米管支撑的二氧化钌(RuO2/MWCNTs),0.05 mA·cm-2电流密度下的充电电压为3.48 V,低于MWCNTs的3.91 V.分析表明:RuO2纳米颗粒促进低结晶度的过氧化锂层覆盖在MWCNTs表面,而在纯MWCNTs表面形成大的Li2O2颗粒(图10).独特的过氧化锂结构提供与导电CNT电极大的接触面积和许多缺陷,在OER低电位下更易分解.这是首次发现金属氧化物在转变Li2O2晶体结构和形貌方面发挥作用,显著降低OER过程中的能量损失.

图10 (a) CNT和 (b) RuO2/CNT阴极Li2O2形成示意图,(c) CNT和 (d) RuO2/CNT首次放电TEM图[110]Fig.10 Schematic illustration of the Li2O2 formation process in (a) CNT and (b) RuO2/CNT cathodes, TEM images of first-discharged (c) CNT and (d) RuO2/CNT cathodes[110]
2.4.2 过渡金属氧化物/纳米碳复合物
具有混合价的过渡金属氧化物有替代贵金属基础电催化剂的潜力[111],但低的导电性和严重的团聚限制其ORR/OER活性,因此,分散催化剂于导电基质上形成复合物是有效的方法,而且催化剂和导电基质间的协同作用能产生卓越的催化性能[112-114].
近来,CAO等[115]在石墨烯纳米片上原位合成α-MnO2纳米线(α-MnO2/GNSs),展现出卓越的ORR和OER催化活性.电流密度200 mA·g-1时的放电容量为11 520 mAh·g-1,远高于α-MnO2和GNSs混合物的7 200 mAh·g-1.除了锰氧化物以外,Fe氧化物[116-118]、Co氧化物[119-121]、Ni氧化物[122]和Zn氧化物[123]与G或CNT的复合材料也被研究为双功能氧催化剂.
另一种复合型双功能氧催化剂由尖晶石型化物和碳材料组成,例如 DAI课题组合成的Co3O4-N-rmGO(掺氮氧化程度低的还原氧化石墨烯)(图11a)[124],展现出卓越的ORR/OER活性,成为高性能非贵金属双功能催化剂(图11c).X射线近边结构吸收测试(XANES)发现:与N-rmGO相比,Co3O4-N-rmGO复合物C的K边缘峰强度在约288 eV处明显增加(图11d),表明在复合物界面存在Co-O-C 和Co-N-C键,以及催化剂和基质间形成协同作用.而且,GO的氧化程度也对复合物性能产生重要影响[125],传统的GO氧化不能兼顾无机物-碳间耦合作用和复合物的导电性,因此,调控石墨烯适中的氧化程度同时提供丰富的官能团和导电性是达到高ORR/OER性能的关键.复合材料结构的合理设计对氧电催化活性至关重要.LI等[126]制备石榴状Co3O4纳米晶-掺氮部分石墨化碳框架复合催化剂,独特的结构使其具有丰富活性位、强协同耦合作用和快速电子转移能力,是高效的ORR/OER催化剂.类似的,YAN等[127]用SiO2球作为模板合成共价耦合的FeCo2O4-中空结构还原氧化石墨烯球(FCO/HrGOS).与纯FCO和HrGOS相比,FCO/HrGOS复合物展现杰出的电催化活性,ORR性能与20% Pt/C催化剂媲美,OER活性也胜过RuO2/C,归因于FCO和HrGOS间共价耦合作用.而且,3D中空结构的石墨烯球提供高比表面积,同时促进电解液中氧和反应物的有效传输.YAN等[128]简易制备CoFe2O4纳米颗粒/氮、硫双掺杂3D还原氧化石墨烯复合物(CFO/NS-rGO),展现出优异的双功能活性,归因于CFO和NS-rGO间耦合作用和分级多孔的结构.
钙钛矿氧化物和碳材料的复合物在氧电催化方面的应用也备受关注.LEE等[129]合成相互交错的核冠结构双功能催化剂(IT-CCBC),多孔交错的网状NCNTs很好的覆盖LaNiO3纳米颗粒.与纯LaNiO3纳米颗粒和NCNT相比,IT-CCBC展现出高的ORR/OER活性和出色的电化学稳定性,其独特的形貌以及LaNiO3和NCNT间的协同作用提升可充电锌-空电池的性能.此外,La0.58Sr0.4Fe0.2Co0.8O3/NCNT复合物[130]和Nd0.5Sr0.5CoO3-δ纳米线/云状石墨烯纳米片[131]等都具有ORR/OER双功能活性,应用于可充电金属-空气电池.

图11 (a) Co3O4-N-rmGO复合物的TEM图,插图是石墨烯担载Co3O4纳米晶的电子衍射图,(b) Co3O4-N-rmGO复合物的XPS图,(c)分散在碳纸上Co3O4-N-rmGO复合物、Co3O4纳米晶和Pt/C催化剂的ORR和OER活性 (O2饱和的0.1 mol/L KOH), (d) Co3O4-N-rmGO和N-rmGO的C的K边缘XANES图[124]Fig.11 (a) TEM images of the Co3O4-N-rmGO hybrid.The electron diffraction pattern of the Co3O4 nanocrystals on graphene is showed in the inset,(b) XPS spectrum of the Co3O4-N-rmGO hybrid,(c) ORR and OER activities of the Co3O4-N-rmGO hybrid,Co3O4 nanocrystal,and Pt/C catalysts dispersed on carbon fiber paper in O2-saturated 0.1 mol/L KOH,(d) C K-edge XANES of the Co3O4-N-rmGO hybrid and N-rmGO[124]
2.4.3 M-N/C复合物
过渡金属和杂环氮配位化合物形成另一种复合物,展现优异的ORR/OER催化活性[132-134].SUN等[135]报道有机电解液溶解的酞菁铁(FePc)作为锂-空电池溶液相双功能催化剂,提出ORR和OER反应机制:FePc是碳导体以及Li2O2位点间传输O2-和电子的载体,由于Li2O2的生长和分解都未与碳接触,电催化性能明显提升.后来发现简单热解含过渡金属、碳和氮前驱体材料可以制备催化活性物种M-Nx/C,这为涉及廉价前驱体材料的研究提供了新方向.
LI等[136]通过高温热处理法制备富含石墨烯/石墨烯管的N-Fe-MOF催化剂,含纳米笼的金属有机框架(MOF)和双氰胺分别作为模板和碳氮前驱体,研究发现:N-Fe-MOF的放电电压约2.80 V,远高于Pt/C催化剂(2.71 V).而且在电流密度为50 mA·g-1时,最高放电容量为5 300 mAh·g-1.这篇报道为使用MOF新模板合成碳基纳米复合物高效ORR/OER催化剂提供新视野.MENG等[137]热解长在碳布上串有珍珠状ZIF-67的聚吡咯纳米纤维网,原位耦合Co4N和交错的N-C纤维,形成3D自支撑柔性氧电极Co4N/CNW/CC,具有优异的ORR/OER活性和稳定性,归因于Co4N和Co-N-C间协同效应以及3D连通导电网状结构.自支撑柔性氧电极Co4N/CNW/CC在便携式和可穿戴式电子设备领域具有广泛的应用前景.
3 结论
金属-空气电池具有超高能量密度已成为最有发展前景的能源存储与转换技术之一[138],但在投入实际商业化应用之前还有许多问题亟需解决[139-142],如:放电容量低、实际能量密度低和循环性能差等.因此,寻找活性高和稳定性好的ORR/OER双功能催化剂至关重要.贵金属及其合金通常具有高活性和稳定性,但成本高和丰度低;对于碳基材料而言,适当的掺杂能有效提高催化性能,但是碳在OER过程高电位下易腐蚀导致衰减;过渡金属氧化物多价态、低成本、环境友好,但导电性差,因此,过渡金属氧化物-纳米碳复合材料成为新一代具有高催化活性的氧催化材料,两者间的强耦合作用显著提升电化学活性和稳定性.
双功能催化剂的开发及应用面临可充电金属-空气电池性能价格比和稳定性问题.因此,寻求新的电极材料和设计特殊的结构降低阴极过电势,是未来发展可充电金属-空气电池的首要任务.可充电金属-空气电池阴极的未来发展方向是:
1)通过新颖的制备方法,探索出新的阴极材料.
2)合理地设计双功能催化剂的形貌和组分.设计形貌可以增加活性位暴露程度和孔隙率,从而提升催化性能.另一方面,微调催化剂的组分可以调整活性位的电子结构,优化反应过程中与氧分子的相互作用.
3)电极结构的合理设计也对催化剂的运用和能量转换效率的提高至关重要.金属-空气电池的电化学反应包含氧扩散和放电产物的沉积,因此,需要优化空气电极的多孔结构和催化剂的分布以实现反应物的快速传输.
4)理论计算和实验相结合,深入研究充放电过程催化剂表面的氧反应机理,明确各种催化剂活性位,这是发展高效和长寿命电池的先决条件.
[1] ARMAND M,TARASCON J M.Building better batteries [J].Nature,2008,451(7179):652-657.
[2] BRUCE P G,FREUNBERGER S A,HARDWICK L J,et al.Li-O2and Li-S batteries with high energy storage [J].Nature Materials,2012,11(1):19-29.
[3] BRUCE P G,SCROSATI B,TARASCON J M.Nanomaterials for rechargeable lithium batteries [J].Angewandte Chemie International Edition,2008,47(16):2930-2946.
[4] WANG J,LI Y,SUN X.Challenges and opportunities of nanostructured materials for aprotic rechargeable lithium-air batteries [J].Nano Energy,2013,2(4):443-467.
[5] WAGNER F T,LAKSHMANAN B,MATHIAS M F.Electrochemistry and the future of the automobile [J].The Journal of Physical Chemistry Letters,2010,1(14):2204-2219.
[6] NG J W D,GORLIN Y,HATSUKADE T,et al.A precious-metal-free regenerative fuel cell for storing renewable electricity [J].Advanced Energy Materials,2013,3(12):1545-1550.
[7] ABRAHAM K M,JIANG Z.A polymer electrolyte-based rechargeable lithium/oxygen battery [J].Journal of the Electrochemical Society,1996,143(1):1-5.
[8] GIRISHKUMAR G,MCCLOSKEY B,LUNTZ A C,et al.Lithium-air battery:Promise and challenges [J].The Journal of Physical Chemistry Letters,2010,1(14):2193-2203.
[9] KINOSHITA K.Electrochemical oxygen technology [M].New York:Wiley,1992:104-105.
[10] CAO R,LEE J S,LIU M L,et al.Recent progress in non-precious catalysts for metal-air batteries [J].Advanced Energy Materials,2012,2(7):816-829.
[11] NEBURCHILOV V,WANG H J,MARTIN J J,et al.A review on air cathodes for zinc-air fuel cells [J].Journal of Power Sources,2010,195(5):1271-1291.
[12] PADBURY R,ZHANG X W.Lithium-oxygen batteries-limiting factors that affect performance [J].Journal of Power Sources,2011,196(10):4436-4444.
[13] CHRISTENSEN P A,HAMNETT A,LINARES-MOYA D.Oxygen reduction and fuel oxidation in alkaline solution [J].Physical Chemistry Chemical Physics,2011,13(12):5206-5214.
[14] JORISSEN L.Bifunctional oxygen/air electrodes [J].Journal of Power Sources,2006,155(1):23-32.
[15] SPENDELOW J S,WIECKOWSKI A.Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media [J].Physical Chemistry Chemical Physics,2007,9(21):2654-2675.
[16] VIELSTICH W,LAMM A,GASTEIGER H.Handbook of fuel cells-fundamentals,technology and applications [M].Chichester:Wiley,2003:111-112.
[17] RASIYAH P,TSEU C C.The role of the lower metal oxide/higher metal oxide couple in oxygen evolution reactions [J].Journal of the Electrochemical Society,1984,131(4):803-808.
[18] HASEGAWA S,IMANISHI N,ZHANG T,et al.Study on lithium/air secondary batteries-stability of nasicon-type lithium ion conducting glass-ceramics with water [J].Journal of Power Sources,2009,189(1):371-377.
[19] NIMON Y S,VISCO S J.Active metal/aqueous electrochemical cells and systems:US,7645543 [P].2010-04-29.
[20] VISCO S J,KATZ B D,NIMON Y S,et al.Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture:US,7282295 [P].2007-10-16.
[21] WANG Y G,ZHOU H S.A lithium-air fuel cell using copper to catalyze oxygen-reduction based on copper-corrosion mechanism [J].Chemical Communications,2010,46(34):6305-6307.
[22] ZHANG T,IMANISHI N,SHIMONISHI Y,et al.A novel high energy density rechargeable lithium/air battery [J].Chemical Communications,2010,46(10):1661-1663.
[23] LAOIRE C O,MUKERJEE S,ABRAHAM K M,et al.Elucidating the mechanism of oxygen reduction for lithium-air battery applications [J].The Journal of Physical Chemistry C,2009,113(46):20127-20134.
[24] LAOIRE C O,MUKERJEE S,ABRAHAM K M,et al.Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium-air battery [J].The Journal of Physical Chemistry C,2010,114(19):9178-9186.
[25] LU Y C,GASTEIGER H A,CRUMLIN E,et al.Electrocatalytic activity studies of select metal surfaces and implications in Li-air batteries [J].Journal of the Electrochemical Society,2010,157(9):A1016-A1025.
[26] DEBART A,BAO J,ARMSTRONG G,et al.An O2cathode for rechargeable lithium batteries:The effect of a catalyst [J].Journal of Power Sources,2007,174(2):1177-1182.
[27] GIORDANI V,FREUNBERGER S A,BRUCE P G,et al.H2O2decomposition reaction as selecting tool for catalysts in Li-O2cells [J].Electrochemical and Solid-State Letters,2010,13(12):A180-A183.
[28] LU Y C,GASTEIGER H A,PARENT M C,et al.The influence of catalysts on discharge and charge voltages of rechargeable li-oxygen batteries [J].Electrochemical and Solid-State Letters,2010,13(6):A69-A72.
[29] LIU C T,JACKOVITZ J F.Bifunctional gas diffusion electrodes employing wettable,non-wettable layered structure using the mud-caking concept:US,5318862 [P].1994-06-07.
[30] LU Y C,XU Z C,GASTEIGER H A,et al.Platinum-gold nanoparticles:A highly active bifunctional electrocatalyst for rechargeable lithium-air batteries [J].Journal the American Chemical Society,2010,132(35):12170-12171.
[31] KO B K,KIM M K,KIM S H,et al.Synthesis and electrocatalytic properties of various metals supported on carbon for lithium-air battery [J].Journal of Molecular Catalysis A:Chemical,2013,379(1):9-14.
[32] KIM B G,KIM H J,BACK S,et al.Improved reversibility in lithium-oxygen battery:Understanding elementary reactions and surface charge engineering of metal alloy catalyst [J].Scientific Reports,2014,4:4225.
[33] LEE M,HWANG Y,YUN K H,et al.Greatly improved electrochemical performance of lithium-oxygen batteries with a bimetallic platinum-copper alloy catalyst [J].Journal of Power Sources,2015,288:296-301.
[34] SHAO Y Y,PARK S,XIAO J,et al.Electrocatalysts for nonaqueous lithium-air batteries:Status,challenges,and perspective [J].ACS Catalysis,2012,2(5):844-857.
[35] GAO Y,WANG C,PU W H,et al.Preparation of high-capacity air electrode for lithium-air batteries [J].International Journal of Hydrogen Energy,2012,37(17):12725-12730.
[36] LI Y L,WANG J J,LI X F,et al.Superior energy capacity of graphene nanosheets for a nonaqueous lithium-oxygen battery [J].Chemical Communications,2011,47(33):9438-9440.
[37] ZHAO G,ZHANG L,PAN T,et al.Preparation of NiO/multiwalled carbon nanotube nanocomposite for use as the oxygen cathode catalyst in rechargeable Li-O2batteries [J].Journal of Solid State Electrochemistry,2013,17(6):1759-1764.
[38] MEINI S,PIANA M,BEYER H,et al.Effect of carbon surface area on first discharge capacity of Li-O2cathodes and cycle-life behavior in ether-based electrolytes [J].Journal of the Electrochemical Society,2012,159(12):A2135-A2142.
[39] CUI Y M,WEN Z Y,LIANG X,et al.A tubular polypyrrole based air electrode with improved O2diffusivity for Li-O2batteries [J].Energy & Environmental Science,2012,5(7):7893-7897.
[40] XIAO J,HU J Z,WANG D Y,et al.Investigation of the rechargeability of Li-O2batteries in non-aqueous electrolyte [J].Journal of Power Sources,2011,196(13):5674-5678.
[41] XU J J,XU D,WANG Z L,et al.Synthesis of perovskite-based porous La0.75Sr0.25MnO3nanotubes as a highly efficient electrocatalyst for rechargeable lithium-oxygen batteries [J].Angewandte Chemie International Edition,2013,52(14):3887-3890.
[42] CHENG H,SCOTT K.Carbon-supported manganese oxide nanocatalysts for rechargeable lithium-air batteries [J].Journal of Power Sources,2010,195(5):1370-1374.
[43] LI F J,OHNISHI R,YAMADA Y,et al.Carbon supported tin nanoparticles:An efficient bifunctional catalyst for non-aqueous Li-O2batteries [J].Chemical Communications,2013,49(12):1175-1177.
[44] QIN Y,LU J,DU P,et al.In situ fabrication of porous-carbon-supportedα-MnO2nanorods at room temperature:Application for rechargeable Li-O2batteries [J].Energy & Environmental Science,2013,6(2):519-531.
[45] IIJIMA S,ICHIHASHI T.Single-shell carbon nanotubes of 1-nm diameter [J].Nature,1993,364(6430):603-605.
[46] IIJIMA S.Helical microtubules of graphitic carbon [J].Nature,1991,354(6348):56-58.
[47] TIAN G L,ZHANG Q,ZHANG B S,et al.Toward full exposure of “active sites”:Nanocarbon electrocatalyst with surface enriched nitrogen for superior oxygen reduction and evolution reactivity [J].Advanced Functional Materials,2014,24(38):5956-5961.
[48] GEIM A K.Graphene:Status and prospects [J].Science,2009,324(5934):1530-1534.
[49] SOIN N,ROY S S,LIM T H,et al.Microstructural and electrochemical properties of vertically aligned few layered graphene (FLG) nanoflakes and their application in methanol oxidation [J].Materials Chemistry and Physics,2011,129(3):1051-1057.
[50] STOLLER M D,PARK S J,ZHU Y W,et al.Graphene-based ultracapacitors [J].Nano Letters,2008,8(10):3498-3502.
[51] YOO E,ZHOU H S.Li-air rechargeable battery based on metal-free graphene nanosheet catalysts [J].Acs Nano,2011,5(4):3020-3026.
[52] MIRZAEIAN M,HALL P J.Preparation of controlled porosity carbon aerogels for energy storage in rechargeable lithium oxygen batteries [J].Electrochimica Acta,2009,54(28):7444-7451.
[53] SAKAUSHI K,FELLINGER T P,ANTONIETTI M.Bifunctional metal-free catalysis of mesoporous noble carbons for oxygen reduction and evolution reactions [J].ChemSusChem,2015,8(7):1156-1160.
[54] ETACHERI V,SHARON D,GARSUCH A,et al.Hierarchical activated carbon microfiber (ACM) electrodes for rechargeable Li-O2batteries [J].Journal of Materials Chemistry A,2013,1(16):5021-5030.
[55] LIN X J,ZHOU L,HUANG T,et al.Hierarchically porous honeycomb-like carbon as a lithium-oxygen electrode [J].Journal of Materials Chemistry A,2013,1(4):1239-1245.
[56] WANG Z L,XU D,XU J J,et al.Graphene oxide gel-derived,free-standing,hierarchically porous carbon for high-capacity and high-rate rechargeable Li-O2batteries [J].Advanced Functional Materials,2012,22(17):3699-3705.
[57] ZHU Q C,DU F H,XU S M,et al.Hydroquinone resin induced carbon nanotubes on Ni foam as binder-free cathode for Li-O2batteries [J].ACS Applied Materials & Interfaces,2016,8(6):3868-3873.
[58] CHEN Y G,WANG J J,LIU H,et al.Nitrogen doping effects on carbon nanotubes and the origin of the enhanced electrocatalytic activity of supported Pt for proton-exchange membrane fuel cells [J].The Journal of Physical Chemistry C,2011,115(9):3769-3776.
[59] GENG D S,LIU H,CHEN Y G,et al.Non-noble metal oxygen reduction electrocatalysts based on carbon nanotubes with controlled nitrogen contents [J].Journal of Power Sources,2011,196(4):1795-1801.
[60] LIU H,ZHANG Y,LI R Y,et al.Structural and morphological control of aligned nitrogen-doped carbon nanotubes [J].Carbon,2010,48(5):1498-1507.
[61] LI Y L,WANG J J,LI X F,et al.Nitrogen-doped graphene nanosheets as cathode materials with excellent electrocatalytic activity for high capacity lithium-oxygen batte-ries [J].Electrochemistry Communications,2012,18(1):12-15.
[62] YADAV R M,WU J J,KOCHANDRA R,et al.Carbon nitrogen nanotubes as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions [J].ACS Applied Materials & Interfaces,2015,7(22):11991-12000.
[63] LI J C,HOU P X,ZHAO S Y,et al.A 3D bi-functional porous N-doped carbon microtube sponge electrocatalyst for oxygen reduction and oxygen evolution reactions [J].Energy & Environmental Science,2016,9:3079-3084.
[64] VINEESH T V,KUMAR M P,TAKAHASHI C,et al.Bifunctional electrocatalytic activity of boron-doped graphene derived from boron carbide [J].Advanced Energy Materials,2015,5(17):1500658.
[65] QU K,ZHENG Y,DAI S,et al.Graphene oxide-polydopamine derived N,S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution [J].Nano Energy,2016,19:373-381.
[66] ZHANG J T,ZHAO Z H,XIA Z H,et al.A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions [J].Nature Nanotechnology,2015,10(5):444-452.
[67] ZHAO Z H,XIA Z H.Design principles for dual-element-doped carbon nanomaterials as efficient bifunctional catalysts for oxygen reduction and evolution reactions [J].ACS Catalysis,2016,6(3):1553-1558.
[68] OGASAWARA T,DEBART A,HOLZAPFEL M,et al.Rechargeable Li2O2electrode for lithium batteries [J].Journal of the American Chemical Society,2006,128(4):1390-1393.
[69] CHENG F Y,ZHANG T R,ZHANG Y,et al.Enhancing electrocatalytic oxygen reduction on MnO2with vacancies [J].Angewandte Chemie International Edition,2013,52(9):2474-2477.
[70] GORLIN Y,CHUNG C J,NORDLUND D,et al.Mn3O4supported on glassy carbon:An active non-precious metal catalyst for the oxygen reduction reaction [J].ACS Catalysis,2012,2(12):2687-2694.
[71] PICKRAHN K L,PARK S W,GORLIN Y,et al.Active mnox electrocatalysts prepared by atomic layer deposition for oxygen evolution and oxygen reduction reactions [J].Advanced Energy Materials,2012,2(10):1269-1277.
[72] TOMPSETT D A,PARKER S C,BRUCE P G,et al.Nanostructuring ofβ-MnO2:The important role of surface to bulk ion migration [J].Chemistry of Materials,2013,25(4):536-541.
[73] MENG Y T,SONG W Q,HUANG H,et al.Structure-property relationship of bifunctional MnO2nanostructures:Highly efficient,ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media [J].Journal of the American Chemical Society,2014,136(32):11452-11464.
[74] ZHANG J,LUAN Y,LYU Z,et al.Synthesis of hierarchical porousδ-MnO2nanoboxes as an efficient catalyst for rechargeable Li-O2batteries [J].Nanoscale,2015,7(36):14881-14888.
[75] ZHENG Y,SONG K,JUNG J,et al.Critical descriptor for the rational design of oxide-based catalysts in rechargeable Li-O2batteries:Surface oxygen density [J].Chemistry of Materials,2015,27(9):3243-3249.
[76] GORLIN Y,LASSALLE-KAISER B,BENCK J D,et al.In situ X-ray absorption spectroscopy investigation of a bifunctional manganese oxide catalyst with high activity for electrochemical water oxidation and oxygen reduction [J].Journal of the American Chemical Society,2013,135(23):8525-8534.
[77] KUO C H,MOSA I M,THANNEERU S,et al.Facet-dependent catalytic activity of MnO electrocatalysts for oxygen reduction and oxygen evolution reactions [J].Chemical Communications,2015,51(27):5951-5954.
[78] HAMDANI M,SINGH R N,CHARTIER P.Co3O4and Co- based spinel oxides bifunctional oxygen electrodes [J].International Journal of Electrochemical Sciety,2010,5(4):556-577.
[79] RIAZ A,JUNG K N,CHANG W,et al.Carbon-free cobalt oxide cathodes with tunable nanoarchitectures for rechargeable lithium-oxygen batteries [J].Chemical Communications,2013,49(53):5984-5986.
[80] ZHANG J,LYU Z,ZHANG F,et al.Facile synthesis of hierarchical porous Co3O4nanoboxes as efficient cathode catalysts for Li-O2batteries [J].Journal of Materials Chemistry A,2016,4(17):6350-6356.
[81] WU F,ZHANG X,ZHAO T,et al.Hierarchical mesoporous/macroporous Co3O4ultrathin nanosheets as free-standing catalysts for rechargeable lithium-oxygen batteries [J].Journal of Materials Chemistry A,2015,3(34):17620-17626.
[82] MENEZES P W,INDRA A,SAHRAIE N R,et al.Cobalt-manganese-based spinels as multifunctional materials that unify catalytic water oxidation and oxygen reduction reactions [J].ChemSusChem,2015,8(1):164-171.
[83] PENG S J,HU Y X,LI L L,et al.Controlled synthesis of porous spinet cobaltite core-shell microspheres as high-performance catalysts for rechargeable Li-O2batteries [J].Nano Energy,2015,13:718-726.
[84] PRABU M,KETPANG K,SHANMUGAM S.Hierarchical nanostructured NiCo2O4as an efficient bifunctional non-precious metal catalyst for rechargeable zinc-air batteries [J].Nanoscale,2014,6(6):3173-3181.
[85] PRICE S W T,THOMPSON S J,LI X H,et al.The fabrication of a bifunctional oxygen electrode without carbon components for alkaline secondary batteries [J].Journal of Power Sources,2014,259(7):43-49.
[86] LIU Y,CAO L J,CAO C W,et al.Facile synthesis of spinel CuCo2O4nanocrystals as high-performance cathode catalysts for rechargeable Li-air batteries [J].Chemical Communications,2014,50(93):14635-14638.
[87] TAN Y,WU C C,LIN H,et al.Insight the effect of surface Co cations on the electrocatalytic oxygen evolution properties of cobaltite spinels [J].Electrochimica Acta,2014,121(3):183-187.
[88] WANG J,FU Y,XU Y,et al.Hierarchical NiCo2O4hollow nanospheres as high efficient bi-functional catalysts for oxygen reduction and evolution reactions [J].Internoctional Journal of Hydrogen Energy,2016,41(21):8847-8854.
[89] CHENG F Y,SHEN J A,PENG B,et al.Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts [J].Nature Chemistry,2011,3(1):79-84.
[90] CHRONEOS A,VOVK R V,GOULATIS I L,et al.Oxygen transport in perovskite and related oxides:A brief review [J].Journal of Alloys and Compdounds,2010,494(1/2):190-195.
[91] TANAKA H,MISONO M.Advances in designing perovskite catalysts [J].Current Opinion in Solid State and Materials Science,2001,5(5):381-387.
[92] ZHANG H M,SHIMIZU Y,TERAOKA Y,et al.Oxygen sorption and catalytic properties of La1-xSrxCo1-yFeyO3perovskite-type oxides [J].Journal of Catalysis,1990,121(2):432-440.
[93] KANNAN A M,SHUKLA A K,SATHYANARAYANA S.Oxide-based bifunctional oxygen electrode for rechargeable metal/air batteries [J].Journal of Power Sources,1989,25(2):141-150.
[94] SWETTE L L,KACKLEY N D.Oxygen electrodes for rechargeable alkaline fuel cells.Ⅱ [J].Journal of Power Sources,1990,29(3/4):423-436.
[95] SWETTE L L,KACKLEY N D.Oxygen electrodes for rechargeable alkaline fuel cells.Ⅲ [J].Journal of Power Sources,1991,36(3):323-339.
[96] GALLANT B M,KWABI D G,MITCHELL R R,et al.Influence of Li2O2morphology on oxygen reduction and evolution kinetics in Li-O2batteries [J].Energy & Environmental Science,2013,6(8):2518-2528.
[97]LEONARD D N,KUMAR A,JESSE S,et al.Nanoscale probing of voltage activated oxygen reduction/evolution reactions in nanopatterned (LaxSr1-x)CoO3-cathodes [J].Advance Energy Materials,2013,3(6):788-797.
[98] RISCH M,STOERZINGER K A,MARUYAMA S,et al.La0.8Sr0.2MnO3-δdecorated with Ba0.5Sr0.5Co0.8Fe0.2O3-δ:A bifunctional surface for oxygen electrocatalysis with enhanced stability and activity [J].Journal of the American Chemical Society,2014,136(14):5229-5232.
[99] JUNG J I,JEONG H Y,LEE J S,et al.A bifunctional perovskite catalyst for oxygen reduction and evolution [J].Angewandte Chemie International Edition,2014,53(18):4582-4586.
[100] JUNG J I,RISCH M,PARK S,et al.Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis [J].Energy & Environmental Science,2016,9(1):176-183.
[101] CHEN C F,KING G,DICKERSON R M,et al.Oxygen-deficient BaTiO3-xperovskite as an efficient bifunctional oxygen electrocatalyst [J].Nano Energy,2015,13:423-432.
[102] MANDAL T K,GOPALAKRISHNAN J.New route to ordered double perovskites:Synthesis of rock salt oxides,Li4MWO6,and their transformation to Sr2MWO6(M:Mg,Mn,Fe,Ni) via metathesis [J].Chemistry of Materials,2005,17(9):2310-2316.
[103] SCHAAK R E,MALLOUK T E.Perovskites by design:A toolbox of solid-state reactions [J].Chemistry of Materials,2002,14(4):1455-1471.
[104] ZHONG M,XIANXIA Y,LIN L,et al.The double perovskite oxide Sr2CrMoO6-δas an efficient electrocatalyst for rechargeable lithium air batteries [J].Chemical Communications,2014,50(94):14855-14858.
[105] WANG H,YANG Y,LIANG Y,et al.Rechargeable Li-O2batteries with a covalently coupled MnCo2O4-graphene hybrid as an oxygen cathode catalyst [J].Energy & Environmental Science,2012,5(7):7931-7935.
[106] WANG L,ZHAO X,LU Y,et al.CoMn2O4spinel nanoparticles grown on graphene as bifunctional catalyst for lithium-air batteries [J].Journal of the Electrochemical Society,2011,158(12):A1379-A1382.
[107] LI F,CHEN Y,TANG D M,et al.Performance-improved Li-O2battery with Ru nanoparticles supported on binder-free multi-walled carbon nanotube paper as cathode [J].Energy & Environmental Science,2014,7(5):1648-1652.
[108] MA S,WU Y,WANG J,et al.Reversibility of noble metal-catalyzed aprotic Li-O2batteries [J].Nano Letters,2015,15(12):8084-8090.
[109] ROCA-AYATS M,HERREROS E,GARCIA G,et al.Promotion of oxygen reduction and water oxidation at Pt-based electrocatalysts by titanium carbonitride [J].Applied Catalysis B-Environmental,2016,183:53-60.
[110] YILMAZ E,YOGI C,YAMANAKA K,et al.Promoting formation of noncrystalline Li2O2in the Li-O2battery with RuO2nanoparticles [J].Nano Letters,2013,13(10):4679-4684.
[111] XIE X,LI Y,LIU Z Q,et al.Low-temperature oxidation of Co catalysed by Co3O4nanorods [J].Nature,2009,458(7239):746-749.
[112] GUO S,ZHANG S,WU L,et al.Co/CoO nanoparticles assembled on graphene for electrochemical reduction of oxygen [J].Angewandte Chemie International Edition,2012,51(47):11770-11773.
[113] TAN Y,XU C,CHEN G,et al.Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction [J].Advanced Functional Materials,2012,22(21):4584-4591.
[114] WANG H,DAI H.Strongly coupled inorganic-nano-carbon hybrid materials for energy storage [J].Chemical Society Reviews,2013,42(7):3088-3113.
[115] CAO Y,WEI Z,HE J,et al.α-MnO2nanorods grown in situ on graphene as catalysts for Li-O2batteries with excellent electrochemical performance [J].Energy & Environmental Science,2012,5(12):9765-9768.
[116] ANDERSEN N I,SEROV A,ATANASSOV P.Metal oxides/CNT nano-composite catalysts for oxygen reduction/oxygen evolution in alkaline media [J].Applied Catalysis B-Environmental,2015,163:623-627.
[117] CHEN W,ZHANG Z,BAO W,et al.Hierarchical mesoporousγ-Fe2O3/carbon nanocomposites derived from metal organic frameworks as a cathode electrocatalyst for rechargeable Li-O2batteries [J].Electrochimica Acta,2014,134:293-301.
[118] JEE S,CHOI W,AHN C H,et al.Enhanced oxygen reduction and evolution by in-situ decoration of hematite nanoparticles on carbon nanotube cathode for high-capacity nonaqueous lithium-oxygen batteries [J].Journal of Materials Chemistry A,2015,3(26):13767-13775.
[119] LI Y,GONG M,LIANG Y,et al.Advanced zinc-air batteries based on high-performance hybrid electrocatalysts [J].Nature Communications,2013,4(5):1805.
[120] MAO S,WEN Z,HUANG T,et al.High-performance bi-functional electrocatalysts of 3D crumpled graphene-cobalt oxide nanohybrids for oxygen reduction and evolution reactions [J].Energy & Environmental Science,2014,7(2):609-616.
[121] MASA J,XIA W,SINEV I,et al.MnxOy/NC and CoxOy/NC nanoparticles embedded in a nitrogen-doped carbon matrix for high-performance bifunctional oxygen electrodes [J].Angewandte Chemie International Edition,2014,53(32):8508-8512.
[122] LIU X,LIU W,KO M,et al.Metal (Ni,Co)-metal oxides/graphene nanocomposites as multifunctional electrocatalysts [J].Advanced Functional Materials,2015,25(36):5799-5808.
[123] YIN J,CARLIN J M,KIM J,et al.Synergy between metal oxide nanofibers and graphene nanoribbons for rechargeable lithium-oxygen battery cathodes [J].Advanced Energy Materials,2015,5(4):1401412.
[124] LIANG Y,LI Y,WANG H,et al.Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction [J].Nature Materials,2011,10(10):780-786.
[125] LIANG Y,LI Y,WANG H,et al.Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis [J].Journal of the American Chemical Society,2013,135(6):2013-2036.
[126] LI G,WANG X,FU J,et al.Pomegranate-inspired design of highly active and durable bifunctional electrocatalysts for rechargeable metal-air batteries [J].Angewandte Chemie International Edition,2016,55(16):4977-4982.
[127] YAN W,YANG Z,BIAN W,et al.FeCo2O4/hollow graphene spheres hybrid with enhanced electrocatalytic activities for oxygen reduction and oxygen evolution reaction [J].Carbon,2015,92:74-83.
[128] YAN W,CAO X,TIAN J,et al.Nitrogen/sulfur dual-doped 3D reduced graphene oxide networks-supported CoFe2O4with enhanced electrocatalytic activities for oxygen reduction and evolution reactions [J].Carbon,2016,99:195-202.
[129] LEE D U,PARK H W,PARK M G,et al.Synergistic bifunctional catalyst design based on perovskite oxide nanoparticles and intertwined carbon nanotubes for rechargeable zinc-air battery applications [J].ACS Applied Materials & Interfaces,2015,7(1):902-910.
[130] ELUMEEVA K,MASA J,SIERAU J,et al.Perovskite-based bifunctional electrocatalysts for oxygen evolution and oxygen reduction in alkaline electrolytes [J].Electrochimica Acta,2016,208:25-32.
[131] KIM C,GWON O,JEON I Y,et al.Cloud-like graphene nanoplatelets on Nd0.5Sr0.5CoO3-δnanorods as an efficient bifunctional electrocatalyst for hybrid Li-air batteries [J].Journal of Materials Chemistry A,2016,4(4):467-474.
[132] WU G,MORE K L,JOHNSTON C M,et al.High-performance electrocatalysts for oxygen reduction derived from polyaniline,iron,and cobalt [J].Science,2011,332(6028):443-447.
[133] YOO E,ZHOU H.Fe phthalocyanine supported by graphene nanosheet as catalyst in Li-air battery with the hybrid electrolyte [J].Journal of Power Sources,2013,244(4):429-434.
[134] YUAN X,ZENG X,ZHANG H J,et al.Improved performance of proton exchange membrane fuel cells withp-toluenesulfonic acid-doped Co-ppy/C as cathode electrocatalyst [J].Journal of the American Chemical Society,2010,132(6):1754-1755.
[135] SUN D,SHEN Y,ZHANG W,et al.A solution-phase bifunctional catalyst for lithium-oxygen batteries [J].Journal of the American Chemical Society,2014,136(25):8941-8946.
[136] LI Q,XU P,GAO W,et al.Graphene/graphene-tube nanocomposites templated from cage-containing metal-organic frameworks for oxygen reduction in Li-O2batteries [J].Advanced Materials,2014,26(9):1378-1386.
[137] MENG F,ZHONG H,DI B,et al.In situ coupling of strung Co4N and intertwined N-C fibers towards free-standing bifunctional cathode for robust,efficient,and flexible Zn-air batteries [J].Journal of the American Chemical Society,2016,138(32):10226-10231.
[138] ZHOU H.New energy storage devices for post lithium-ion batteries [J].Energy Environ Sci,2013,6(8):2256.
[139] ADAMS J,KARULKAR M,ANANDAN V.Evaluation and electrochemical analyses of cathodes for lithium-air batteries [J].Journal of Power Sources,2013,239(10):132-143.
[140] GRANDE L,PAILLARD E,HASSOUN J,et al.The lithium/air battery:Still an emerging system or a practical reality? [J].Advanced Materials,2015,27(5):784-800.
[141] LI F,ZHANG T,ZHOU H.Challenges of non-aqueous Li-O2batteries:Electrolytes,catalysts,and anodes [J].Energy & Environmental Science,2013,6(4):1125-1141.
[142] PARK M,SUN H,LEE H,et al.Lithium-air batteries:Survey on the current status and perspectives towards automotive applications from a battery industry standpoint [J].Advanced Energy Materials,2012,2(7):780-800.
[责任编辑:吴文鹏]
Progress in cathodic bi-functional catalysts for metal-air battries
WANG Ya,LAI Qingxue,ZHU Junjie,LIANG Yanyu*
(JiangsuKeyLaboratoryofMaterialsandTechnologyforEnergyConversion,CollegeofMaterialsScienceandTechnology,NanjingUniversityofAeronauticsandAstronautics,Nanjing211106,Jiangsu,China)
Rechargeable metal-air batteries are considered as one of the most promising energy storage and conversion devices due to their ultrahigh energy density.Slow kinetics of cathodic electrochemical oxygen reduction/evolution reactions is a key factor affecting the performance of metal-air batteries,which requires a bi-functional catalyst to facilely realize the charge and discharge processes of a metal-air battery.In this review,we discuss the novel bi-functional catalysts developed in recent years,including precious metals,carbon materials,transition metal oxides,and hybrid materials.Among them,transition metal oxide/nanocarbon strong coupled hybrid materials have been developed as a new gene-ration of oxygen catalytic material with promising catalytic activity.Finally,several possible research directions in future are proposed based on the existing problem.
metal-air battery; air cathode; bi-functional catalyst; oxygen reduction/evolution reaction
2016-10-15.
国家自然科学基金项目(21273114),中央高校基本科研业务费(NE2015003),江苏省“六大人才高峰”高层次人才项目(2013-XNY-010),江苏高校优势学科建设工程资助项目.
王 亚(1992-),女,硕士生,研究方向为化学电源与电极材料.*
,E-mail:liangyy403@126.com.
O643.36
A
1008-1011(2017)01-0001-18

